Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase
Abstract
Thiamidol® was the most potent inhibitor of human tyrosinase out of 50 000 screened substances. In vivo, it was well tolerated and improved melasma significantly. This was the first 24-week, randomized, double-blind, vehicle-controlled, cosmetic clinical study to assess the efficacy and tolerability of thiamidol in moderate-to-severe melasma of phototype III–V subjects with subsequent regression phase. Females allocated to verum (n = 23), applied daily Dual Serum followed either by Day Care SPF30 in the morning or by Night Care in the evening, all containing Thiamidol. The vehicle group (25 females) followed the same skin care routine using the corresponding vehicle formulations. Subjects came back for a follow-up visit 13–20 weeks after treatment (regression phase). Assessments included clinical photography, Melasma Area and Severity Index (MASI), skin lightness, quality of life, and tolerability. Baseline demographics and hyperpigmentation were well balanced across the treatment groups. Clinical photography and MASI improved with Thiamidol significantly versus baseline (p < 0.001) and vehicle (p < 0.001–0.043) at all time points up to treatment end. At follow-up, the MASI was still significantly lower than at baseline but similar for verum and vehicle. Skin lightness and quality of life improved significantly versus baseline without significant differences between verum and vehicle. This study demonstrated that Thiamidol is well tolerated and superior in improving melasma compared to baseline and vehicle over a treatment period of 24 weeks.
1 INTRODUCTION
Melasma is a predominantly female and darker skin type disorder with an estimated prevalence of 9–50% in the higher-risk and 1% in the general population.1-3 The abnormal pigmentation is associated with a substantial impact on quality of life.4, 5 Tyrosinase inhibitors are the focus of the research for the treatment of hyperpigmentation.6, 7 Thiamidol® (isobutylamido thiazolyl resorcinol) was recently identified as the most potent strictly competitive inhibitor of human tyrosinase out of 50 000 screened molecules.8, 9 With this mode of action, it has the advantage of lacking the potential side-effects of compounds that are acting via transformation to quinones.8
In vitro, Thiamidol strongly but reversibly inhibited melanin production and was superior to the irreversibly acting hydroquinone.8 In vivo, twice-daily applications for 12 weeks significantly reduced age spots versus vehicle8 and mild-to-moderate melasma in comparison with no treatment or hydroquinone.10 Furthermore, four applications daily for 12 weeks were more effective than only two.11 The substance was efficacious and well tolerated in all 12-week studies conducted so far,8, 10-12 including a real-world study with a three-product regimen consisting of Dual Serum, Day Care SPF30, and Night Care.11
Previous studies were done for 12 weeks in subjects with Fitzpatrick skin phototypes I–V, showing mild-to-moderate melasma. Therefore, the objective of this study was to assess the efficacy and tolerability of the three-product regimen for 24 weeks in darker-complexioned individuals compared to vehicle including SPF30 in the case of the day care. Besides, as melasma has a recurrent and refractory nature, we wanted to evaluate the melasma development after the end of the treatment phase.
2 METHODS
2.1 Trial design
This randomized, vehicle-controlled, double-blind clinical trial was conducted in a study center in Mauritius (CIDP Ltée, Biopark, Socota Phoenicia, Phoenix, Mauritius). In the 24-week treatment phase, the subjects returned every 4 weeks for assessments and between 13 and 20 weeks after treatment discontinuation for a 1-day regression phase visit.
The study was conducted following the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines as applicable to cosmetic products. In Mauritius, studies involving cosmetic products are not governed by the Clinical Trial Act 2011; therefore, they do not need regulatory approval by the Clinical Research Regulatory Council and do not necessarily require the opinion of an independent ethics committee (IEC). Because of the panel and procedures, this cosmetic study was not submitted to an IEC for opinion. All subjects provided written informed consent before inclusion. Subject anonymity in the study documentation was maintained by pseudonymization.
2.2 Study population and treatment
Healthy, female subjects, aged 18–65 years, with Fitzpatrick skin phototypes III–V, demonstrating moderate-to-severe (30–50% severe) melasma according to Melasma Area and Severity Index (MASI) score (MASI score 10–20 as moderate and >20 as severe melasma),13 willing and capable to follow the study rules including avoidance of sun exposure for the duration of the study, and undergoing stable dose hormonal treatment/contraception for at least 6 months before study onset were eligible for the trial. Exclusion criteria are listed in Table S1.
For 24 weeks, the subjects allocated to the verum group applied twice daily (morning and evening) on their face a Dual Serum (Eucerin Anti-Pigment) containing Thiamidol followed by a Day Care (Eucerin Anti-Pigment) containing Thiamidol and SPF30 in the morning, and a Night Care (Eucerin Anti-Pigment) with Thiamidol in the evening. The vehicle group applied the same skin care routine using the Thiamidol-free vehicle formulations, namely the vehicle Day Care included SPF30. The vehicle formulations did not contain any other substances that could improve melasma.
In the washout period of approximately 3 days before baseline and during the entire study, the subjects were not allowed to use any skin care product on the face (including care-providing cleansers, soaps, or shower oils) other than the products provided by the sponsor. The first application of the study products was performed at baseline in the study center under supervision by trained personnel. During the 24 h preceding each scheduled measurement, subjects had to refrain from intensive sports and visits to saunas and swimming pools. No sunscreen was allowed in the washout period and subjects had to refrain from ultraviolet exposure.
2.3 Assessments
The primary outcome of the study was the evaluation of the hyperpigmentation-reducing effect of the test products through MASI score at each of the eight visits (baseline, weeks 4, 8, 12, 16, 20, and 24, and regression), and through skin pigmentation (individual typology angle [ITA°] measurements with Chromameter® Minolta CR400)14 and digital standardized clinical photography15 with Facial Imaging System VISIA CR and the lighting modes standard 2 and cross-polarized (Canfield Imaging Systems) at all visits except the regression visit. In addition, subjects completed a quality of life questionnaire at baseline, week 12, and week 24 with 10 questions (Table S2) on a 7-point scale (1 = no discomfort, 7 = always discomforts). Before any measurements, the subjects acclimatized under controlled room conditions (24 ± 2°C, 40–60% relative humidity) for 30 min.
The secondary outcome was the skin tolerability as assessed by the subjects and the investigator (baseline, weeks 12 and 24).
Treatment adherence was controlled by weighing the products at each of the visits 1–7.
2.4 Statistical analyses
Statistical analyses of efficacy variables were based on the full analysis set (FAS) consisting of all randomized subjects having completed the study without any major protocol deviation. Tolerability analyses were based on the tolerability set (TAS), which included all subjects who applied the products at least once. Quantitative variables, or those that can reasonably be treated as such, were summarized using the mean, minimum, maximum, standard deviation, and variation coefficient. Qualitative variables were summarized using counts and percentages. Data normality for each comparison was tested using a Shapiro–Wilk test at 5% significance. The null hypothesis (rejected for p ≤ .05) was that there is no difference between the treatment groups or the time points compared. For instrumental measurements, the significance of the difference between two groups at baseline was investigated using the independent samples t-test, and the evolution across time was investigated with Student’s paired t-test.
For the quality of life, the sum of all questions’ scores was calculated for each subject at each time point. The average of the sum across all subjects was used for analysis purposes. The significance of the difference between two treatment groups at baseline was investigated using the Mann–Whitney U-test, the evolution across time with the Wilcoxon signed rank test, and the significance of the difference between two treatment groups with the Mann–Whitney U-test.
Statistical analysis was conducted using SPSS version 19.0 for Windows.
3 RESULTS
3.1 Demographics and baseline characteristics
Between August 2019 and June 2020, 51 female subjects aged 38–64 years (mean: 53 ± 1) were enrolled (TAS), of whom 26 were randomized to group A (vehicle) and 25 to group B (verum) (Table 1). Of those, 48 subjects (25 group A, 23 group B) completed the treatment phase. One subject was excluded from group A because of pregnancy and two from group B, one due to local intolerance to the treatment and one due to consent withdrawal. One subject (group B) showed inconclusive data in MASI assessment and was excluded from MASI analysis. For the regression phase analysis, 43 subjects were traceable (22 group A, 21 group B). The randomized subjects’ demographic and baseline characteristics were balanced among the two treatment groups (Table 1).
| Characteristic | Group A (vehicle) (n = 26) | Group B (verum) (n = 25) |
|---|---|---|
| Age, years | 54 (1) | 52 (1) |
| Phototype, n | ||
| III | 1 | 1 |
| IV | 18 | 16 |
| V | 7 | 8 |
| Race, n | ||
| Caucasian | 1 | – |
| Mixed race | 17 | 19 |
| Indian | 8 | 8 |
| Skin type, n | ||
| Normal | 16 | 16 |
| Combination | 5 | 2 |
| Dry | 1 | 2 |
| Very dry | – | 1 |
| Oily | 4 | 4 |
| Melasma severity, n (%) | ||
| Moderate (MASI score 10–20) | 19 (73) | 18 (72) |
| Severe (MASI score > 20) | 7 (27) | 7 (28) |
| Melasma duration before study start, years | 8.2 (4.7) | 9.8 (6.7) |
- Abbreviation: MASI, Melasma Area and Severity Index.
- a Data are presented as mean (standard deviation) unless otherwise stated.
3.2 Efficacy
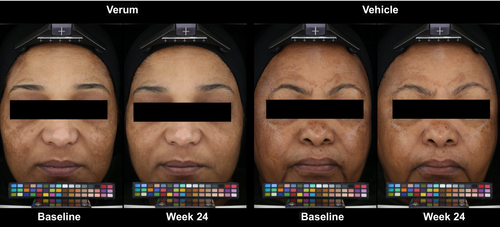
Clinical photography demonstrated superior visible improvement of the melasma in subjects applying the verum compared to baseline and vehicle at all time points measured (Figure 1).
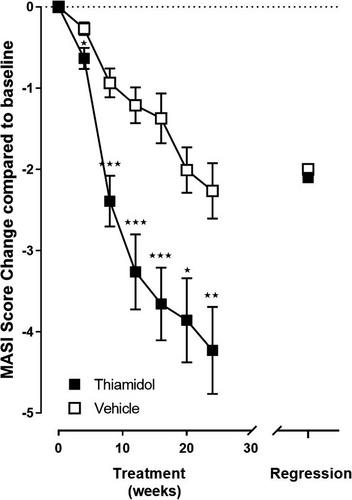
The MASI at baseline was 16.2 ± 4.0 in the vehicle group and 16.4 ± 5.1 in the verum group (Figure S1). The verum treatment led to statistically significant reduction of MASI compared with vehicle (p < 0.001–0.043) and baseline (p < 0.001) at all time points (week 4: −0.6 ± 0.6, week 8: −2.4 ± 1.4, week 12: −3.2 ± 2.1, week 16: −3.6 ± 2.0, week 20: −3.8 ± 2.3, week 24: −4.2 ± 2.4) in addition to the corresponding statistically significant improvement of MASI by the vehicle (week 4: −0.3 ± 0.4, week 8: −0.9 ± 0.9, week 12: −1.2 ± 1.1, week 16: −1.4 ± 1.5, week 20: −2.0 ± 1.4, week 24: −2.3 ± 1.7) in comparison to baseline (p < 0.001) (Figure 2). The MASI increased significantly during the regression phase in comparison to treatment end (week 24) with the absolute increase being higher for verum (2.1 ± 3.0, p < 0.005) than for vehicle (0.5 ± 1.6, p = 0.123). However, 13–20 weeks after treatment end (regression phase), both MASI scores were still statistically significantly lower than at baseline (verum −2.1 ± 3.0, p < 0.005; vehicle −2.0 ± 2.0, p < 0.001), with a marginal advantage for the verum group (Figure 2).
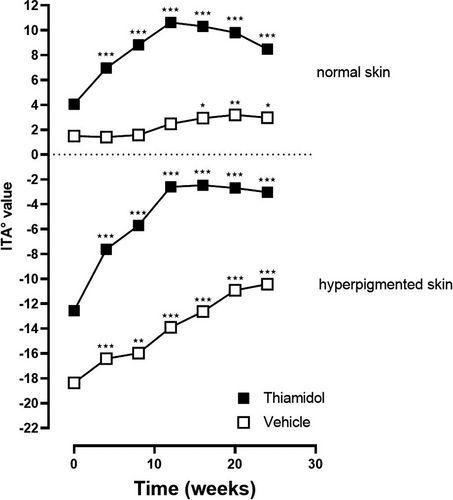
On hyperpigmented skin, the skin lightness value ITA° improved statistically significant compared to baseline in both groups at every time point. For verum, the ITA° values were at baseline: −12.56, at week 4: −7.62, week 8: −5.70, week 12: −2.59, week 16: −2.47, week 20: −2.70, and week 24: −3.03 (p < 0.001 compared to baseline for all time points) and for vehicle at baseline: −18.36, week 4: −16.42 (p < 0.001), week 8: −15.97 (p < 0.004), week 12: −13.90 (p < 0.001), week 16: −12.63 (p < 0.001), week 20: −10.91 (p < 0.001), and week 24: −10.43 (p < 0.001) (Figure 3). On non-hyperpigmented skin, ITA° values significantly improved for verum at all points in time for vehicle starting at week 16. ITA° values were at baseline: 4.06, at week 4: 6.95, week 8: 8.83, week 12: 10.63, week 16: 10.32, week 20: 9.81, and week 24: 8.48 (p < 0.001 compared to baseline for all time points) for verum and for vehicle at baseline: 1.49, at week 4: 1.41 (p = 0.826), week 8: 1.57 (p = 0.853), week 12: 2.47 (p = 0.056), week 16: 2.93 (p = 0.020), week 20: 3.19 (p = 0.005), and week 24: 2.97 (p = 0.013). The differences between the treatment groups were not significant.
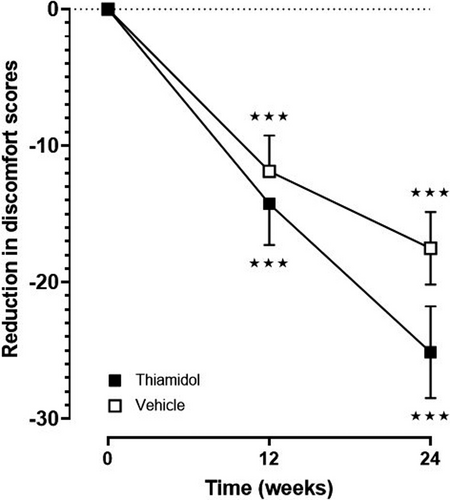
The quality of life improved significantly (p < 0.001) in both groups and at each evaluated time point in comparison to baseline (vehicle, week 12: −11.9 ± 13.1 and week 24: −17.5 ± 13.3; verum, week 12: −14.3 ± 14.4, and week 24: −25.1 ± 16.1) (Figure 4). Within 24 weeks, total scores dropped from 45 to 20 for Thiamidol and from 39 to 21 for the vehicle (Figure S1). There was no significant difference between the two groups.
3.3 Tolerability
The tolerability assessment by subjects and clinical investigator has shown that all of the tested products are very well tolerated on the face after 24 weeks of skin care regimen usage.
4 DISCUSSION
This was the first randomized, double-blind, controlled clinical study that evaluated the efficacy and tolerability of a skin care regimen with Thiamidol for 24 weeks compared to vehicle in subjects with a darker complexion and moderate-to-severe melasma. Both skin care regimens (with/without Thiamidol) were well tolerated and resulted in significant improvement of MASI and clinical photography compared with the skin’s initial state. While the efficacy in the vehicle group reflects the well-known effect of efficient sun protection, the verum group showed significantly superior improvement of melasma over the entire study duration of 24 weeks in analogy to earlier studies of shorter duration.8, 10-12 Of note, the subjects had symptoms for several years (vehicle: 8.2 ± 4.7; verum: 9.8 ± 6.7) before enrolment.
Although the MASI scores increased during the regression phase, they remained below the baseline values for both treatments, indicating some lasting effect. However, after the regression phase, there was no significant difference between the vehicle and the verum group anymore. This can be explained by the reversibility of tyrosinase inhibition by Thiamidol.8 These results emphasize the crucial need of sunscreen maintenance in melasma to prevent relapses. They also show the absence of any rebound effect after the discontinuation of Thiamidol treatment. Due to the lock-down caused by coronavirus disease 2019, the individual regression phase varied between 13 and 20 weeks and it was not possible to analyze for how long after the treatment the verum efficacy would still be measurable. Future studies are needed to elucidate this point.
The results of clinical photography corresponded to MASI. Also, skin lightness and quality of life showed statistically significant improvement versus baseline with higher levels in the verum group. However, the difference to the vehicle was not statistically significant. The number of subjects was possibly too low to achieve significance in assessments with small differences between the two groups. Furthermore, different baseline level in skin lightness for verum and vehicle group, despite comparable baseline mean MASI scores for both groups, point to the fact that skin lightness measurements are limited to small areas and thus do not reflect the clinical phenotype of the patient like the clinical parameter MASI does.
A significant skin lightening of non-lesional skin by verum, but not by vehicle, within the first 12 weeks demonstrates that Thiamidol induces a faster skin lightening of non-hyperpigmented skin than SPF30 alone. This observation of Thiamidol-induced skin lightening in non-lesional skin in subjects with skin phototypes IV–V was not seen in a previous study with subjects with skin phototypes V–VI.12 Following study could focus on the effect of Thiamidol on non-hyperpigmented skin in different skin types to further investigate this observation.
The positive results of this trial in skin phototypes IV–V subjects with moderate-to-severe melasma supplement confirm previous studies in lighter skin types and mild-to-moderate melasma and support the efficacy of the treatment in clinical presentations of various origin, skin type, and severity. The study covered the hottest and more sunny periods of the year in a tropical area, demonstrating that the regimen is efficacious and well tolerated even under demanding climatic conditions. Besides, subjects, including those randomized to the vehicle, applied SPF30. Thus, confounding by sun, humidity, or temperature that was not necessarily fully considered in every preceding study can be excluded. Confounding by other skin care products is not expected, as the subjects could use only the study products on their face.
This study has several strengths, like the focus on skin types IV–V subjects with moderate-to-severe melasma, for whom data are rare, and the double-blinded, vehicle-controlled design, the long duration of treatment, and the regression phase. Limitations of the study are the monocentric study approach as well as the relatively low number of patients resulting in descriptive, yet not significant, differences between treatment groups in certain parameters such as quality of life assessment. This needs to be addressed in more detail in future studies.
In conclusion, this single-center, randomized, double-blind, vehicle-controlled, parallel-group study demonstrated that Thiamidol, applied as three-product skin care regimen, is well tolerated, and reduced significantly long-lasting, moderate-to-severe melasma. The findings suggest Thiamidol is safe for at least 24 weeks of application and adds to the vehicle and SPF30 sun protection effect.
ACKNOWLEDGMENTS
This clinical study was financially supported by Beiersdorf AG. The authors would like to express their sincerest appreciation to all investigators, clinical trial staff, and participants. The authors also thank Helena Karajiannis for providing medical writing assistance.
CONFLICT OF INTEREST
The sponsor has borne the costs for the preparation and submission of the manuscript, including third-party medical writing assistance. D.R., A.S., M.F., M.K., and L.K. are employees of Beiersdorf.




