Efficacy and safety of the selective TYK2 inhibitor, deucravacitinib, in Japanese patients with moderate to severe plaque psoriasis: Subgroup analysis of a randomized, double-blind, placebo-controlled, global phase 3 trial
Abstract
Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor that demonstrated superior efficacy versus placebo and apremilast in a global phase 3 trial (POETYK PSO-1; NCT03624127) in patients with moderate to severe plaque psoriasis (N = 666). This report describes efficacy and safety in Japanese patients from this study (N = 66) who were randomly assigned to treatment with deucravacitinib 6 mg once daily (n = 32), placebo (n = 17), or apremilast 30 mg twice daily (n = 17). Patients randomized to placebo crossed over to deucravacitinib at Week 16. Patients randomized to apremilast who did not achieve ≥50% reduction from baseline in Psoriasis Area and Severity Index (PASI 50) score at Week 24 switched to deucravacitinib. The proportion of Japanese patients achieving ≥75% reduction from baseline in PASI (PASI 75) score was numerically higher with deucravacitinib versus placebo and apremilast at Week 16 (78.1% vs. 11.8% and 23.5%, respectively) and versus apremilast at Week 24 (78.1% vs. 29.4%). A numerically higher proportion of patients achieved a static Physician's Global Assessment score of 0 or 1 (clear or almost clear) with at least a two-point improvement from baseline (sPGA 0/1) with deucravacitinib versus placebo or apremilast at Week 16 (75.0% vs. 11.8% and 35.3%) and versus apremilast at Week 24 (75.0% vs. 29.4%). Findings for other clinical and patient-reported outcomes also favored deucravacitinib. Response rates were maintained through 52 weeks in the deucravacitinib group. Incidence rates for adverse events per 100 person-years (PY) in the Japanese patients were comparable across treatment groups through Week 52 (deucravacitinib, 336.8/100 PY; placebo, 321.0/100 PY; apremilast, 358.6/100 PY). The most frequently reported adverse event with deucravacitinib was nasopharyngitis. The efficacy and safety of deucravacitinib in Japanese patients was consistent with those in the global population in POETYK PSO-1.
1 INTRODUCTION
Plaque psoriasis is a chronic, immune-mediated inflammatory disease that impairs physical and mental health and reduces work productivity and quality of life in affected patients.1 Globally, psoriasis affects more than 125 million people, and the incidence is increasing.1 Psoriasis prevalence in Japan is 0.34% (95% confidence interval [CI], 0.34%–0.34%).2 Epidemiological analyses reveal that psoriasis is more common among Japanese men than Japanese women: Of the more than 400 000 Japanese patients with psoriasis in 2015, 59.1% were male.2, 3 While psoriasis can occur at any age, the most common age of disease onset among Japanese patients is between 30 and 39 years.4
Several therapeutic approaches have been approved for moderate to severe plaque psoriasis, including vitamin A derivatives (retinoids), phosphodiesterase 4 inhibitors (apremilast), immunosuppressants (cyclosporine and methotrexate), and biologics, including tumor necrosis factor, interleukin (IL)-12/23, IL-23, and IL-17 inhibitors.1 Among the patients eligible for systemic therapies, apremilast appears to be the most frequently used in Japan,3, 4 presumably reflecting patient preference for oral treatment4, 5 and regulated use of biologics based on the guidance from the Japanese Dermatological Association.6 Current treatment options for moderate to severe plaque psoriasis, including biologic and other oral treatments, have improved patient outcomes.1, 5 However, unmet needs exist in terms of long-term efficacy maintenance, safety and tolerability concerns, dosing convenience, and cost. Novel treatments with improved risk–benefit profiles based on selective targeting of psoriasis signaling pathways are necessary.1, 5
Tyrosine kinase 2 (TYK2) is an intracellular enzyme that mediates signaling of specific cytokines involved in the pathogenesis of psoriasis, including Type I interferons, IL-23, and IL-12.7, 8 Deucravacitinib is an oral agent that selectively inhibits TYK2 via an allosteric mechanism by binding to the less conserved regulatory pseudokinase domain of the enzyme, in contrast to the more conserved catalytic domain where Janus kinase (JAK) 1/2/3 inhibitors bind.7, 9 Deucravacitinib is approved in the United States and other countries for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.10 It is also approved by Japan's Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency for patients with plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis who have had an inadequate response to conventional therapies.11 The efficacy and safety of deucravacitinib were investigated in two global phase 3 clinical trials, POETYK PSO-1 (NCT03624127) and POETYK PSO-2 (NCT03611751), in patients with moderate to severe plaque psoriasis.12, 13 Results from these trials demonstrated that deucravacitinib was significantly more efficacious than placebo and apremilast based on the coprimary endpoints of proportions of patients achieving ≥75% reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) and static Physician's Global Assessment score of 0 or 1 (clear or almost clear skin) with at least a two-point improvement from baseline (sPGA 0/1), at Week 16.14, 15 In addition, deucravacitinib was well tolerated.14-16 This report details the specific findings among Japanese patients who participated in PSO-1 (PSO-2 did not include Japanese patients).
2 METHODS
2.1 Study design
This was a subgroup analysis of data from the POETYK PSO-1 trial; a detailed description of the study has been published previously.14, 15 PSO-1 was a 52-week, global, randomized, double-blind, placebo- and active-comparator– controlled trial in which patients with stable moderate to severe plaque psoriasis were randomly assigned 2:1:1 to treatment with deucravacitinib 6 mg once daily (QD), placebo, or apremilast 30 mg twice daily (BID) (Figure 1). Apremilast was titrated, as per approved labeling, from 10 mg QD to 30 mg BID for the first 5 days of dosing, with appropriate blinding across treatment arms. At Week 16, patients receiving placebo crossed over in a blinded manner to deucravacitinib, and at Week 24, patients receiving apremilast who did not achieve a PASI 50 response were switched in a blinded manner to deucravacitinib. Patients initially assigned to deucravacitinib maintained the same treatment through Week 52, and patients initially randomized to apremilast who achieved PASI 50 at Week 24 continued with apremilast through Week 52.
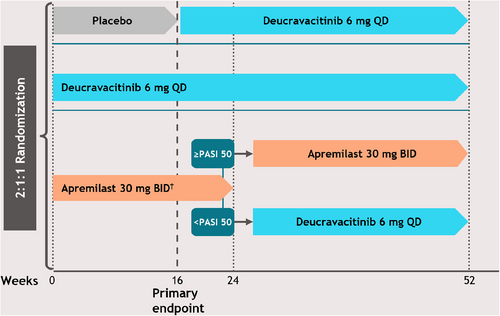
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, as defined by the International Council for Harmonization. Eligible patients provided written informed consent, and each study site's institutional review board or independent ethics committee provided study approval.
2.2 Patients
Eligible patients were ≥ 18 years old with a diagnosis of moderate to severe plaque psoriasis (ie, PASI ≥12, sPGA ≥3, and body surface area involvement ≥10% at baseline) for ≥6 months and were candidates for phototherapy or systemic treatment. Prior use of any systemic treatment or biologic therapy, except deucravacitinib or apremilast, was permitted with an appropriate washout period. The current subgroup analysis included only the subset of patients from study sites in Japan.
2.3 Outcomes
The coprimary endpoints were the proportions of patients achieving PASI 75 and sPGA 0/1 at Week 16 for deucravacitinib versus placebo. Secondary endpoints included the proportions of patients achieving PASI 90, PASI 100, sPGA 0/1, and sPGA 0 responses with deucravacitinib compared with placebo through Week 16, and compared with apremilast through Week 24. Efficacy on scalp psoriasis through Week 24 was assessed by the proportions of patients achieving a scalp-specific Physician's Global Assessment (ss-PGA) score of 0 or 1 (ss-PGA 0/1), with at least a two-point improvement from baseline. Patient-reported outcomes were also captured through Week 24, including the proportion of patients with a Dermatology Life Quality Index score of 0 or 1 (DLQI 0/1), and change from baseline in the Psoriasis Symptoms and Signs Diary (PSSD) symptom score, which is a composite score of itching, burning, stinging, pain, and skin tightness reported by the patients.17, 18 Week 52 outcomes were examined in patients who received continuous deucravacitinib treatment for the duration of the study, including PASI 75, PASI 90, PASI 100, sPGA 0/1, and sPGA 0.
Safety endpoints included adverse events (AEs) and serious AEs (SAEs) that occurred during the Week 0–16 and Week 0–52 periods. Specific AEs of interest based on results from previous studies with deucravacitinib and its mechanism of action included infections (including influenza), skin-related events, creatine phosphokinase levels ≥2.5× the upper limit of normal, malignancies, and cardiovascular events, including major adverse cardiovascular events. Week 52 safety data are reported as exposure-adjusted incidence rates (EAIRs) per 100 person-years (PY) to account for variable periods of treatment exposure in the three investigational treatment groups.
2.4 Statistical analysis
This was a subgroup analysis but with no preplanned statistical comparisons across treatment groups for any outcome. Non-responder imputation was used for binary efficacy endpoints for patients who discontinued treatment, left the study prior to completion, or had missing endpoint data for any reason. A modified baseline observation-carried-forward approach was used for continuous secondary endpoints for patients who discontinued treatment due to lack of efficacy or AEs. For patients who discontinued or who had a missing value for other reasons, the last valid observation was carried forward.
3 RESULTS
3.1 Patients
This PSO-1 subgroup analysis included 66 Japanese patients (ie, ~10% of the global study population) who were randomized to treatment with deucravacitinib (n = 32), placebo (n = 17), or apremilast (n = 17). Patients had a mean (SD) age of 49.5 (11.0) years at the time of enrollment and 25.8% were female (Table 1). Baseline patient demographics and disease characteristics were generally balanced across treatment groups (Table 1), although a greater proportion of patients randomized to deucravacitinib had no experience with prior systemic treatments (46.9%) than in the placebo (35.3%) and apremilast (23.5%) treatment groups. In addition, patients receiving deucravacitinib had numerically higher mean (SD) DLQI scores (12.0 [6.2]) and lower mean (SD) PSSD symptom scores (44.9 [22.0]) compared with placebo (DLQI, 8.9 [3.9]; PSSD symptom score, 53.3 [27.3]) and apremilast (DLQI, 9.6 [5.3]; PSSD symptom score, 54.4 [30.5]). Baseline disease characteristics in the Japanese population were mostly consistent with the global population,14 with the exceptions of lower body weight (mean [SD], 72.5 [15.6] kg vs. 88.1 [21.7] kg, respectively), lower body mass index (mean [SD], 25.8 [4.8] kg/m2 vs. 29.9 [7.0] kg/m2), and later age at disease onset (mean [SD], 37.0 [12.0] years vs. 29.6 [14.6] years) in the Japanese patients.
| Placebo (n = 17) | Deucravacitinib (n = 32) | Apremilast (n = 17) | Total (n = 66) | |
|---|---|---|---|---|
| Age, mean (SD), years | 52.5 (12.0) | 48.2 (11.7) | 48.8 (8.4) | 49.5 (11.0) |
| Weight, mean (SD), kg | 71.7 (17.4) | 74.4 (16.4) | 69.6 (12.3) | 72.5 (15.6) |
| BMI, mean (SD), kg/m2 | 25.5 (5.0) | 26.4 (4.7) | 25.0 (5.0) | 25.8 (4.8) |
| Female, n (%) | 5 (29.4) | 7 (21.9) | 5 (29.4) | 17 (25.8) |
| Age at disease onset (years), mean (SD) | 39.1 (11.6) | 35.6 (13.7) | 37.4 (8.9) | 37.0 (12.0) |
| Duration of disease (years), mean (SD) | 14.4 (10.1) | 13.4 (7.7) | 12.4 (9.1) | 13.4 (8.6) |
| Psoriasis-related history, n (%) | ||||
| Scalp | 16 (94.1) | 31 (96.9) | 17 (100) | 64 (97.0) |
| Nails | 12 (70.6) | 16 (50.0) | 11 (64.7) | 39 (59.1) |
| Psoriatic arthritis | 2 (11.8) | 2 (6.3) | 3 (17.6) | 7 (10.6) |
| Prior systemic treatment use, n (%) | ||||
| Biologic | 4 (23.5) | 8 (25.0) | 6 (35.3) | 18 (27.2) |
| Nonbiologic | 7 (41.2) | 9 (28.1) | 7 (41.2) | 23 (34.8) |
| None | 6 (35.3) | 15 (46.9) | 4 (23.5) | 25 (37.9) |
| PASI (0–72), mean (SD) | 23.0 (7.9) | 22.4 (8.9) | 20.7 (5.9) | 22.1 (7.9) |
| sPGA score (0–4), n (%) | ||||
| 3 = moderate | 14 (82.4) | 27 (84.4) | 15 (88.2) | 56 (84.8) |
| 4 = severe | 3 (17.6) | 5 (15.6) | 2 (11.8) | 10 (15.2) |
| BSA involvement, % | 28.3 (16.3) | 27.2 (14.3) | 29.2 (14.1) | 28.0 (14.6) |
| ss-PGA ≥3, n (%) | 12 (70.6) | 21 (65.6) | 12 (70.6) | 45 (68.2) |
| PSSD symptom score (0–100), mean (SD) | 53.3 (27.3) | 44.9 (22.0) | 54.4 (30.5) | 49.6 (25.8) |
| DLQI (0–30), mean (SD) | 8.9 (3.9) | 12.0 (6.2) | 9.6 (5.3) | 10.6 (5.6) |
- Abbreviations: BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PSSD, Psoriasis Symptoms and Signs Diary; sPGA, static Physician's Global Assessment; ss-PGA, scalp-specific Physician's Global Assessment.
3.2 Efficacy
Clinical efficacy outcomes through Week 24 are summarized in Figure 2. The proportion of Japanese patients achieving PASI 75 was numerically higher with deucravacitinib versus placebo and apremilast at Week 16 (78.1% vs. 11.8% and 23.5%, respectively) and versus apremilast at Week 24 (78.1% vs. 29.4%). Consistent with the PASI findings, a greater proportion of patients achieved sPGA 0/1 with deucravacitinib versus placebo and apremilast at Week 16 (75.0% vs. 11.8% and 35.3%, respectively) and versus apremilast at Week 24 (75.0% vs. 29.4%). Numerical differences in PASI 75 response rates with deucravacitinib versus placebo were apparent by Week 4; numerical differences in sPGA 0/1 responses were apparent by Week 2.
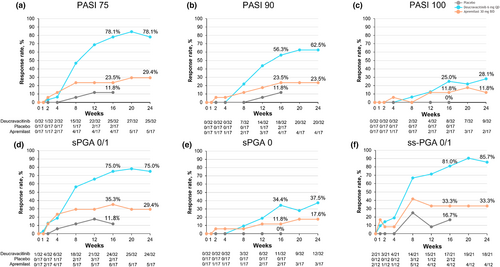
Patients were also more likely to achieve PASI 90 with deucravacitinib versus placebo and apremilast at Week 16 (56.3% vs. 11.8% and 23.5%, respectively), and versus apremilast at Week 24 (62.5% vs. 23.5%). Similar results were seen for the more stringent PASI 100 outcome at Week 16 (25.0% vs. 0% and 11.8%, respectively) and at Week 24 (28.1% vs. 11.8%).
Deucravacitinib was also associated with higher response rates for sPGA 0 versus placebo and apremilast at Week 16 (34.4% vs. 0% and 11.8%, respectively) and versus apremilast at Week 24 (37.5% vs. 17.6%). Improvements in scalp psoriasis, as determined by ss-PGA 0/1 response, were greater with deucravacitinib versus placebo and apremilast at Week 16 (81.0% vs. 16.7% and 33.3%, respectively) and versus apremilast at Week 24 (85.7% vs. 33.3%).
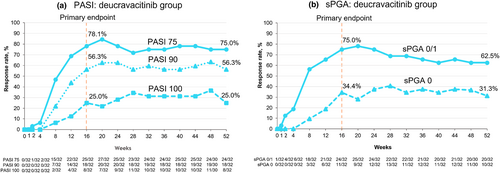
Sustained clinical efficacy was observed in deucravacitinib-treated patients for up to 52 weeks (Figure 3). At Week 52, PASI 75, PASI 90, and PASI 100 response rates were 75.0%, 56.3%, and 25.0%, respectively. Week 52 response rates for sPGA 0/1 and sPGA 0 were 62.5% and 31.3%, respectively.
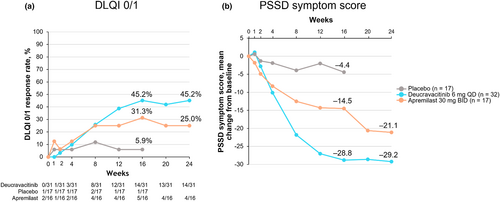
In addition to clinical benefits, deucravacitinib was also associated with greater improvements in patient-reported outcomes (Figure 4). The proportion of patients achieving DLQI 0/1 (ie, no effect on patient's life) was greater with deucravacitinib versus placebo and apremilast at Week 16 (45.2% vs. 5.9% and 31.3%, respectively) and versus apremilast at Week 24 (45.2% vs. 25.0%); differences versus placebo and apremilast were apparent by Weeks 8 and 12, respectively. Patients receiving deucravacitinib also showed greater mean reductions from baseline in PSSD symptom score at Week 16 versus placebo and apremilast (−28.8 vs. −4.4 and − 14.5, respectively) and at Week 24 versus apremilast (−29.2 vs. −21.1); differences from placebo and apremilast occurred at Weeks 4 and 8, respectively.
3.3 Safety
Safety outcomes are shown in Table 2. Overall, AEs were more frequent with deucravacitinib than placebo over Weeks 0–16, but incidence rates were similar overall with deucravacitinib and apremilast over Weeks 0–16 and Weeks 0–52 with minor imbalances between groups due to small numbers. The incidence rates for AEs leading to treatment discontinuation were similar across treatment groups.
| Weeks 0–16a | Weeks 0–52 | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 17) | Deucravacitinib (n = 32) | Apremilast (n = 17) | Placebo (n = 17, PY = 5.0) | Deucravacitinib (n = 56, PY = 44.7) | Apremilast (n = 17, PY = 11.2) | |
| n (%) | n (EAIR) | |||||
| Any AEs | 10 (58.8) | 22 (68.8) | 12 (70.6) | 10 (321.0) | 48 (336.8) | 14 (358.6) |
| Serious AEs | 0 | 1b (3.1) | 0 | 0 | 5c (11.7) | 0 |
| Treatment related AEs | 3 (17.6) | 7 (21.9) | 5 (29.4) | 3 (64.0) | 13 (34.1) | 5 (63.4) |
| AEs leading to discontinuationd | 1 (5.9) | 2 (6.3) | 1 (5.9) | 1 (19.7) | 2 (4.5) | 1 (9.0) |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs occurring in ≥5% in deucravacitinib-treated patients during Weeks 0–16 or EAIR ≥7 in deucravacitinib-treated patients during Weeks 0–52 | ||||||
| Nasopharyngitis | 1 (5.9) | 4 (12.5) | 3 (17.6) | 1 (20.3) | 16 (43.0) | 5 (49.4) |
| Arthralgia | 0 | 1 (3.1) | 0 | 0 | 5 (11.6) | 1 (9.2) |
| Folliculitis | 0 | 2 (6.3) | 0 | 0 | 4 (9.3) | 1 (8.9) |
| Influenza | 0 | 0 | 0 | 0 | 4 (9.2) | 0 |
| Diarrhea | 0 | 2 (6.3) | 2 (11.8) | 0 | 4 (9.4) | 2 (21.5) |
| Urticaria | 0 | 1 (3.1) | 0 | 0 | 4 (9.4) | 0 |
| Psoriasis | 4 (23.5) | 2 (6.3) | 4 (23.5) | 4 (93.3) | 3 (7.0) | 4 (42.5) |
| Increased blood CPK | 0 | 2 (6.3) | 0 | 0 | 3 (7.0) | 0 |
| Conjunctivitis | 1 (5.9) | 2 (6.3) | 0 | 1 (20.5) | 2 (4.6) | 0 |
- Note: Safety data in the Week 0–52 period are expressed as EAIRs (events/100 PY) to account for variable periods of exposure to treatment. AEs are sorted by deucravacitinib data for Weeks 0–52.
- Abbreviations: AEs, adverse events; CPK, creatine phosphokinase; EAIR, exposure-adjusted incidence rate; PY, person-years.
- a Fifteen and 9 patients in the placebo group and the apremilast group, respectively, crossed over to receive deucravacitinib at Week 16 and Week 24, respectively.
- b Pyelonephritis (considered not related to the study drug).
- c Pyelonephritis, cataract, clavicle fracture, osteoarthritis, dyspnea exertional (1 event each; all considered not related to the study drug).
- d For patients in the deucravacitinib group: abnormal hepatic function and folliculitis (1 event each). For patients in the placebo and apremilast groups: psoriasis (1 in each group). The abnormal hepatic function was diagnosed on Day 58 in a 41-year-old male with moderate fatty liver disease and was considered related to treatment. Peak ALT was 3.8 × upper limit of normal (158 U/L) at Day 62, with a concurrent elevation in total bilirubin (2.2 × upper limit of normal [2.6 mg/dL]). No symptoms were reported. The patient discontinued the study on Day 91, with last treatment received on Day 59. Hepatic function resolved on Day 92. The folliculitis was diagnosed on Day 42 in a 35-year old male and was considered mild and related to study drug. The subject received treatment with nadifloxacin, ketoconazole, minocycline and guaiazulene and discontinued the study on Day 197 due to folliculitis on the nose that was resolving, with the last treatment received on Day 181.
No deaths occurred in any group. The only SAE reported through Week 16 was a case of pyelonephritis in a patient receiving deucravacitinib. Additional SAEs occurring on deucravacitinib beyond Week 16 through Week 52 included cataract, clavicle fracture, osteoarthritis, and dyspnea on exertion (n = 1 each). All SAEs were considered to be unrelated to the study drug.
3.4 AEs of interest over 52 weeks
The AEs of interest that occurred over the 52-week study period are reported in Table 3. Infections and infestations reported with deucravacitinib (n = 5; EAIR, 11.6/100 PY) included influenza (n = 4; EAIR, 9.2/100 PY) and herpes zoster (n = 1; EAIR, 2.2/100 PY), which were not serious. No opportunistic infections or tuberculosis were reported in the study. Skin events occurred at a higher rate with deucravacitinib (n = 15; EAIR, 40.0/100 PY) than placebo (n = 0) and apremilast (n = 2; EAIR, 18.5/100 PY) in the Japanese population, which is consistent with the trend of higher rates of skin events observed with deucravacitinib (EAIR, 17.0/100 PY compared with placebo (EAIR, 6.3/100 PY) and apremilast (EAIR, 8.8/100 PY) in the global population in this study (see Table 2). Folliculitis (n = 4; EAIR, 9.3/100 PY) and urticaria (n = 4; EAIR, 9.4/100 PY) were the most frequent skin events observed with deucravacitinib in the Japanese population. Elevations in creatine phosphokinase occurred only in patients treated with deucravacitinib (n = 3; EAIR, 7.0/100 PY), were in general due to recent exercise, and none were considered to be related to treatment (Table 2). No malignancies or cardiovascular events (eg, major adverse cardiovascular events, venous thromboembolic events) were reported in any group.
| Placebo (n = 17) | Deucravacitinib (n = 56) | Apremilast (n = 17) | ||||
|---|---|---|---|---|---|---|
| n | EAIRa | n | EAIRa | n | EAIRa | |
| Skin events | 0 | 0 | 15 | 40.0 | 2 | 18.5 |
| Folliculitis | 0 | 0 | 4 | 9.3 | 1 | 8.9 |
| Urticaria | 0 | 0 | 4 | 9.4 | 0 | 0 |
| Contact dermatitis | 0 | 0 | 2 | 4.5 | 0 | 0 |
| Eczema | 0 | 0 | 2 | 4.5 | 0 | 0 |
| Acne | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Dyshidrotic eczema | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Paronychia | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Pruritus | 0 | 0 | 1 | 2.3 | 0 | 0 |
| Rash | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Rosacea | 0 | 0 | 1 | 2.3 | 0 | 0 |
| Seborrheic dermatitis | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Skin erosion | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Skin infection | 0 | 0 | 1 | 2.3 | 0 | 0 |
| Atopic dermatitis | 0 | 0 | 0 | 0 | 1 | 9.1 |
| Asteatotic eczema | 0 | 0 | 0 | 0 | 1 | 9.0 |
| Infection and infestations | 0 | 0 | 5 | 11.6 | 0 | 0 |
| Influenza | 0 | 0 | 4 | 9.2 | 0 | 0 |
| Herpes zoster | 0 | 0 | 1 | 2.2 | 0 | 0 |
| Malignancies | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiovascular events | 0 | 0 | 0 | 0 | 0 | 0 |
- Abbreviation: EAIR, exposure-adjusted incidence rate.
- a Safety data are expressed as EAIRs (events/100 person-years) to account for variable periods of exposure to treatment.
4 DISCUSSION
Findings from this subgroup analysis of Japanese patients in the phase 3 POETYK PSO-1 trial suggest that treatment with deucravacitinib would be effective with acceptable safety for Japanese patients with moderate to severe plaque psoriasis. Clinical response rates for PASI, sPGA, and ss-PGA outcomes were better with deucravacitinib versus placebo through 16 weeks and versus apremilast through 24 weeks. Response rates for PASI and sPGA were maintained through 52 weeks in patients who received continuous deucravacitinib treatment. Japanese patients who received deucravacitinib treatment also had better responses on patient-reported outcomes, including the DLQI 0/1 and PSSD symptom scores, compared with placebo and apremilast.
The overall trends in efficacy outcomes in the Japanese subgroup of POETYK PSO-1 were generally consistent with the overall PSO-1 study population, although values for the individual endpoints tended to be higher in the Japanese population, specifically in the deucravacitinib group. For example, 78.1% of Japanese patients randomized to deucravacitinib achieved PASI 75 at Week 16 compared with 58.4% of the overall global PSO-1 population. Efficacy outcomes in the apremilast group were generally similar to the overall population and previous reports from clinical trials.19
Safety outcomes in the Japanese patients in PSO-1 were consistent with findings from the overall study population. No safety signals specific to the Japanese population were detected. Discontinuation rates due to AEs were low, demonstrating acceptable tolerability of deucravacitinib in the Japanese population.
This analysis was limited by the small number of Japanese patients enrolled in PSO-1. The POETYK PSO-4 trial (NCT03924427), a prospective, open-label, single-arm, phase 3 trial that enrolled approximately 70 Japanese patients with psoriasis, will provide additional analyses to inform the use of deucravacitinib in Japan.20 Real-world evidence studies will also be beneficial to understanding the benefits and risks of using deucravacitinib in this patient population.
In conclusion, deucravacitinib showed a favorable benefit–risk balance in Japanese patients with moderate to severe psoriasis in a global phase 3 study. While the lack of statistical comparison limits the conclusions that can be drawn from these analyses, findings were consistent with the global trial population.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided by Liz Rockstein, Ph.D., of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, funded by Bristol Myers Squibb.
CONFLICT OF INTEREST STATEMENT
None declared.
Open Research
DATA AVAILABILITY STATEMENT
Bristol Myers Squibb details their data sharing request process via the following website: https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.




