EDIII-DENV3 nanospheres drive immature dendritic cells into a mature phenotype in an in vitro model
ABSTRACT
Domain III of E protein of dengue virus (DENV) is a target for vaccine development. Unfortunately, this protein based platform has low general immunogenicity. To circumvent this problem, the use of an adjuvant-nanoparticle delivery system to facilitate immunogenicity of soluble DENV-EDIII protein was investigated. One of the key features of this delivery system is its ability to simultaneously deliver antigens and exert adjuvanticity on specialized immune cells. In this study, N-trimethyl chitosan (TMC) nanoparticles (NPs) were generated to be used as adjuvant and carrier for soluble E-domain III of dengue virus serotype 3 (sEDIII-D3). Using ionotropic gelation, purified sEDIII-D3 was encapsulated into TMC NPs to form EDIII-D3 TMC NPs. After optimization, EDIII-D3 TMC particles exhibited a loading efficiency of 81% and a loading capacity of 41%. The immunogenicity of EDIII-D3 TMC NPs was tested using monocyte-derived dendritic cells (MoDCs). It was found that EDIII-D3 TMC NPs were well taken up by MoDCs. In addition, EDIII-D3 TMC NP treated MoDCs significantly upregulated maturation markers (CD80, CD83, CD86 and HLA-DR) and induced secretion of various cytokines and chemokines (IFN-α, IL-1β, IL-6, IL-2, IL-12p70, IFN-γ, IL-4, IL-10, IL-8, MCP-1, macrophage inflammatory protein-1β, granulocyte-colony stimulating factor, granulocyte–macrophage colony-stimulating factor and IL-7). These results indicate that EDIII-D3 TMC NPs are potent immunogens, at least in vitro, with the ability to induce maturation of DCs and highlight the potential use of TMC NPs for enhancing immunogenicity of a non-replicating dengue vaccine.
List of Abbreviations
-
- DENV
-
- dengue virus
-
- E
-
- envelope
-
- EDIII-D3
-
- domain III of dengue serotype-3 envelope protein
-
- EDIII-D3 TMC NPs
-
- domain III of dengue serotype-3 envelope protein loaded into trimethyl chitosan nanoparticles
-
- G-CSF
-
- granulocyte-colony stimulating factor
-
- GM-CSF
-
- granulocyte–macrophage colony-stimulating factor
-
- MFI
-
- mean fluorescence intensity
-
- MIP-1β
-
- macrophage inflammatory protein-1β
-
- MoDC
-
- monocyte-derived dendritic cell
-
- NP
-
- nanoparticle
-
- sEDIII-D3
-
- soluble domain III of dengue serotype-3
-
- RPLP0
-
- large ribosomal protein P0
-
- TMC
-
- trimethyl chitosan
Dengue virus belongs to the Flaviviridae family and has four antigenically distinct serotypes (DENV-1 to -4). All four serotypes can cause a spectrum of symptoms ranging from mild fever up to life-threatening shock syndrome 1. The emergence and re-emergence of DENV is not only a major public health problem, but also a significant economic burden in more than 100 affected countries 2. An effective vaccine is therefore urgently needed. Among the vaccines currently being developed, a live-attenuated tetravalent dengue-yellow fever 17D chimeric virus vaccine has recently been registered in a few countries 3, 4. This licensed vaccine provides an overall protection of 60% and is not recommended for children aged under 9 years, the group that is most in need of it 5, 6. Thus, a more effective vaccine is still needed.
Despite their high immunogenicity, use of live-attenuated vaccines involves problems that are yet to be solved, including interference between serotypes of replicating DENV vaccine and lack of suitability for immunocompromised individuals. Thus, there is a need for a subunit vaccine containing only the antigens that are essential for stimulating a protective immune response. Unfortunately, subunit vaccines are usually poor immunogens and thus require exogenous adjuvants and appropriate delivery systems 7. There has been growing interest in using nanospheres as vehicles for vaccines 8, 9. The distinctive advantages of nanosphere-delivered vaccines over conventional subunit vaccines are their ability to stabilize antigens, enhance antigen uptake by antigen presenting cells, and their designed adjuvanticity 10-12. Several vaccines based on nanotechnology are now in the preclinical phase 13-16; hence, this approach could be used to develop a dengue subunit vaccine.
DENV E-protein contains serotype-specific and conformation-dependent neutralizing epitopes 17-19, which elicit long-lasting protective antibody responses 20, 21. The protein consists of 495 amino acids that are organized into three discrete domains: a central domain (EDI), a dimerization domain (EDII) and an immunoglobulin-like domain (EDIII) 22. Of these three domains, EDIII is the target of choice for vaccine development because it contains the host cell receptor recognition site and serotype-specific neutralizing epitopes 17. Thus, several forms of DENV-EDIII vaccine candidates have been developed and been found to have the ability to stimulate protective, broad immune responses in mice and non-human primates 23-25.
N-trimethyl chitosan chloride, a cationic water-soluble compound, is biocompatible, biodegradable, non-toxic, muco-adhesive and enhances absorption. When treated with TMC, DCs upregulate maturation markers, highlighting its immunopotentiating activity 26, 27. In addition, TMC NPs have been found to enhance immunogenicity of encapsulated antigens 28-30. Therefore, we sought to use TMC NPs as an adjuvant delivery system for the EDIII-DENV immunogens.
We encapsulated yeast-derived EDIII-D3 into TMC NPs and the physicochemical properties, adjuvanticity and immunogenicity of dengue-nanospheres were investigated using a human ex vivo system, primary human MoDCs. DCs are the frontline of immune cells encountering invading pathogens and serve as the principal antigen-presenting cells that crosstalk between innate and adaptive immunity. They are well recognized as one of the main target cells of DENV infection 31. Therefore, responses of DCs to dengue immunogens would be a useful tool for validating selected vaccine platforms before developing more complicated in vivo models. In this study, it was demonstrated that EDIII of DENV-3 encapsulated in NPs safely and strongly drives immature DCs into a mature phenotype that produce high concentrations of IFN type I and other mediators of antiviral innate immunity.
MATERIALS AND METHODS
Virus
DENV-3 (strain 16562) was propagated in C6/36 cells.
Preparation of EDIII-D3 antigen
EDIII-D3 was produced as previously described, with minor modifications 32, 33. Briefly, viral genomic RNA was extracted from DENV-3 infected C6/36 cells using TRIzol (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed using AMV reverse transcriptase (Promega, Madison, WI, USA). A DENV-3 EDIII cDNA fragment was amplified using a high fidelity DNA polymerase (Toyobo Life Science, Osaka, Japan) using primers: 5ʹ-TTT CTG CAG TCA AGG GGA TGA GCT ATGC-3ʹ (sense) and 5ʹ-TTT TCT AGA GAG CTT CCC TTC CTG TACC-3ʹ (antisense). The amplified product was purified and cloned into the pGEM-T vector. Purified pGEM-T EDIII-D3 plasmid was sub-cloned into plasmid pPICZαB before being electroporated into Pichia pastoris. Transformed colonies were selected and confirmed according to the protocol recommended by the manufacturer (Invitrogen). Secreted, his-tagged sEDIII-D3 was purified by immobilized metal affinity chromatography using a nickel-chelating resin (Invitrogen). Purified EDIII-D3 was characterized by immunoblotting analysis using a mouse anti-EDIII DENV-3 mAb (clone 8A1) and an anti-polyhistidine antibody. The contaminated endotoxin in purified sEDIII-D3 was tested using the Limulus amebocyte lysate assay (QCL-1000; Pierce, Rockford, IL, USA) and found to be <0.1 EU/mg.
Virus blocking assay
Cultures of Vero cells were pre-incubated with either various concentrations of purified sEDIII-D3 (0–400 μg/mL) or BSA at 37°C for 30 min. After treatment, cultures were inoculated with DENV-3 (MOI 0.01). The inoculated cultures were washed three times with PBS and cultured in fresh medium (1 mL/well) for 3 days at 5% CO2, 37°C. Supernatants were collected on Day 3 of infection and subjected to standard plaque assay. DENV-3 infected cultures were used as control.
Preparation of EDIII-D3 N-trimethyl chitosan nanoparticles
Chitosan powder (Seafresh Chitosan (Lab), Bangkok, Thailand) with a degree of deacetylation of 94% was used for N-TMC synthesis. Methylation of chitosan was carried out by a single treatment with iodomethane in the presence of sodium hydroxide and N-methyl pyrrolidone at 60°C for 45 min. The TMC product was dialyzed against deionized water and lyophilized. The product was then analyzed by 1H-nuclear magnetic resonance spectroscopy (Unity Inova 400; Varian, Palo Alto, CA, USA) and the degree of quaternization calculated.
EDIII-D3 TMC NPs was prepared by ionotropic gelation as previously described 34. Briefly, various amounts of sEDIII-D3 protein (0.1, 0.2, 0.4 and 0.8 mg/mL) were dissolved in a sodium tripolyphosphate solution (0.416 mg/mL). This solution was then mixed under continuous stirring with a TMC chloride solution (2 mg/mL) containing 0.5% (w/w) Tween 80 in HEPES, pH 7.4, for 1 hr. The NPs were collected by centrifugation at 10,000 g for 15 min and resuspended in HEPES. The amount of encapsulated EDIII-D3 was calculated by measuring the difference between the initial amount of sEDIII-D3 and the quantity of non-encapsulated sEDIII-D3 remaining in the supernatants after centrifugation. The amount of non-entrapped protein remaining in the supernatant was measured by µBCA assay using BSA as a standard. The measured amount of EDIII-D3 was used to calculate the loading efficiency and loading capacity. A suspension of non-loaded NPs without Tween 80 was used as a blank. The contaminated endotoxin in EDIII-D3 TMC NPs and TMC NPs was <0.1 EU/mg.
Characterization of nanoparticles
The hydrodynamic diameter (nm) and zeta-potential (mV) of NPs were determined by dynamic light scattering and laser Doppler electrophoresis using a Malvern Zetasizer Nano Series Nano-ZS (Malvern Instruments, Westborough, MA, USA). The particle size is reported as a polydispersity index. Morphology and average size of the dried NPs were analyzed by transmission electron microscopy (Tecnai G2 F20 S-TWIN; FEI, Hillsboro, OR, USA) as previously described 35.
To investigate NP stability, NP suspensions were sealed in ampoules immediately after preparation and stored at either −20°C or 4°C. The particle size was measured as described above.
EDIII-D3 release study
The release profile of EDIII-D3 from TMC NPs was investigated under two physiological conditions, pH 7.4 and pH 5.0. Briefly, EDIII-D3 TMC NPs were resuspended in 6 mL of 0.1 M PBS (50 μg/mL), and kept at 37°C for 42 days under constant shaking (100 rpm). At specified time points, 0.5 mL of the suspensions were aliquoted and centrifuged at 18,000 g for 15 min. An equal volume of PBS was added to maintain a constant volume. The protein concentration in the supernatants was analyzed using a µBCA protein assay. A sample consisting of empty TMC NPs in PBS was used as a negative control.
Generation of MoDCs
CD14+ cells were purified from peripheral blood mononuclear cells of healthy volunteers using CD14-antibody-magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) 36. CD14+ cells (1 × 106 cells/mL) were differentiated by cultivation in RPMI 1640 supplemented with 10% heat-inactivated human AB serum, 1% (v/v) penicillin/streptomycin, 100 ng/mL of GM-CSF and 100 ng/mL of IL-4 at 37°C, 5% CO2. The medium was changed every other day for 7 days. MoDCs were identified as cells positive for both CD11c and CD209, these being detected by using specific antibodies (R&D Systems, Minneapolis, MN, USA). The purity of the MoDCs was determined by flow cytometry.
Cytotoxicity test
MoDC cultures were incubated with various amounts of TMC NPs or EDIII-D3 TMC NPs (0–450 μg/mL). The viability of cells was evaluated at 48 hr using a trypan blue exclusion assay.
Measurement of NPs uptake
MoDCs cellular up-take of NPs was performed as previously described 37. Cultures of MoDCs (1 × 106 cells/mL) were treated with EDIII-D3 TMC NPs (25–112.5 μg/mL) or sEDIII-D3 (25 µg/mL). Cells were harvested after 24 and 48 hr of treatment and washed with perm wash (BD Biosciences, Franklin Lakes, NJ, USA) three times prior to permeabilization with Cytofix/Cytoperm (BD Biosciences). Intracellular EDIII-D3 was detected using a mouse anti-EDIII DENV-3 mAb, whereas an Alexa Fluor 488-conjugated goat anti-mouse antibody was used as a secondary antibody. The MFI and frequency of Alexa Fluor 488 positive cells were determined by flow cytometry.
Immunostimulatory effect of EDIII-D3 TMC nanoparticles
MoDC cultures were treated for 2 days with sEDIII-D3 (25 μg/mL), TMC NPs (25–112.5 μg/mL), EDIII-D3 TMC NPs (25–112.5 μg/mL) or 1 μg/mL of LPS as a positive control. Cells and supernatants were harvested at specified time points. Surface expression of DC maturation markers (CD80, CD83, CD86 and HLA-DR) were measured by flow cytometry.
Amounts of IL-1β, IL-6, TNF-α, IL-2, IL-12p70, IL-17, IFN-γ, IL-4, IL-5, IL-10, IL-13, MCP-1, MIP-1β, IL-8, G-CSF, GM-CSF and IL-7 in harvested supernatants were quantified using the Bio-Plex human cytokine assay kit (Bio-Rad, Hercules, CA, USA) following the manufacturer's protocol. The concentration of IFN-α was determined using an ELISA kit (VeriKine; PBL Interferon Source, Piscataway, NJ, USA).
Detection of cytokines/chemokine gene expression by qRT-PCR
MoDCs were stimulated with EDIII-D3 TMC NPs (112.5 μg/mL), TMC NPs (112.5 μg/mL), sEDIII-D3 (25 μg/mL) or LPS (1 μg/mL) for 8 and 18 hr. Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. First strand cDNA was prepared using Superscript II Reverse Transcriptase (Invitrogen) with oligo(dT) primers (Sigma–Aldrich, St Louis, MO, USA). The synthesized cDNA was used as template in a real-time PCR mix as described in the manufacturer's protocol (iQ SYBR Green Supermix reagents and iQ5 detection system; Bio-Rad, Hercules, CA, USA). The reaction was performed in 20 µL with 2 µL of the respective cDNA samples with annealing temperatures of 60°C–65°C. All measurements were performed in triplicate. All primer sequences are provided as follows: TNF-α sense 5ʹ-ATG AGC ACT GAA AGC ATG ATCC-3ʹ, antisense 5ʹ-GAG GGC TGA TTA GAG AGA GGTC-3ʹ; IL-6 sense 5ʹ-AAC CTG AAC CTT CCA AAG ATGG-3ʹ, antisense 5ʹ-TCT GGC TTG TTC CTC ACT ACT-3ʹ; IL-8 sense 5ʹ-ACT GAG AGT GAT TGA GAG TGG AC-3ʹ, antisense 5ʹ-AAC CCT CTG CAC CCA GTT TTC-3ʹ; IL-10 sense 5ʹ-GCC TAA CAT GCT TCG AGA TC-3ʹ, antisense 5ʹ-TGA TGT CTG GGT CTT GGT TC-3ʹ; RPLP0 sense 5′-GGC ACC ATT GAA ATC CTG AGT GAT GTG-3′, antisense 5′-TTG CGG ACA CCC TCC AGG AAG-3′. mRNA content was calculated using the formula 2-Δct, where Δct represents the difference between the gene of interest and the reference gene, RPLP0.
Statistical analysis
Statistical analysis was performed using Stat View for Windows. Student's t-test or one-way anova was used when appropriate. Simple regression was used to verify linearity of the correlations between variables. Differences were considered significant for P < 0.05.
RESULTS
Preparation and characterization of EDIII-D3
Purified yeast-derived EDIII-D3 was subjected to immunoblotting. As expected, the molecular weight of sEDIII-D3 was 14 kDa (Fig. 1a,b). The ability of sEDIII-D3 to block DENV-3 entry was measured and it was found that treatment with sEDIII-D3 before infection with DENV-3 significantly reduced DENV-3 production by 70%, 46% and 30% when the cells had been pretreated with 400, 100 or 25 μg/mL of sEDIII-D3, respectively (Fig. 1c). These results indicate that sEDIII-D3 is properly folded when expressed in P. pastoris.

Characterization of EDIII-D3 TMC NPs
TMC NPs and EDIII-D3 TMC NPs were generated using ionotropic gelation. The average size, zeta-potential, loading efficiency and loading capacity of the EDIII-D3 TMC NPs are shown in Table 1. The results suggest that NP size and charge tend to diminish with the amount of encapsulated protein as evidenced by the size of particles decreasing from 346.9 ± 3.3 nm to 255.1 ± 4.2 nm at 0.1 and 0.8 mg/mL of sEDIII-D3, respectively. Moreover, it was found that the tested protein concentrations had no effect on the size distribution of particles, a narrow size distribution being observed with the polydispersity index ranging between 0.21 ± 0.01 and 0.28 ± 0.02. In contrast, the amount of protein used contributed significantly to the surface charge of the particles. The higher the amount of protein encapsulated, the lower the positive surface charge obtained. Loading efficiency and loading capacity increased from 14.9% to 81.4% and 15.7% to 41.4%, respectively, as the amount of EDIII-D3 increased. Hence, NPs produced with 2 mg/mL of TMC, 0.416 mg/mL of sodium tripolyphosphate and 0.8 mg/mL of sEDIII-D3 were used in the subsequent experiments.
| [sEDIII-D3] (mg/mL) | Size (nm) | PDI | ZP (mV) | LE (%) | LC (%) |
|---|---|---|---|---|---|
| 0.1 | 346.9 ± 3.3 | 0.211 ± 0.011 | +34.3 ± 0.2 | 0.00 | 15.7 ± 2.2 |
| 0.2 | 410.9 ± 12.8 | 0.256 ± 0.017 | +30.3 ± 0.3 | 14.9 ± 2.1 | 26.5 ± 0.6 |
| 0.4 | 294.6 ± 6.5 | 0.282 ± 0.023 | +27.8 ± 1.1 | 28.4 ± 2.3 | 36.1 ± 2.0 |
| 0.8 | 255.1 ± 4.2 | 0.211 ± 0.019 | +25.5 ± 0.4 | 81.4 ± 2.8 | 41.4 ± 1.1 |
- Data are presented as mean values ± SD, n = 3.
- LC, loading capacity; LE, loading efficiency; PDI, polydispersity index; ZP, zeta-potential.
The morphology of empty and EDIII-D3 TMC NPs was observed by transmission electron microscopy. Both types of NPs were spherical in shape and homogenous in size. The TMC NPs had smooth surfaces (Fig. 2a,b), whereas the EDIII-D3 TMC NPs had rough surfaces and dense cores (Fig. 2c,d). The average particle diameters were 214 nm and 243 nm for TMC NPs and EDIII-D3 TMC NPs, respectively. The sizes measured by Zetasizer were 225.9 nm for TMC NPs and 255.1 nm for EDIII-D3 TMC NPs.
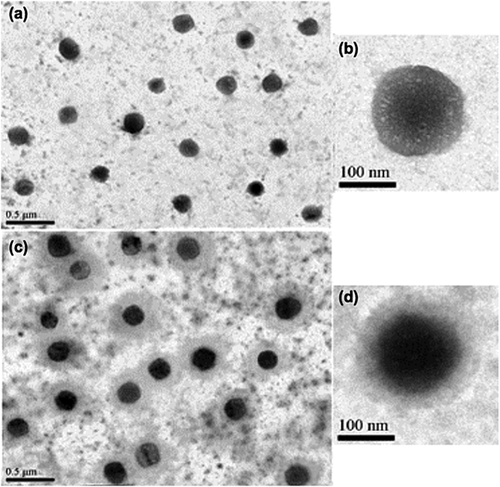
To determine the effect of the encapsulation process on EDIII-D3 antigenicity, NPs were destabilized with 10% (w/v) NaCl solution. The released proteins were subjected to dot enzyme immunoassay and immunoblotting (Supporting Information Fig. S1). It was found that anti-EDIII-D3 mAb reacted with the released EDIII-D3 detected by both assays (Supporting Information Fig. S1a,c). In addition, the size of EDIII-D3 released from the NPs was comparable to the size of purified sEDIII-D3. These results indicate that the encapsulation process was suitable for EDIII-D3 TMC NPs preparation.
Stability of EDIII-D3 TMC NPs and kinetics of EDIII-D3 release
The stabilities of NPs at −20°C and 4°C were tested. When frozen suspensions of NPs were thawed, NP aggregations were clearly observed (data not shown). In contrast, the size of the NPs kept in suspensions at 4°C was stable for at least 3 weeks. TMC NPs average size was 226.7 ± 3.9 nm and 242.9 ± 7.2 nm at time 0 and in the third week, respectively. The size of EDIII-D3 TMC NPs changed from 255.7 ± 3.6 nm at time 0 to 267.3 ± 7.2 nm in Week 3. In the fourth week, the particle size increased dramatically to 350 nm. Therefore, in the present study the control and EDIII-D3 TMC NPs were kept at 4°C and used within a week.
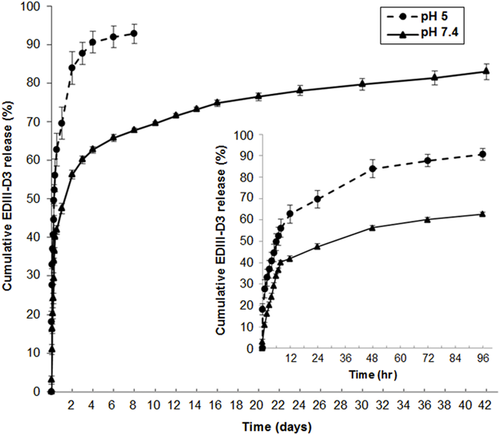
To assess the EDIII release profile, the kinetics of sEDIII-D3 released from the NPs was monitored at pH 5 and pH 7.4 over 42 days. As shown in Figure 3, at pH 7.4 around 40% of sEDIII-D3 was released within the first 12 hr and 70% was released within 10 days, followed by progressive release of up to 85% after 42 days. The release profile at pH 5 showed a characteristic burst release in which 60% and up to 90% of their original contents were released within the first 12 hr and Day 4 of experimentation, respectively. This difference may be due to the burst effect of the protonated amine groups of TMC in acidic conditions 38, 39.
Toxicity and cellular uptake of EDIII-D3 TMC NPs
To explore the safety characteristics of TMC NPs, the percentages of viable cells were quantified after 48 hr of exposure to NPs. The percentages of viable MoDCs were 90.0 ± 0.5, 89.7 ± 5.7 and 89.7 ± 3.2% for untreated, TMC NPs-treated and EDIII-D3 TMC NPs-treated cultures, respectively. These results indicate that the particles are not toxic for MoDCs.
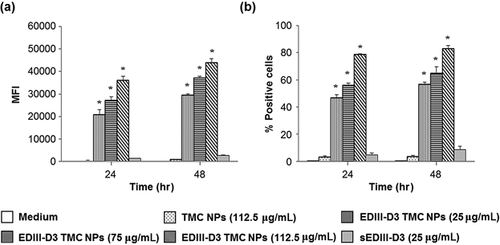
Next, whether TMC NPs facilitate EDIII-D3 uptake was assessed. Using an EDIII-D3 specific antibody and flow cytometry, it was found that the MFI of cells treated with EDIII-D3 TMC NPs was 29,500 ± 600, 37,050 ± 750 and 43,925 ± 1705 at 25, 75 and 112.5 µg/mL treatment, respectively (Fig. 4a). The percentages of EDIII-D3 positive cells were 56.9 ± 1.3, 65.1 ± 4.7 and 82.9 ± 2.3%, respectively, after 48 hr of EDIII-D3 TMC NPs treatment (Fig. 4b). In contrast, sEDIII-D3 uptake was much lower, as evidenced by the low MFI (1416 ± 119 and 2746 ± 187 at 24 and 48 hr of treatment, respectively) and low percentage of EDIII-D3 positive cells (4.7 ± 1.5 and 8.6 ± 2.5% at 24 and 48 hr, respectively). These results indicate that the TMC NPs effectively delivered EDIII-D3 into MoDCs.
EDIII-D3 TMC NPs and TMC NPs strongly upregulated maturation markers on MoDCs
To determine whether this particulate form of dengue antigens has the potential to be a strong immunogen in humans, the ability of EDIII-D3 TMC NPs to induce maturation of MoDCs was investigated. The strength of expression of CD80, CD83, CD86 and HLA-DR on MoDCs treated with TMC NPs, EDIII-D3 TMC NPs or sEDIII-D3 was measured using flow cytometry and it was found that EDIII-D3 TMC NPs upregulate expression of all four molecules significantly more strongly than do TMC NPs, mock-treated and sEDIII-D3 treated MoDCs. It was also demonstrated that upregulation of these surface molecules was dose-dependent and could be detected within 24 hr of treatment. These findings show that MoDCs cultures treated with 25 μg/mL of sEDIII-D3 upregulate these markers to the same degree as mock-treated cultures (Fig. 5a–d and Supporting Information Fig. S2). This may be due to the poor uptake of sEDIII-D3 by MoDCs.
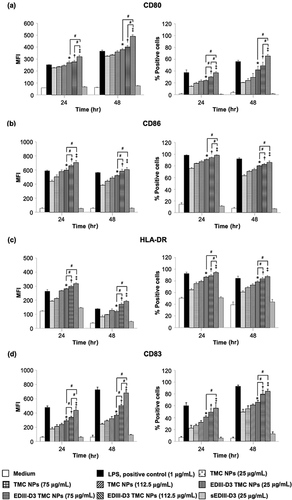
Interestingly, EDIII-D3 TMC NPs activated expression of all measured maturation markers as extensively as observed with LPS, a positive control. In addition, the MFI of all measured markers of MoDCs treated with 112.5 μg/mL of TMC NPs were similar to that of these cells treated with LPS at 24 hr, suggesting that TMC NPs may act as a danger signal and have the potential to act as an adjuvant. These data indicate the potential role of TMC NPs as an adjuvant delivery system that can improve the ability of sEDIII-D3 to induce maturation of MoDCs.
Production of cytokines and chemokines
The maturation status of the NP-treated MoDCs was further investigated by determining amounts of cytokines/chemokines in the supernatants of MoDCs treated with EDIII-D3 TMC NPs, TMC NPs, sEDIII-D3 or mock-treated cultures. As shown in Figure 6a, proinflammatory cytokines such as IL-1β, IL-6 and TNF-α were induced significantly more strongly in the EDIII-D3 TMC NPs treated cells than in the TMC NPs- or sEDIII-D3-treated cells. The TMC NPs were a stronger inducer of these cytokines than sEDIII-D3. Finally, sEDIII-D3 treated cells produced more IL-1β, IL-6, and TNF-α than did mocked-treated cells. Remarkably, TMC NPs rapidly and strongly upregulated IL-1β production, implying that TMC NPs activates an inflammasome.
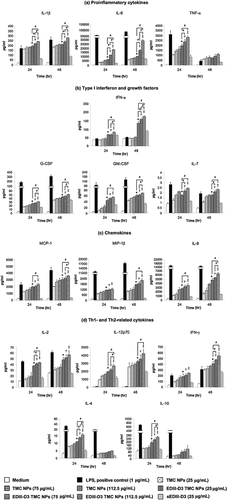
The production of growth factors (G-CSF, GM-CSF and IL-7), chemokines (MCP-1, MIP-1β and IL-8), Th1-related cytokines (IL-2, IL-12p70 and IFN-γ) and Th2-related cytokines (IL-4 and IL-10) followed a similar pattern, the greatest amounts being generated by EDIII-D3 TMC NPs-treated cells with a dose-dependent profile across all time points (Fig. 6b–d).
Interestingly, large amounts of the antiviral cytokine IFN-α were released by EDIII-D3 TMC NPs treated cells (174.5 ± 3.4 pg/mL), whereas sEDIII-D3 induced greater amounts of IFN-α than of TMC NPs (Fig. 6b). Hence, it is plausible that stimulation of IFN-α expression by EDIII-D3 TMC NPs resulted from encapsulated EDIII-D3.
Quantitative real-time PCR was used to confirm the effect of these forms of immunogens on the strength of transcription of cytokines encoding genes. The data demonstrated that EDIII-D3 TMC NPs significantly induce transcription of IL-6, IL-8, IL-10 and TNF-α genes (Fig. 7), suggesting that kinetics of amounts of cytokine mRNA are consistent with the kinetic of protein accumulation in the culture supernatant.
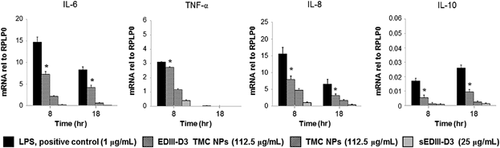
In summary, the up-regulation of maturation markers and cytokines/chemokines indicated that TMC NPs and EDIII-D3 TMC NPs were able to drive maturation of MoDCs, whereas sEDIII-D3 alone induced maturation of MoDCs much more weakly. Thus, encapsulated EDIII-D3 is more immunogenic than the soluble form.
DISCUSSION
The major concern for replicating dengue vaccine is the inter-serotype interference, which can result in unbalanced responses to the four serotypes. Although non-replicating vaccines are not subject to this limitation, they generally have limited immunogenicity because of their “minimalist” compositions. An interesting approach to circumventing this hurdle is the use of NP-based vaccines, which enhance antigen uptake, exert adjuvant effects and can even dictate the type of immune response elicited 40. One example of this platform as applied to a potential dengue vaccine that used UV-inactivated DENV absorbed into chitosan NPs has recently been published. This form of candidate dengue vaccine stimulated robust immune responses in a mouse model and proved very immunogenic in human MoDCs 37, 41. In this study, we explored the immunogenicity of EDIII-D3 encapsulated into adjuvant nanospheres, having recently reported that EDIII-D3 administered via a nanosphere delivery system powerfully stimulates mucosal epithelial cell responses 42.
We investigated the biological properties of yeast-derived sEDIII-D3 and found it remained intact, as illustrated by its ability to inhibit the entry and production of DENV-3 in Vero cells. Moreover, we succeeded in encapsulating purified sEDIII-D3 into TMC NPs. The EDIII-D3 TMC NPs particles were smaller in our system, suggesting tight interactions between sEDIII-D3 and TMC matrix, which may be triggered by the cross-linking activities of sEDIII-D3 and TPP 43. In addition, the high loading may result from ionic interactions between the negatively charged EDIII-D3 and positively charged quaternary ammonium groups of TMC and the crosslinking activity of TPP 44, 45.
Consistent with other reports, we demonstrated that TMC NPs aid efficient internalization of EDIII-D3, as shown by the higher percentage of antigen positive cells and the amount of antigen internalized in each cell in the EDIII-D3 TMC NPs-treated group. Yue and colleagues have shown that polylactide NPs enhance hepatitis B surface antigen uptake via interaction between NPs and mannose surface receptors on macrophages 46. The route of entry of EDIII-D3 TMC NPs was not investigated in the present study; however, we believe that uptake of EDIII-D3 TMC NPs may be mediated through interaction between the positively charged surfaces of NPs and the negatively charged cell membranes. Enhancement of antigen uptake through NPs has previously been reported for hepatitis B surface antigen and DENV 37, 47.
The release profile of encapsulated EDIII is of interest. The slow release at pH 7.4 and a burst release at endosomal pH could be beneficial for vaccine delivery. Slow release at pH 7.4 may reduce loss of antigens before being delivered into target cells, whereas the increased release of antigens at the phagolysosomal pH would strongly activate the target cells.
We found that TMC NPs not only enhance antigen uptake by MoDCs but also exert adjuvanticity. An adjuvant is typically a danger signal that works by activating TLRs and inflammatory cytokine receptors to induce maturation of DCs and initiate adaptive immunity. Our data show that MFIs of maturation markers of DCs treated with TMC NPs are similar to those induced by LPS. In addition, we found that TMC NPs strongly activates IL-1β production, suggesting that TMC NPs can serve as an adjuvant. The N-acetyl glucosamine moieties on chitosan particles act as pathogen-associated molecular patterns involved in activation of innate immune responses upon interaction with C-type lectins on the surface of antigen-presenting cells 48, 49. Moreover, chitosan NPs are known to stimulate DCs via TLR4, mannose receptors and NLRP3 inflammasomes 50, 51. Given that TMC is a derivative of chitosan, it is possible that they share similar modes of action.
Here, we investigated the antigenicity of EDIII-D3 TMC NPs to determine whether this form of EDIII has the potential to stimulate immune responses in human. Using an ex vivo model of human MoDCs, we found that EDIII-D3 TMC NPs are potent stimulators of proinflammatory cytokines, antiviral cytokine, chemokines, Th1 and Th2 cytokines and growth factors. These findings confirm the capacity of EDIII-D3 TMC NPs, at least in vitro, to initiate the cellular processes that are essential for generation of both adaptive and innate immunity.
Its pronounced effect on immune responses renders TMC a remarkable adjuvant in the area of vaccine development. However, some aspects of TMC, including uniform reproduction of bulk quantities with reliable sources, the ability to control antigen release, toxicity and some ambiguous outcomes regarding production of proinflammatory effects, still need to be improved and investigated 52.
In conclusion, we have developed a biocompatible adjuvant delivery system for EDIII protein of DENV. This system is not only able to efficiently deliver dengue immunogens into antigen presenting cells, but also exerts strong adjuvanticity, as evidenced by its ability to drive immature DCs into a mature stage. These findings indicate that this platform makes soluble dengue immunogens very immunogenic in human MoDCs. Thus, this form of DENV-EDIII protein deserves to be further investigated in an in vivo model.
ACKNOWLEDGEMENTS
This work was supported by the National Vaccine Institute, Thailand (Grant No. 2258.1/7) to S.U. and by the Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. PHD/0269/2552) to N.N.
DISCLOSURE
The authors declare that they have no conflicts of interest.