Real World Experience With Dupilumab in Eosinophilic Esophagitis in Children and Young Adults at a Tertiary Care Pediatric Medical Center
The author's institution is a study site for phase 3 trials entitled “A randomized, double-blind, placebo-controlled study to investigate the efficacy and safety of dupilumab in pediatric patients with active eosinophilic esophagitis” sponsored by Regeneron Pharmaceuticals. While the authors will be listed as sub-investigators in this trial, neither author is receiving financial compensation from this study, nor do they have any other competing financial interests.
Abstract
Dupilumab is one of a number of biologics currently under investigation for the treatment of eosinophilic esophagitis (EoE). We report on a group of 7 pediatric and young adult patients with EoE who were treated with dupilumab for a primary indication of asthma or atopic dermatitis, all of whom previously failed swallowed topical corticosteroid therapy dietary for management of their EoE. All 7 patients demonstrated histologic improvement in their EoE while on dupilumab, with a drop in median peak esophageal eosinophil count from 50 eosinophils per high-powered field (eos/hpf) (IQR 48–95 eos/hpf) to 2 eos/hpf (IQR 0–5 eos/hpf) off swallowed topical corticosteroid. Additionally, improvements in EoE symptoms and endoscopic findings were noted. This report highlights the effectiveness of dupilumab in a group of multiply atopic pediatric and young adult patients with difficult-to-treat EoE in real world practice.
What is Known
- Proton pump inhibitors and swallowed topical corticosteroids are the current mainstays of pharmacologic therapy in eosinophilic esophagitis and have a 50% to 78% success rate.
- Dupilumab is an IL-4 receptor α antibody effective in treating a number of Th2-mediated allergic conditions and is currently in phase 3 trials to evaluate its effectiveness in EoE.
What is New
- A cohort of 7 pediatric and young adult patients with atopy and difficult-to-treat EoE demonstrated histologic, endoscopic, and symptomatic improvement in their EoE on dupilumab.
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic gastrointestinal disease characterized by esophageal dysfunction with eosinophilic infiltration of the esophageal epithelium. EoE is a Th2-mediated response triggered by antigens, likely in foods, which results in stimulation and release of proinflammatory cytokines IL-4, IL-5, IL-13, causing eosinophil and mast cell infiltration, epithelial barrier disruption, and tissue remodeling (1). Despite increasing incidence over the past 30 years (2), treatment options for EoE up until this point have been quite limited, with no approved FDA treatments to date. Common practice pharmacologic therapies for EoE include proton pump inhibitors (PPI) or swallowed topical corticosteroids (STC), with treatment success rates around 50% for PPIs (3) and 68% and 77% for swallowed topical fluticasone and budesonide respectively (4). Given limitations of the currently available treatment options, other pharmacologic options for EoE are needed. Dupilumab is one of several biologic medications being studied for EoE treatment. It is a monoclonal antibody that acts to inhibit IL-4 and IL-13 via IL-4 receptor α targeting. It is currently FDA approved for treatment of moderate-to-severe atopic dermatitis (AD) in children 6 years and older, asthma in children 12 and older, and chronic rhinosinusitis with nasal polyposis in patients 18 and older. Results of phase 2 trials in adults with EoE have been promising, demonstrating significantly improved dysphagia symptoms, reduction in eosinophil counts, improved endoscopic features, and improved esophageal distensibility after 12 weeks of treatment (5). Phase 3 trials in children and adults are under way and preliminary data from the adult cohort suggests that dupilumab also significantly decreases symptom burden in EoE (6). Real world data on dupilumab effectiveness in EoE is limited, with only 2 single patient case reports documenting the effectiveness of dupilumab as a stand-alone pharmacologic therapy for EoE. The first documents the case of a 9-year-old male with EoE with up to 25 eosinophils per high-powered field (eos/hpf) at initial diagnosis, AD, and asthma, who demonstrated symptomatic and histologic improvement in EoE in addition to asthma and AD while on dupilumab (7). The second is a case of 42-year-old male with EoE, asthma, chronic urticaria, allergic rhinitis, and AD who was reported to remain in clinical and histologic remission on dupilumab after weaning off STC (8).
METHODS
Institutional review board approval was obtained (IRB-P00036678). A search of the electronic medical record from a large tertiary care pediatric medical center yielded 172 patients prescribed dupilumab, 12 of whom had histologically confirmed diagnosis of EoE with ≥15 eos/hpf. Of these 12 patients, 7 had a follow-up esophagogastroduodenoscopy (EGD) completed after dupilumab initiation, all of whom are included in this report. Chart review was performed within the electronic medical record to obtain patient demographics, medication records, pathology reports, EGD reports, and clinic notes.
RESULTS
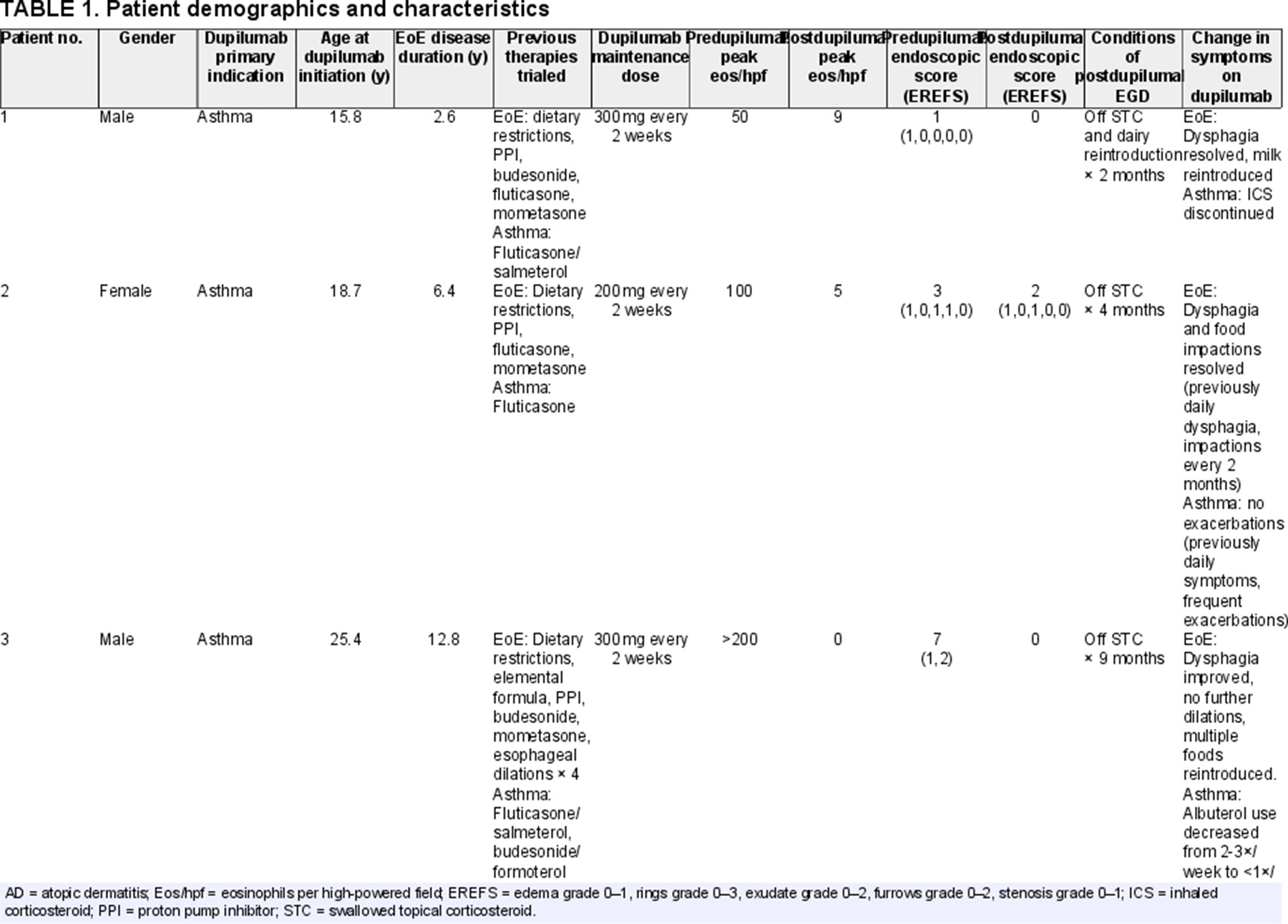
Seven patients with EoE were treated with subcutaneous dupilumab every 2 weeks for the primary indication of asthma and/or AD. The median age was 15.8 years (IQR 9.3–19.5 years) at time of dupilumab initiation and 5 (71%) were male. Patients had a median EoE disease duration of 2.7 years (IQR 1.8–5.3 years). All patients had trialed other EoE therapies before initiation of dupilumab, including proton pump inhibitor (6 of 7 patients), one or more STCs (7 of 7), and dietary restrictions (6 of 7). One patient had previously undergone 4 esophageal dilations in addition to pharmacologic and dietary interventions. None of the patients had achieved healing (<15 eos/hpf) on prior therapies. Just prior to dupilumab initiation, median peak esophageal eosinophil count was 50 eos/hpf (IQR 48–95 eos/hpf), with edema (4 of 6 patients), rings (1 of 6 patients), exudates (3 of 6 patients), furrows (3 of 6 patients), and strictures (1 of 6 patients) documented on most recent EGD completed while on STC or dietary restrictions for EoE treatment, before dupilumab initiation. On follow-up EGD done on dupilumab, median peak esophageal eosinophil count was 2 eos/hpf (IQR 0–5 eos/hpf) with edema and exudate in 2 patients and no rings, furrows, or strictures in any of the 7 patients. Follow-up EGD was performed a median of 5.3 months (IQR 4.6–9.8) after dupilumab initiation, all patients were off STCs and 4 of the 7 patients had reintroduced one or more food groups into their diet at the time of follow-up EGD (Table 1).
DISCUSSION
Our chart review revealed 7 pediatric and young adult patients with atopic conditions and EoE, all of whom demonstrated histologic response in their EoE and response in their asthma or AD after treatment with dupilumab. Not only were these patients previously unresponsive to STCs, but many also had relatively severe EoE phenotypes, which shows hope that dupilumab may be an effective treatment strategy in those with difficult-to-treat EoE. In addition to improvement in EoE, all patients demonstrated improvement in their primary indication for dupilumab initiation, with reports of less frequent exacerbations and decreased albuterol and inhaled corticosteroid use in asthma and fewer AD exacerbations, suggesting that dupilumab has the potential to be effective in patients with multiple concurrent atopic conditions.
This report represents the largest known case series to demonstrate effectiveness of dupilumab in the treatment of EoE in real world practice in both children and young adults but does have limitations. Because this study is retrospective and based on chart review, clinician-to-clinician variability in symptom reporting made it difficult to fully assess changes in symptoms after dupilumab initiation in some patients. Because all patients started dupilumab relatively recently, long-term follow-up data from subsequent EGDs was not available, though we look forward to continuing to follow this cohort to further evaluate dupilumab's long-term effectiveness in sustaining EoE remission.