Joint ESPGHAN/NASPGHAN Guidelines on Childhood Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis
Sources of Funding: This study was supported by ESPGHAN, NASPGHAN, and LaCache Chair in Gastrointestinal Allergic and Immunological Diseases, Children's Hospital Colorado (GTF).
CME module may be found at https://learnonline.naspghan.org/jpgn2
Disclaimer: See complete disclaimers on page 152.
Amil-Dias received Speaker's honoraria from Ferrer, Takeda, and Danone; and advisory honorarium from Adacyte. Auth received research grant from Guts UK/BSPGHAN/Falk Pharma GmbH; honoraria from Falk Pharma and AbbVie; educational grants from Nutricia, Abbott, and RD/Mead Johnson. Bredenoord received research funding from Nutricia, Norgine, Falk Pharma, Thelial, and SST; and received speaker and/or consulting fees from Laborie, Arena, EsoCap, Medtronic, Falk Pharma, Calypso Biotech, Alimentiv, Sanofi/Regeneron, Reckett, and AstraZeneca. Chehade received consultant fees from Regeneron, Allakos, Adare/Ellodi, Shire/Takeda, AstraZeneca, Sanofi, Bristol Myers Squibb, and Phathom; and research funding from Regeneron, Allakos, Shire/Takeda, AstraZeneca, Adare/Ellodi, and Danone. Collins received research funding from AstraZeneca, Celgene/Receptos/BMS, Regeneron Pharmaceuticals, Inc., and Shire, a Takeda company; Consultant for Allakos, Arena Pharmaceuticals, AstraZeneca, BMS, Calypso Biotech, EsoCap, GSK, Regeneron Pharmaceuticals, Inc., and Shire, a Takeda company. Gutiérrez-Junquera received research grants from Pharma GmbH and speaker's honorarium for Danone Nutricia. Dellon received research funding from Adare/Ellodi, Allakos, Arena, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, and Shire/Takeda; Consultant for Abbott, AbbVie, Adare/Ellodi, Aimmune, Akesobio, ALK, Allakos, Amgen, Arena, Aslan, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, Eupraxia, GSK, Gossamer Bio, Invea, Landos, LucidDx, Morphic, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE; Educational grant from Allakos, Banner, and Holoclara. Gonsalves received consulting fees from Allakos, AstraZeneca, Nutricia, BMS, Sanofi-Regeneron, Takeda, and Knopp; Royalties: Up to Date; Speaker bureau: Takeda, Sanofi-Regeneron. Furuta is the Chief Medical Officer of EnteroTrack. Koletzko: Grants given the institution from Mead Johnson, BioGaia, and personal fees from Nestle, Danone, Mead Johnson, AbbVie, Novalac, Janssen, Sanofi, Takeda, and Pfizer outside the submitted work. Gupta: Consultant/DSMB member/Author for Abbott, Adare, Celgene, Gossamer Bio, QOL, Takeda, MedScape, ViaSkin, UpToDate. Research support: Allakos, Ellodi, AstraZeneca. Liacouras: Consultant and/or speaker's honorariums from Abbott; speaker's honorarium from Regeneron. Oliva received consultant fees from Sanofi, Bristol Myers Squibb, and Medtronic; and research funding from Medtronic and Alfa Sigma. Orel received speaker's fees from Ewopharma, AbbVie, Nutricia, Lek Sandoz, and Medis. Papadopoulou received research grants from AbbVie, United Pharmaceuticals, Falk Pharma GmbH, Takeda, AstraZeneca; Speaker's honorariums SYN Innovation Laboratories AE, Uni-Pharma Pharmaceuticals Laboratories S.A., Cross Pharmaceuticals, Petsiavas, Nestle; Touch Independent Medical Education; Sanofi-Regeneron Pharmaceuticals; Advisory board: Falk Pharma GmbH and Specialty Therapeutics. Rothenberg is Consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Nextstone One, Bristol Myers Squibb, Astra Zeneca, Ellodi Pharma, GlaxoSmithKline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first seven listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate. Rothenberg is an inventor of patents owned by Cincinnati Children's Hospital. Strauman has Consultant contracts with Alakos, Astra-Seneca, Calypso, EsoCap, Falk-Pharma, Gossamer, Nutricia, Pfizer, Receptos-Celgene, Regeneron-Sanofi, Roche-Genentec, and Shire. Vieira received consultant and/or speaker fees for Danone Nutricia, Nestlé Nutrition Institute, and Aché Laboratories. Thapar received speaker and/or consulting fees from Danone, Nutricia, Reckitt Benckiser, Biogaia, and Takeda. Zevit received consultation fees from Adare Pharmaceuticals, AstraZeneca, Sanofi, and Falk Pharma; speakers’ fees from Rafa Inc. and Sanofi.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpn3.org).
Abstract
Introduction
Eosinophilic gastrointestinal disorders beyond eosinophilic esophagitis (non-EoE EGIDs) are rare chronic inflammatory disorders of the gastrointestinal (GI) tract. Diagnosis is based on clinical symptoms and histologic findings of eosinophilic inflammation after exclusion of a secondary cause or systemic disease. Currently, no guidelines exist for the evaluation of non-EoE EGIDs. Therefore, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) formed a task force group to provide consensus guidelines for childhood non-EoE EGIDs.
Methods
The working group was composed of pediatric gastroenterologists, adult gastroenterologists, allergists/immunologists, and pathologists. An extensive electronic literature search of the MEDLINE, EMBASE, and Cochrane databases was conducted up to February 2022. General methodology was used in the formulation of recommendations according to the Appraisal of Guidelines for Research and Evaluation (AGREE) II and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system to meet current standards of evidence assessment.
Results
The guidelines provide information on the current concept of non-EoE EGIDs, disease pathogenesis, epidemiology, clinical manifestations, diagnostic and disease surveillance procedures, and current treatment options. Thirty-four statements based on available evidence and 41 recommendations based on expert opinion and best clinical practices were developed.
Conclusion
Non-EoE EGIDs literature is limited in scope and depth, making clear recommendations difficult. These consensus-based clinical practice guidelines are intended to assist clinicians caring for children affected by non-EoE EGIDs and to facilitate high-quality randomized controlled trials of various treatment modalities using standardized, uniform disease definitions.
Graphical Abstract
Eosinophilic gastrointestinal disorders beyond eosinophilic esophagitis (non-EoE EGIDs) are rare chronic inflammatory disorders of the gastrointestinal (GI) tract with unknown long-term consequences. Patients with non-EoE EGIDs suffer from a variety of upper and lower GI symptoms such as vomiting, abdominal pain, and diarrhea and may develop anemia and hypoalbuminemia. Although the natural history of non-EoE EGIDs is poorly defined, several studies support the concept that these diseases are chronic but usually not life-threatening.
Non-EoE EGIDs are composed of a group of diseases subdivided according to the anatomic location of inflammation. They include eosinophilic gastritis (EoG), eosinophilic duodenitis (EoD), eosinophilic enteritis [EoN, a term that can be further subdivided into eosinophilic duodenitis (EoD), eosinophilic jejunitis (EoJ), and eosinophilic ileitis (EoI)], and eosinophilic colitis (EoC). The clinical presentation of the different non-EoE EGIDs depends on the affected GI site and the extent and depth of eosinophilic infiltration through the bowel wall. In the absence of biological markers, the diagnosis is based on clinical symptoms and histologic findings of eosinophilic inflammation after ruling out a secondary cause of inflammation or systemic disease. Treatment strategies depend on various medical and social factors.
A number of factors pose challenges to non-EoE EGIDs guideline development. First, non-EoE EGIDs are rare conditions, so clinical experience is limited and an extensive literature is lacking. Since this guideline focuses on pediatric non-EoE EGIDs, this problem becomes even more apparent because much of the current literature reports adult experiences. Second, unlike the esophagus, which does not contain eosinophils, the immune milieu of the GI tract distal to the esophagus contains a resident population of eosinophils. It is likely that these eosinophils are involved in various forms of innate immunity, and as such, their numbers may rise and fall depending also on which part of the GI tract is being examined. Therefore, determining the diagnostic number of eosinophils for non-EoE EGIDs remains a moving target. Because the underlying pathogenesis of non-EoE EGIDs remains elusive and likely has multiple causes depending on the part of the GI tract examined, current treatment options are limited and have not been thoroughly investigated.
To address timely issues related to improving care of pediatric patients with non-EoE EGIDs, we have taken a 2-pronged approach, namely a thorough literature review and a series of electronic and virtual discussions. The statements and recommendations are intended to support the care of children with non-EoE EGIDs. They are based in part on the adult literature, which is currently more robust.
AIMS AND METHODOLOGY
Participants and Structure
European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) requested submission of a non-EoE EGIDs clinical guideline and contacted representatives of each Society to develop a proposal, which was approved by each Society. The Task Force Group (TFG) leader from ESPGHAN (AP) and NASPGHAN (GTF) invited various representatives with expertise in non-EoE EGIDs to participate in generating this document. This TFG of 24 physicians and researchers was assembled virtually to address specific clinically relevant topics (Appendix 1, Supplemental Digital Content 3, http://links.lww.com/MPG/D225). Originally, the TFG was scheduled to meet at the World Congress of Pediatric Gastroenterology, Hepatology and Nutrition in 2020, but due to the COVID-19 pandemic, most of the subsequent 2-year work was accomplished virtually. The TFG was divided into core groups with at least 1 assigned leader per group that focused on different aspects of the non-EoE EGIDs literature (Appendix 2, Supplemental Digital Content 4, http://links.lww.com/MPG/D226). The ESPGHAN and NASPGHAN leaders and the core group leaders (Amil-Dias, Auth, Chehade, Collins, Gupta, Gutiérrez-Junquera, Orel, Vieira, Zevit) formed the TFG Steering Committee. The TFG leaders identified a set of core topics that were determined by the Steering Committee. The themes were reviewed and finalized by all participating authors, and a set of clinically relevant questions was developed to form the basis of this guideline. Guidelines will be reviewed by the ESPGHAN Council and the NASPGHAN Clinical Care and Quality Committee and their relevant stakeholders. ESPGHAN and NASPGHAN will utilize guidelines to improve patient care through citation referencing.
Literature Search, Review, and Evaluation of Evidence
Literature Search
A comprehensive systematic review of the literature was conducted by a librarian working at Tel Aviv University using the electronic databases MEDLINE (accessed through PubMed) and EMBASE, as well as the Cochrane Database of Systematic Reviews (The Cochrane Library) and the Cochrane Central Register of Controlled Trials (CENTRAL) from 1935 through February 2022. Core leaders performed a review of the provided list of articles and abstracts and selected those publications that were published in English, included children and if not available, publications in adults as well, histologic documentation of GI eosinophilia, case reports and case series, and clinical trials to address the defined topic area. Primary citations were obtained and distributed in PDF form to all participants and recorded in ENDNOTE.
Because this is an evolving field and new nomenclature has recently been developed, we attempted to use the definitions in each citation rather than updating them. For example, based on the recent publication by Dellon et al (1), the term eosinophilic gastroenteritis (EGE) has been replaced by the terms EoG and EoN. If a patient has both gastric and small bowel involvement, he or she would have EoG and EoN. For the purposes of this document, therefore, the acronym EGE may continue to be used when it was used in the original citation, although it is often not known whether it means involvement of the stomach, small intestine, or both. Unless otherwise stated, the term non-EoE EGIDs is used in this document to describe EoG, EoN, and EoC. Finally, since the natural history of non-EoE EGIDs has yet to be fully defined, we look forward to the results of future natural history studies that will permit derivation of more comparative analyses and extrapolation from studies of adults.
Review and Evaluation of the Evidence
Core group leaders distributed relevant literature to their groups and a review of publications followed. The TFG followed the methodology of GRADE (2) (see www.gradeworkinggroup.org) to rate the quality of evidence (QoE; high, moderate, low, or very low quality) and classify recommendations into 4 clear categories (3): a strong recommendation for an intervention, meaning the physician should do it; a weak recommendation for an intervention, meaning the physician probably should do it; a weak recommendation against an intervention, meaning the physician probably should not do it; and a strong recommendation against an intervention, meaning the physician should not do it. Finally, the Agree tool II (www.agreecollaboration.org) was used to ensure the high quality of our clinical practice guideline.
Consensus Process
After a series of virtual meetings, an electronic vote was held between February 22 and March 6, 2022 to rate each of the statements and recommendations using a 6-point Likert scale (1: strongly disagree; 2: quite disagree; 3: somewhat disagree; 4: somewhat agree; 5: quite agree; 6: strongly agree) with an opportunity to comment. A statement/recommendation was approved if more than 75% of the participants agreed with it (Likert score of 4–6). All Statements and Recommendations reached consensus (Appendix 3a–c, Supplemental Digital Content 5, http://links.lww.com/MPG/D227). A second vote was conducted between September 21 and October 9, 2022 on a recommendation on definition of remission which was not included in the first vote but consensus was not achieved and the recommendation was not included in the manuscript. All statements and recommendations that emerged from the vote were discussed and approved in an online meeting.
Statements and Recommendations
Each statement is labeled with the QoE (high, moderate, low, or very low) and the result of the vote (percent agreement). Each recommendation is labeled with the strength of recommendation (SoR: strong or weak) and also the result of the vote (percent agreement). The SoR using the GRADE approach was indicated only for studies on the accuracy of diagnostic procedures or the evaluation of the effectiveness of a treatment, as mentioned above. Each recommendation started with the words “We recommend” when the SoR was strong and “We conditionally recommend” when the SoR was weak.
STATEMENTS, SUMMARY OF EVIDENCE AND RECOMMENDATIONS
The following are clinically relevant questions followed by a statement, summary of evidence, and a clinical recommendation. Depending on the type of question, statements and recommendations are not always provided and the answer to the question is embedded in the summary of evidence followed by a conclusion and open questions for research. The list of statements and recommendations can be found in Table 1 and Table 2, respectively.
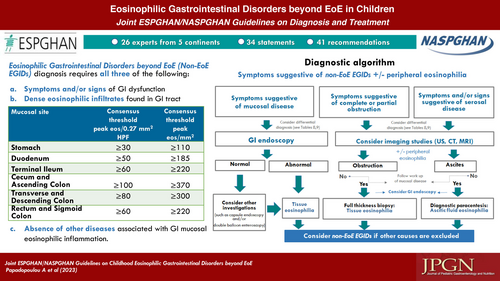
1. The presenting symptoms of non-EoE EGIDs depend on the GI segment involved, the extent of eosinophilic inflammation within the GI tract and the depth of inflammation through the bowel wall (See Table 3). QoE: Moderate, Agreement: 100% |
2. The described symptoms and signs are not specific for non-EoE EGIDs, and detailed alternative conditions should be considered before the confirmation of the diagnosis. QoE: Moderate, Agreement: 100% |
3. Presently no validated symptoms severity assessment tools exist thus making correlation of symptoms with severity of eosinophilic inflammation inconclusive. QoE: Very Low, Agreement: 95% |
4. Studies assessing the impact of non-EoE EGIDs on quality of life of children and their families are lacking. QoE: Very low, Agreement: 95% |
5. Peripheral eosinophilia may occur in patients with non-EoE EGIDs but is neither a specific nor a sensitive indicator for non-EoE EGIDs. QoE: Moderate, Agreement: 95% |
6. The available data do not allow conclusions to be drawn regarding the use of peripheral eosinophilia as a marker for the resolution of tissue inflammation. QoE: Very low, Agreement: 95% |
7. There is lack of evidence on the usefulness of fecal calprotectin for diagnosing or monitoring non-EoE EGIDs. QoE: Very low, Agreement: 100% |
8. Various non-specific endoscopic findings have been described in patients with EoG/EoN, (See Table 4). QoE: Moderate, Agreement: 95% |
9. In many patients with EoG/EoN, the GI mucosa looks macroscopically normal. QoE: Low, Agreement: 100% |
10. In patients with EoC, the colonoscopy findings include mucosal nodularity, oedema and mucosal friability but many patients may have normal macroscopic appearance of the colonic mucosa. QoE: Low, Agreement: 100% |
11. Imaging studies such as abdominal ultrasound, computed tomography, magnetic resonance imaging and contrast series do not directly contribute to the diagnosis of non-EoE EGIDs. QoE: Low, Agreement: 100% |
12. Imaging studies such as abdominal ultrasound, computed tomography, magnetic resonance imaging and contrast series give important additional information about the depth of inflammation through the bowel wall (muscular, serosal layers), the extent of involvement, and the presence of complications. QoE: Low, Agreement: 100% |
13. Complete blood count with differential, hemoglobin, ferritin, serum albumin, immunoglobulin G concentrations and total immunoglobulin E levels may be abnormal in selected patients with non-EoE EGIDs, but these abnormalities are not specific for non-EoE EGIDs and may be secondary to other diseases that need to be excluded. QoE: Low, Agreement: 100% |
14. Assessment of complete blood count with differential, hemoglobin, ferritin, serum albumin and immunoglobulin G as well as fecal a1-antitrypsin concentrations may be helpful to monitor non-EoE EGIDs response to treatment if they were abnormal at diagnosis. QoE: Low, Agreement: 95% |
15. When analyzing ascitic fluid, a predominance of eosinophils amongst inflammatory cells is highly suggestive of the serosal form of non-EoE EGIDs. QoE: Very low, Agreement: 100% |
16. A paucity of studies have investigated the eosinophilic infiltration of the GI mucosa in children with no organic diseases reporting the area of high- power field (See Table 5). QoE: Low Agreement: 100% |
17. Non-EoE EGIDs are clinico-pathological entities, therefore, histology alone is not enough to diagnose them without compatible symptoms and signs. QoE: Low, Agreement: 95% |
18. Some pathologic features are not normally associated with non-EoE EGIDs but do not necessarily rule out that diagnosis. Such features include acute neutrophilic inflammation, neutrophilic glandulitis/cryptitis and granulomas that are characteristic of inflammatory bowel disease but may be also seen in biopsies taken from non-EoE EGIDs-related ulcers/erosions or from patients with parasitic infection. QoE: Low, Agreement: 95% |
19. Histological features in favor of non-EoE EGIDs in the presence of eosinophilic infiltration of the GI mucosa are eosinophil glandulitis/cryptitis, eosinophils in muscularis mucosa/submucosa, fibrosis/fibroplasia of the lamina propria, degranulation of eosinophils and lymphoid aggregates (See Table 7). However, the diagnostic/prognostic value of these ancillary findings remains unclear. QoE: Low, Agreement: 100% |
20. In the presence of eosinophilic infiltration of the GI mucosa, the presence of signs of chronicity (such as atrophy, fibrosis and smooth muscle hyperplasia in the stomach and duodenum and architectural abnormalities such as villous blunting in the small intestine, and crypt elongation/branching/distortion in the small and large intestines) are helpful features to confirm the histological part of the diagnosis, especially if the endoscopic appearance is normal. QoE: Low, Agreement: 82% |
21. Differential diagnosis of eosinophilic inflammation of the GI tract occurring as segmental disease or as part of more diffuse involvement of the GI tract, includes a wide range of conditions (See Table 8). QoE: Low, Agreement: 100% |
22. The initial evaluation of a patient with mucosal eosinophilia depends on the presenting symptoms, history, physical examination, laboratory findings as well as the involved GI segment(s) and may include a combination of tests (See Table 9). QoE: Low, Agreement: 100% |
23. In limited numbers of case series, systemic oral steroids have been effective in inducing clinical and histological remission in non-EoE EGIDs. QoE: Low, Agreement: 95% |
24. There are no data on the selection criteria of which patients with non-EoE EGIDs should be treated with oral steroids, nor on the optimal dose or duration of treatment. LE: Very low, Agreement: 95% |
25. Elimination diets may induce clinical improvement or remission in a proportion of children with non-EoE EGIDs but there are very limited data on histological response. QoE: Very low, Agreement: 95% |
26. There is insufficient data on which foods should be eliminated, but case series suggest that avoidance of cow's milk may be effective in some children. QoE: Very low, Agreement: 91% |
27. There is no evidence to support the use of IgE-based food allergy tests to guide dietary restriction therapy. QoE: Very low, Agreement: 100% |
28. Evidence supporting the use of proton pump inhibitors or H2 receptor antagonists in the treatment of children with EoG/EoD is lacking. QoE: Very low, Agreement: 95% |
29. Limited number of case series describe the use of endoscopic dilation to manage partial obstruction in adults with non-EoE EGIDs. QoE: Very low, Agreement: 95% |
30. Surgical intervention is used for non-EoE EGIDs-related clinically significant bowel obstruction that does not respond to treatment with systemic steroids, whereas pyloromyotomy has rarely been shown to be effective in children with EoG and associated pyloric stenosis. QoE: Low, Agreement: 91% |
31. Combination therapy has been used in a proportion of patients with non-EoE EGIDs with variable effects. QoE: Low, Agreement: 82% |
32. There is lack of randomised controlled trials assessing the efficacy of the available treatment options of non-EoE EGIDs. QoE: Low, Agreement: 100% |
33. Treatment with systemic steroids at appropriate doses followed by timely tapering is an effective initial approach to the treatment of most patients with non-EoE EGIDs. QoE: Very low, Agreement: 100% |
34. There are no studies that have examined the role of maintenance treatment in patients with non-EoE EGIDs. QoE: Very low, Agreement: 100% |
1. We recommend the term Eosinophilic Gastrointestinal Disorders beyond Eosinophilic Esophagitis (non-EoE EGIDs) to describe chronic inflammatory disorders of the gastrointestinal (GI) tract beyond the esophagus characterized clinically by the presence of gastrointestinal symptoms and histologically by eosinophilic predominant inflammation of the GI tract, in the absence of an identifiable secondary cause. SoR: Strong, Agreement: 100% |
2. We conditionally recommend using the prefix “Eo” followed by the specific organ involved as a convention to name non-EoE EGIDs: EoG for eosinophilic gastritis, EoD for eosinophilic duodenitis, EoJ for eosinophilic jejunitis, EoN for eosinophilic enteritis, EoI for eosinophilic ileitis, and EoC for eosinophilic colitis. SoR: Weak, Agreement: 100% |
3. We conditionally recommend that when multiple parts of the gastrointestinal tract are affected by non-EoE EGIDs, they are referred to the segment involved; for instance, Eosinophilic gastritis and eosinophilic duodenitis: EoG and EoD and Eosinophilic gastritis and jejunitis: EoG and EoJ. SoR: Weak, Agreement: 100% |
4. We conditionally recommend that when clinically known, subclassification of the different layers of the GI tract should be also described as mucosal, muscular or serosal. SoR: Weak, Agreement: 100% |
5. We recommend that peripheral blood eosinophilia in the clinical context, not be used as the sole criterion to make the diagnosis of non-EoE EGIDs. SoR: Strong, Agreement: 95% |
6. We conditionally recommend that when consistently associated with mucosal eosinophilia in an individual patient, peripheral eosinophilia may be considered as an adjunct to monitor non-EoE EGIDs disease activity. SoR: Weak, Agreement: 91% |
7. We recommend that fecal calprotectin concentrations not be used to make the diagnosis of non-EoE EGIDs or to monitor non-EoE EGIDs disease activity. SoR: Strong, Agreement: 95% |
8. We recommend that assessment of the gross appearance of the mucosa be documented during endoscopic assessment. SoR: Strong, Agreement: 95%. |
9. We recommend multiple biopsies including gastric antrum, gastric body and duodenum to be obtained in case of symptoms suggestive of EoG/EoD, taken from the involved segments of the GI tract, from normal and abnormal appearing areas of the mucosa. SoR: Strong, Agreement: 95% |
10. We conditionally recommend multiple biopsies from terminal ileum and from at least three sites (cecum/ascending colon, transverse/descending colon, and sigmoid/rectum) in case of symptoms suggestive of EoC, to be obtained from both normal and abnormal appearing areas of the mucosa. SoR: Weak, Agreement: 100% |
11. We conditionally recommend biopsies be labelled as such in separate containers to help interpret eosinophil numbers based on threshold diagnostic numbers. SoR: Weak, Agreement: 95% |
12. We recommend that imaging studies be considered in selected cases for providing information on the depth of bowel wall inflammation and disease extent. SoR: Strong, Agreement: 95% |
13. We recommend that imaging studies be considered in selected cases to localize involved areas for targeted tissue diagnosis. SoR: Strong, Agreement: 95% |
14. We conditionally recommend that blood tests not be used to make the diagnosis of non-EoE EGIDs but may be useful to monitor treatment responses in selected cases. SoR: Weak, Agreement: 95% |
15. We recommend that in the appropriate clinical context, ascitic fluid should be assessed and the finding of eosinophilic predominant inflammation will support the non-EoE EGIDs diagnosis. SoR: Strong, Agreement: 100% |
16. We recommend that GI segment specific threshold peak eosinophil counts (Table 6) be considered prior to making a non-EoE EGIDs diagnosis (expert opinion). SoR: Strong, Agreement: 91% |
17. We conditionally recommend that evaluation of acute and chronic features of mucosal inflammation should be recorded as these can be supportive of the non-EoE EGIDs diagnosis (See Table 7). SoR: Weak, Agreement: 100%, |
18. We recommend that other clinically relevant diseases associated with mucosal eosinophilia be evaluated prior to making a non-EoE EGIDs diagnosis (See Table 8). SoR: Strong, Agreement: 100% |
19. We conditionally recommend the initial evaluation of a patient with symptoms suggestive of non-EoE EGIDs be individualized based on history and clinical examination and associated laboratory testing (See Table 9). SoR: Weak, Agreement: 100% |
20. We conditionally recommend that during the initial evaluation of a patient with GI mucosal eosinophilia one should consider allergic diseases, parasite infections, drug administration (especially immunosuppressants), inflammatory bowel diseases, and malignancy as a part of the differential diagnosis (See Table 8). SoR: Weak, Agreement: 95% |
21. We recommend that the choice of endoscopic examination(s) of the gastrointestinal tract should be guided by symptoms, laboratory, and radiographic findings. SoR: Strong, Agreement: 95% |
22 We recommend that the diagnosis of non-EoE EGIDs in children and adolescents must include all three of the following: a. Symptoms and/or signs of GI dysfunction including but not limited to vomiting, abdominal pain/cramping, bloating, anorexia, weight loss, early satiety, hematemesis, heartburn, dyspepsia, tenesmus, diarrhea or constipation, hematochezia or melena, abdominal distention, ascites, iron deficiency, protein loss. b. Dense eosinophilic infiltrates found in mucosal or full thickness biopsies above organ specific threshold values (See Table 6). c. Absence of other diseases associated with GI mucosal eosinophilic inflammation (See Table 8). SoR: Strong, Agreement: 91% |
23. We conditionally recommend using an Algorithm to guide in the diagnostic approach of children and adolescents with symptoms suggestive of non-EoE EGIDs (See diagnostic Algorithm). SoR: Weak, Agreement: 100% |
24. We conditionally recommend that the goals of treatment in non-EoE EGIDs include achieving resolution of symptoms, improving gross endoscopic and histological abnormalities, promoting normal childhood growth and development, and preventing disease complications. SoR: Weak, Agreement: 100% |
25. We conditionally recommend that the timing of endoscopic and histological re-assessment should be decided on an individualized basis. Agreement: 100% |
26. We recommend that the use of oral systemic steroids be considered to induce remission in individual patients with non-EoE EGIDs and that their use should be undertaken after thorough discussion with the patient and parents about their benefits and risks (expert opinion). SoR: Strong, Agreement: 100% |
27. We conditionally recommend that topical steroids may be considered in selected patients with non-EoE EGIDs (expert opinion). See General approach to treatment. SoR: Weak, Agreement: 96% |
28. We conditionally recommend that empiric elimination diets may be considered in selected patients with non-EoE EGIDs (expert opinion). See General approach to treatment. SoR: Weak, Agreement: 100% |
29. We conditionally recommend not using food allergy tests to guide dietary restriction therapy for the treatment of non-EoE EGIDs. SoR: Weak, Agreement: 100% |
30. There is insufficient data to make a recommendation for or against the use of proton pump inhibitors or H2 receptor antagonists for treating childhood EoG/EoD. Agreement: 100% |
31. We conditionally recommend that proton pump inhibitors may be considered for treating upper GI ulcerations in children with EoG/EoD. SoR: Weak, Agreement: 90% |
32. There is insufficient data to make a recommendation for or against the use of antihistamines, leukotriene inhibitors or mast cell stabilizers as a sole treatment of non-EoE EGIDs. Agreement: 90% |
33. There is insufficient data to make a recommendation for or against the use of immunomodulating drugs for the treatment of non-EoE EGIDs. Agreement: 100% |
34. There is insufficient data to make a recommendation for or against the use of biological drugs in treating childhood non-EoE EGIDs. Agreement: 90% |
35. We conditionally recommend that in addition to medical/dietary treatment, endoscopic dilation may be considered in selected cases with significant objective signs of obstruction. SoR: Weak, Agreement: 91% |
36. We conditionally recommend that surgical treatment of non-EoE EGIDs may be useful for patients with refractory ulcers, intestinal perforation or bowel obstruction which cannot be controlled otherwise. SoR: Weak, Agreement: 100% |
37. There is insufficient data to make a recommendation for or against the use of combination therapy for treating non-EoE EGIDs. Agreement: 90% |
38. We conditionally recommend that combination therapy may be useful for treating concomitant allergic diseases. SoR: Weak, Agreement: 85% |
39. We conditionally recommend that the initial treatment of children with non-EoE EGIDs be individualized based on the symptoms, impact on growth and development and other co-morbid features with an attempt to involve patients and parents/caregivers in shared decision making. SoR: Weak, Agreement: 95% |
40. We conditionally recommend that changes in symptoms and histology should be monitored, preferably with objective tools to allow meaningful conclusions on treatment effects. SoR: Weak, Agreement: 100% |
41. Since the natural history of non-EoE EGIDs is uncertain, we conditionally recommend that the long-term treatment should be discussed with patients and parents/caregivers and include the benefits and risks of long- term treatments as well as their impact on health-related quality of life and financial costs. SoR: Weak, Agreement: 100% |
Section A. Definition and Epidemiology
1. What are the definitions of non-EoE EGIDs including eosinophilic gastritis, eosinophilic enteritis and eosinophilic colitis?
Summary of Evidence
Eosinophilic gastrointestinal disorders beyond eosinophilic esophagitis (non-EoE EGIDs) are chronic, immune-mediated disorders of the GI tract characterized by eosinophilic inflammation of the mucosa that can lead to organ dysfunction (4, 5). These clinicopathologic disorders require both clinical symptoms and histologic inflammation to establish the diagnosis.
There is a broad differential diagnosis for intestinal eosinophilia in any part of the GI tract that includes hypersensitivity reactions to drugs or foods, malignancies, inflammatory bowel disease (IBD) (Crohn's disease, ulcerative colitis, IBD-unclassified), infections (viral, bacterial, helminths, parasites), drug-induced disease, especially tacrolimus-induced disease after solid organ transplantation, and primary immunodeficiencies (eg, common variable immunodeficiency, and several monogenic diseases) and hypereosinophilic syndrome (HES) (6). The diagnosis of non-EoE-EGIDs requires exclusion of these conditions when clinically indicated.
Some patients with non-EoE EGIDs may have mucosal eosinophilia in more than one segment of their GI tract. For example, in a recent retrospective multicenter series, of 373 subjects (317 children and 56 adults) diagnosed with non-EoE EGIDs, 38% had EoG, 33% EGE, and 29% EoC, while 41% had eosinophilic inflammation outside of their primary disease location with the esophagus the second most common GI segment involved. Multisite inflammation was more common in children than in adults (68% vs 37%; P < 0.001) (7). The colon presents a diagnostic challenge as eosinophil density decreases from the cecum to the rectum.
Recommendation 1: We recommend the term eosinophilic gastrointestinal disorders beyond eosinophilic esophagitis (non-EoE EGIDs) to describe chronic inflammatory disorders of the gastrointestinal (GI) tract beyond the esophagus characterized clinically by the presence of gastrointestinal symptoms and histologically by eosinophilic predominant inflammation of the GI tract, in the absence of an identifiable secondary cause.
SoR: Strong, Agreement: 100%
2. What is the recommended terminology to describe non-EoE EGIDs that involve one or different GI segments, one or more layers of the GI tract wall?
Summary of Evidence
Recently, updated nomenclature for non-EoE EGIDs has been published based on the location of eosinophilic inflammation and the organ involved in the inflammatory process: EoG; EoN with subcategories of EoD, EoJ, EoI; and EoC (1) by a group of 92 experts from various fields (gastroenterology, allergy, pediatrics, pathologists, researchers) to conduct a series of surveys using the Delphi process and to develop an expert consensus for non-EoE EGIDs nomenclature. This was necessary because a variety of terms are used to describe non-EoE EGIDs patients, particularly those with gastric and/or intestinal eosinophilia. Based on this effort, the term eosinophilic gastrointestinal diseases was established to encompass all eosinophilic GI diseases and then subdivide them according to the site of predominant involvement. For example, if the disease involves the stomach or colon, it would be referred to as “eosinophilic gastritis-EoG” and “eosinophilic colitis-EoC.” When multiple organs are involved, including the esophagus, the nomenclature here remains somewhat controversial. For example, when eosinophils are higher than normal in the stomach, duodenum, jejunum, ileum, or colon and esophagus, and the primary symptoms/diagnosis involve the stomach, duodenum, jejunum, ileum, or colon, it is recommended to use the nomenclature “eosinophilic gastritis or duodenitis, ‘eosinophilic ileitis or colitis’” with “esophageal involvement.”
Recommendation 2 We conditionally recommend using the prefix “Eo” followed by the specific organ involved as a convention to name non-EoE EGIDs: EoG for eosinophilic gastritis, EoD for eosinophilic duodenitis, EoJ for eosinophilic jejunitis, EoN for eosinophilic enteritis, EoI for eosinophilic ileitis, and EoC for eosinophilic colitis.
SoR: Weak, Agreement: 100%
Recommendation 3 We conditionally recommend that when multiple parts of the gastrointestinal tract are affected by non-EoE EGIDs, they are referred to by the segment involved; for instance, eosinophilic gastritis and eosinophilic duodenitis: EoG and EoD and eosinophilic gastritis and jejunitis: EoG and EoJ.
SoR: Weak, Agreement: 100%,
Recommendation 4 We conditionally recommend that when clinically known, subclassification of the different layers of the GI tract should be also described as mucosal, muscular or serosal.
SoR: Weak, Agreement: 100%
3. What is the current incidence and prevalence of non-EoE EGIDs?
Summary of Evidence
Non-EoE EGIDs are considered rare disorders of the gastrointestinal tract. Accurate data on incidence and prevalence are difficult to ascertain because most publications to date have focused on case reports and small retrospective series. In addition, there are limitations in extrapolating incidence and prevalence based on coding or insurance databases. For example, a recent study suggested that the incidence of EoC in their centers is much lower than diagnosed based on chart review with International Classification of Diseases, Ninth Revision (ICD-9) codes. After reviewing clinical data, most patients did not meet the criteria for EoC (8).
Nevertheless, recent estimates of prevalence based on information from insurance databases in North America, over a 2-year period (2009–2011) with data from more than 75 million individuals (ages 0–64 years) using the International Classification of Diseases, Ninth Revision suggest that the prevalence of EoG, EGE, and EoC is 6.3 per 100,000, 8.4 per 100,000, and 3.3 per 100,000, respectively, while in individuals younger than 20 years the prevalence of EGE was 10.7 per 100,000 (9). In 2011, Spergel et al published the results of an electronic survey of US pediatric and adult allergists, immunologists, and gastroenterologists that included 1836 responses (17%) of 10,874 inquiries. The estimated prevalence of EGE or EoC was 28 per 100,000 based on patients seen by pediatric or adult gastroenterologists and 2 per 100,000 among patients seen by allergists and immunologists (10). In another population-based study in the United States, which analyzed information from electronic records from 26 major health systems between 2012 and 2017, Mansoor et al (8) estimated an overall prevalence of EGE of 5.1 per 100,000. In a retrospective study of 7457 endoscopic procedures with biopsies performed over a 10-year period at a tertiary pediatric hospital, 17 children (0.23%) were diagnosed with primary EGIDs and 13 (0.17%) with EGE (11). Turner et al (12) identified 194 patients with high eosinophil counts (166–5050/mm) in the absence of other underlying disease from a database of 1.2 million patients with colonic biopsies and calculated a prevalence of primary colonic eosinophilia in adults of less than 1 in 6000 patients. Mansoor et al (8) used the database of 26 US electronic health records from 1999 to March 2017 and found that of the 35,826,830 individuals, 770 had EoC, which was an overall prevalence rate of 2.1/100,000 individuals. Jensen et al used a similar approach to calculate prevalence based on medical codes and then standardized the estimates to the US population by age and sex. However, the reported data may represent an overestimation of the primary EoC because both asymptomatic colonic eosinophilia and secondary forms of EoC were included in the calculation. Furthermore, in the US study by Jensen et al (9) the estimated prevalence of EoG, age- and sex-standardized to the US population, decreased from 6.3/100,000 to 6.0/100,000 after exclusion of patients with IBD codes. Although non-EoE EGIDs are considered rare diseases, there is increasing evidence that EGE may be underdiagnosed. In a recent collaborative study of 6 centers in the United States involving 373 individuals with non-EoE EGIDs (317 children and 56 adults), an increase in the rate of diagnosis of all non-EoE-EGIDs was observed from 2005 to 2016 (7). Limited natural history studies suggest that some non-EoE EGIDs, particularly EoG, have increased in the past decade, similar to EoE 15 years ago (9).
In a study from Turkey, Egritas Gurkan et al (11) examined the pathology reports of all endoscopy and colonoscopy procedures performed in children between 2008 and 2018. Of 7457 biopsies taken in 8262 procedures, 17 children were diagnosed with primary non-EoE EGIDs, of whom only 1 had an EoG (16.6 years, male) (11). In a retrospective observational study from Colombia, 35 (23.2%) of 151 children (0–12 years) were found to have a much higher rate of eosinophilic gastroenteropathy and the majority of them (78.8%; 60.9% were males) had only one segment affected (13).
4. What are the key demographic features of non-EoE EGIDs including age of onset, sex, ethnicity, co-morbid features and socioeconomic factors?
Summary of Evidence
Jensen et al (9) analyzed adult and pediatric data from a national database (>75 million individuals from across the United States), and reported a mean (std) age of patients with EoG (ICD-9) of 39.8 (±17.4) years and a prevalence predominant in females with 7.9 cases/100,000 compared to 5.4 cases/100,000 for males. Notably, in women, prevalence increased with age, being highest in the oldest age group (14.4 cases/100,000 in women aged 60–64 years). Although there was no socioeconomic analysis, the prevalence of EoG in the US South and Midwest was nearly double that in the Northeast and West.
EGE is diagnosed more commonly in children than in adults with a prevalence of 10.7 per 100,000 in subjects younger than 20 years and 7.1 per 100,000 in subjects between 20 and 64 years, with the highest prevalence in children younger than 5 years (17.6 per 100,000 in boys and 16.7 per 100,000 in girls) (9).
Mansoor et al (8) reported the highest prevalence of EGE (7.8 per 100,000) in children and adolescents aged 10–14 years. Regarding gender, unlike EoE, male predominance of EGE was not observed. In a retrospective multicenter study that included 123 patients with EGE, 52% were males and 47% were females (7). In addition, population-based studies have described a slightly higher prevalence of EGE in women: 5.3 versus 4.8 per 100,000 (8) and 8.8 versus 7.6 per 100,000 (9). EGE was more prevalent in White than Black Americans and Asians, with reported prevalence of 6.3, 5.5, and 4.3 per 100,000, respectively (8). Pesek et al (7) reported that of 123 patients with EGE, 67% were White, 14% were Black, and 3% were Asian.
More recently, Pesek et al (14) conducted a retrospective cohort study in which they reviewed clinical and research databases for non-EoE-EGIDs diagnoses from 2005 to 2016 at 6 US centers affiliated with the Consortium of Eosinophilic Gastrointestinal Researchers (CEGIR). Of the 373 subjects, there were 317 children [mean age at diagnosis of 7.3 years (range 0.5–17, median 7)] and 56 adults [mean age at diagnosis of 36 years (range 18–77, median 32)]. Of these 373 patients, 142 patients had EoG; 52% were female and 71% were Whites (4% Asian, 10% Black, 14% missing data). It should be noted however that all of the above prevalence studies were carried out in North America, while prevalence data from other populations, such as Asian populations, are lacking, although many case reports of patients with non-EoE EGIDs are from the above populations (15-18). A recent nationwide hospital-based survey in Japan of patients who visited 2906 hospitals that performed endoscopies and answered the questionnaire from January 2013 to December 2017 revealed a total number of 151 patients with non-EoE EGIDs (19). Age at onset of non-EoE EGIDs showed 2 peaks: 0–14 years and in the 50s. Non-EoE EGIDs showed no sex difference (19).
Coexisting allergic conditions (rhinitis 28%–30%; asthma 16%) were reported in 30.5% of EoG cases, which was significantly higher than in the baseline population. The proportion of EoG patients reporting a concomitant allergic disease was higher in pediatric patients (age < 19 years; 58.9%) than in adults (33.6%) (9).
Atopic disease was commonly observed in retrospective studies and case series of patients with EGE, with approximately 41%–73% of patients with concomitant asthma, dermatitis, or seasonal allergies or food allergies (7, 20-22). In a study from a national database, 45.6% of patients with EGE had coexisting allergic conditions (rhinitis, dermatitis, sinusitis, asthma, and food allergy), with rhinitis being the most reported concomitant allergic condition. Concomitant allergic diseases were more common in pediatric patients (51.6% vs 41.8%) (9). Mansoor et al (8) reported that EGE patients were more likely than control patients to have drug allergy, rhinitis, asthma, sinusitis, dermatitis, food allergy, eczema, and urticaria, with the odds ratio highest for food allergy (OR 12.20; 95% CI: 10.97–13.57). Consistent with the study by Jensen et al (9), the study by Pesek et al found that 57% of 142 adults and children with EoG had a history of at least 1 atopic disease (7, 14). Concomitant allergic disease has been found in about 40%–45% of children by EoC. In addition to atopy, immunodeficiency, such as selective IgA deficiency, may also be associated with GI eosinophilia (23).
Regarding comorbidity, both functional abdominal pain disorders (FAPDs, Rome IV, formerly known as abdominal pain-related functional gastrointestinal disorders; AP-FGIDs; Rome III) and IBD have been associated with eosinophilic infiltration of the GI tract. Lee et al (24) retrospectively studied 105 patients with AP-FGIDs. The number of eosinophils in the gastric antrum and body was significantly higher in these children compared with normal pediatric reference values, but there were no differences among the 4 subtypes of AP-FGID (functional dyspepsia, irritable bowel syndrome, abdominal migraine, and functional abdominal pain or syndrome). More recently, Lee et al (25) performed a quantitative comparison of eosinophils within the GI tract of children with FAPDs, IBD, and control subjects. The number of eosinophils in the stomach (antrum and body) was significantly higher in children with Crohn disease (but not ulcerative colitis) than in FAPDs. Similarly to the previous study, eosinophil counts in the gastric antrum of children with FAPD were significantly higher than in normal controls, with no differences noted between FAPD subgroups. A comparison between GI eosinophil counts of children with IBD (52 with Crohn disease and 23 with ulcerative colitis) and normal reference GI eosinophil counts (26, 27) showed that eosinophil counts in all segments between the stomach and rectum were significantly higher in IBD than in controls (25). In contrast, Koutri et al (28) studied children in 3 referral pediatric gastroenterology units (Athens, Madrid, and Rome) but found no differences in eosinophil density in the GI tract (including the stomach) between children with or without functional GI disorders.
Conclusions of Section A and open questions for research: To date, non-EoE EGIDs are considered rare diseases, but further studies using standardized definitions and global diagnostic codes will be critical to identify trends in incidence and prevalence. Well-defined documentation of the demographic and comorbid characteristics associated with different patterns of non-EoE EGIDs will allow better recognition of the disease.
Section B. Pathogenesis/Natural History
1. What are the underlying mechanisms of non-EoE EGIDs?
The underlying pathogenesis of non-EoE EGIDs is not entirely certain but is probably different for each of the clinicopathologic entities. EoG, EoN, and EoC each share symptoms that reflect the dysfunction of the respective organs associated with the diagnostic tissue eosinophilia. For instance, EoG may present as a manifestation of its mucosal phenotype with bleeding, EoN with diarrhea, and EoC with hematochezia. Since non-EoE EGIDs themselves are rare and the mucosal form of non-EoE EGIDs is the most common, this summary of pathogenetic mechanisms focuses on the mucosal forms of non-EGIDs based on clinical and molecular studies (29).
Eosinophilic Gastritis
Recent evidence supports dysregulation of the gastric immune milieu with alterations in molecular profile. Early work on the pathogenesis of the disease reveals similarities between EoG and EoE. EoG appears to be driven by a similar TH2 mechanism compared to EoE, with higher levels of IL-4, IL-5, and IL-13 compared to control subjects (30). Prussin et al (31) found that TH2 cells in patients with EGE were associated with high expression of IL-5, in contrast to TH2 cells in patients with immunoglobulin E (IgE)-mediated peanut anaphylaxis, in whom IL-4 + TH2 cells were more abundant. More recent work (32) has identified an EoG transcriptome diagnostic panel based on studies of 18 gastric genes called the EoG-Diagnostic Panel (EGDP). The EGDP identified patients with active EoG; monitored disease activity in longitudinal samples; and inversely correlated with peak gastric eosinophil levels, periglandular circumferential collars, and endoscopic nodularity. Notably, the levels of TH2-associated cytokines in blood, eotaxin-3, thymus and activation-regulated chemokines (TARC), IL-5, and thymic stromal lymphopoietin (TSLP) were significantly increased compared with control subjects. Interestingly, a study of omalizumab in patients with EoG/EGE showed that the number of eosinophils in the stomach and duodenum decreased and clinical symptoms improved in treated subjects, suggesting that IgE, although not the primary effector of disease, may play a role in a subset of patients, although this does not appear to be the case in EoE (5). Transcriptomic analysis of the stomach in EoG shows upregulation of IL-4, IL-5, IL-13, eotaxin-3 (CCL26), and mast cell signature genes (eg, CPA3), consistent with findings in patients with EoE (30). However, more than 90% of the genes from EoG diverge from those of patients with EoE and differ from those in patients with other gastric diseases, such as Helicobacter pylori and gastric cancer. IL-17 is also upregulated, a finding not typically associated with EoE but rather with asthma and eczema.
Eosinophilic Enteritis
In a phase 2 trial (33), 43 adults with EoG, EoD, or both conditions were randomly assigned to receive AK002 (lirentelimab) and 22 were assigned to receive placebo. The mean percentage change in GI eosinophil count was -86% in the combined AK002 group, as compared with 9% in the placebo group (P < 0.001). Treatment response occurred in 63% of the patients who received AK002 and in 5% of the patients who received placebo (P < 0.001) (33). Other work shows that antigen presentation at distant host mucosal surfaces can lead to GI mucosal eosinophilia, suggesting that systemic communication can lead to gut dysfunction (34).This suggests that sensitization and allergen challenge may lead to EoN.
Eosinophilic Colitis
Recent data suggest a different inflammatory profile in EoC patients compared with patients with upper non-EoE EGIDs. Of note is the absence of strong TH2 immunity and the demonstration of lower cell proliferation in mucosal biopsies compared with control subjects (32).
2. Do food allergies cause non-EoE EGIDs?
Summary of Evidence
As with the above discussion, non-EoE EGIDs can present with distinct clinicopathological features. The role of food allergy in the pathogenesis of EoG, EoN, and EoC is likely as diverse as has been suggested for the mechanistic features of EoE. For example, recent work suggests that at least three endotypes distinguish EoE that can be defined clinically and molecularly (35). One endotype is clearly atopic in nature, while another is fibrostenotic. With respect to the non-EoE EGIDs, patterns are likely to emerge. Clinical observations and response to treatments will be key to our future understanding.
EoG is characterized by several nonspecific symptoms related to gastric dysfunction, such as nausea, early satiety, vomiting, hematemesis, and abdominal pain. The fact that these symptoms may or may not occur in association with the ingestion of certain foods does not rule out some form of food allergy but does not support an IgE-mediated reaction. EoG patients show clinical responses to dietary restrictions and topical steroids (36), but identification of the specific protein allergen can be elusive and there is no treatment platform yet. Whether this is a non-IgE mediated food allergy or changes in the inflammatory milieu related to the microbiome is not certain.
EoN is a very rare clinical entity that may or may not be associated with symptoms of either diarrhea or pain that may or may not occur with certain foods. These symptoms reflect inflammation of the small intestinal mucosa leading to malabsorption, as well as muscle contraction and luminal distention. In other cases, mucosal eosinophilia is seen in asymptomatic patients with severe anemia or hypoalbuminemia (37). Since some patients with EoN respond to dietary avoidance treatment, a non-IgE-mediated food allergy may be responsible, whereas other patients require the use of topical or systemic steroids, suggesting an alternative inflammatory response.
EoC is the least well understood and described of the non-EoE EGIDs, as its clinical presentation beyond infancy can be confused with IBDs such as ulcerative colitis or Crohn disease (38). Nevertheless, symptoms such as diarrhea, tenesmus, urgency, hematochezia, and lower abdominal pain suggest colonic dysfunction. Recent evidence suggests that the molecular profile of patients is not consistent with a strong TH2 inflammatory pattern, while a clinical series has identified children with non-IgE-mediated food allergic reactions (39).
Since the clinical entity of allergic proctocolitis in infancy, is transient, benign, and appears to be triggered by soy or cow's milk, it will not be considered as one of non-EoE EGIDs here.
3. Do non-EoE EGIDs spread to other parts of the GI tract?
Summary of Evidence
Limited clinical experience and reports suggest that the organ specificity of eosinophilia observed in non-EoE EGIDs remains constant over time. Sometimes, clinically indicated endoscopic examination may reveal eosinophilia in another organ, but it remains to be seen whether this was simply missed on sentinel endoscopy, represents a normal innate response, or is evidence of spread of pathologic eosinophilia (14, 19).
When eosinophilia is observed on follow-up endoscopy, clinical interpretation, especially in relation to new symptoms and endoscopic findings, is important for at least 2 reasons. First, a complete interpretation of the histologic specimen should be performed to determine whether only mucosal eosinophilia is present or whether additional features have developed that are suggestive of another disease process such as IBD. Second, the clinicopathologic findings will determine whether additional testing or treatment is indicated and what type of follow-up, if any, is required.
4. Are non-EoE EGIDs premalignant conditions?
Summary of Evidence
There is no evidence that non-EoE EGIDs are a premalignant or malignant condition. Mucosal eosinophilia, when found with additional clinicopathologic features, can occur in some malignancies (40) and should be evaluated before a diagnosis of non-EoE EGIDs is made.
5. Are non-EoE EGIDs chronic?
Summary of Evidence
The definition of chronicity may be based on symptoms, endoscopic findings, and histologic abnormalities. For example, several studies have shown that patients with non-EoE EGIDs present with symptoms over a long period of time. Second, limited data and experience suggest that endoscopic and mucosal eosinophilia persists in patients with non-EoE EGIDs when observed over a long period of time. Finally, several studies have shown that some patients with non-EoE EGIDs may have waxing and waning courses, underscoring the need for long-term follow-up. Overall, the evidence to date suggests that non-EoE EGIDs have a chronic course similar to EoE, but with some important caveats (14, 19, 21, 22, 29, 41, 42).
First, while most clinical experience and data support the fact that non-EoE EGIDs are chronic, some studies suggest that non-EoE EGIDs can resolve. Pineton et al (41) reviewed files from 43 patients diagnosed with EGE who were followed from January 1988 to April 2009 and reported that 18 patients (42%; 9 with subserosal disease) had an initial flare of the disease without relapse, 16 (37%) had multiple flares that were separated by periods of full remission (recurring disease), and 9 (21%) had chronic disease. Another center reported that 18 of 35 patients with EGE, had remission without chronic symptoms, whereas 10 had chronic symptoms requiring chronic medical therapy (42). Whether these findings represent a transient innate response manifested by mucosal eosinophilia or a clinical pattern of waxing and waning non-EoE EGIDs is uncertain. Long-term studies in a large number of patients are needed. There is also uncertainty regarding the need for follow-up endoscopy as part of routine care in all patients with non-EoE EGIDs and the timing of endoscopy, whether it should be performed only during relapses or also during periods of remission. There are no data to answer this question, and clinical judgment should be individualized.
Conclusions of Section B and open questions for research: The pathogenesis of non-EoE EGIDs is not yet fully understood, and it is likely that various disease patterns will emerge in the future. Studies of the natural history of non-EoE EGIDs will determine the frequency of spread of mucosal eosinophilia from originally predominant GI segments to other segments. Allergic reactions to food antigens may be the cause of some non-EoE EGIDs, but future studies may clarify how to identify these allergens and the impact of their restriction on short- and long-term prognosis. Malignant potential is not currently supported as part of the natural history of non-EoE EGIDs. Future research and long-term follow-up of patients with non-EoE EGIDs will provide further insight into pathogenesis and identification of therapeutic targets.
Section C. Symptoms/Endoscopy/Imaging Studies/Other Tests
1. What are the presenting symptoms and signs associated with non-EoE EGIDs?
Statement 1 The presenting symptoms of non-EoE EGIDs depend on the GI segment involved, the extent of eosinophilic inflammation within the GI tract and the depth of inflammation through the bowel wall (See Table 3).
QoE: Moderate, Agreement: 100%
Statement 2 The described symptoms and signs are not specific for non-EoE EGIDs, and detailed alternative conditions should be considered before the confirmation of the diagnosis.
QoE: Moderate, Agreement: 100%
| Clinical symptoms and signs | |
|---|---|
| Mucosal involvement | EoG: abdominal pain/cramping, bloating, vomiting, anorexia, weight loss, early satiety, hematemesis, heartburn, dyspepsia, melena, iron deficiency anemia, protein loss, and ulceration with or without perforation. EoD/EoJ/EoI/EoN: diarrhea, abdominal pain, nausea, vomiting, ulceration with or without perforation, iron deficiency anemia and protein loss. EoC: abdominal pain, nausea, vomiting, tenesmus, diarrhea and hematochezia or constipation. |
| Muscular involvement | EoG: abdominal pain and persisting vomiting, pyloric stenosis. EoD/EoJ/EoI/EoN/EoC: obstructive symptoms (abdominal pain, persistent vomiting, abdominal distension, severe constipation), intussusception, perforation. |
| Subserosal involvement | EoD/EoJ/EoI/EoN/EoC: abdominal distention, ascites. |
- EoC = eosinophilic colitis; EoD = eosinophilic duodenitis; EoG = eosinophilic gastritis; EoI = eosinophilic ileitis; EoJ = eosinophilic jejunitis; EoN = a term that can be further subdivided into EoD, EoJ, and EoI.
Summary of Evidence
In a retrospective study of 373 patients, including children and adults with non-EoE EGIDs, treated at 6 hospitals, abdominal pain (51%), nausea and/or vomiting (49%), and diarrhea (30%) were the most common symptoms (7). Diarrhea was more common in patients with EoC than in those with EoG. Hematochezia occurred in 11% of all patients and in 24% of patients with EoC. In other retrospective studies (20, 21, 43-45) abdominal pain occurred in 46%–100%, diarrhea in 27%–59%, nausea and/or vomiting in 31%–70%, and flatulence in 27%. Other symptoms described include loss of appetite, weight loss, reflux/regurgitation, and rarely ascites. Laboratory abnormalities were observed and included eosinophilia in peripheral blood (15%–92%), elevated IgE (62%), anemia (15%–54%), and hypoalbuminemia (62%). In EoC, symptoms may include abdominal pain, nausea, vomiting, tenesmus, constipation and/or diarrhea, and mucus or blood in the stool (46, 47). In 50 pediatric patients with EoC, recurrent abdominal pain occurred in 66%, chronic diarrhea in 64%, and chronic constipation in 8% (46). A study of 78 patients with colonic eosinophilia found that abdominal pain, hematochezia, and diarrhea occurred in 59%, 47%, and 39%, respectively (47). In rare cases, obstructive symptoms may occur due to intussusception or perforation of the bowel. Vomiting as an isolated symptom may also be merely a consequence of vagal stimulation due to colonic dysmotility (48). If the inflammatory process is deep within the colon wall and involves the subserosal level, ascites and abdominal distension may also occur (43). However, the clinical signs may be nonspecific (7, 49).
2. Does symptom severity reflect severity of eosinophilic inflammation in non-EoE EGIDs?
Statement 3 Presently no validated symptom severity tools exist thus making correlation of symptoms with severity of eosinophilic inflammation inconclusive.
QoE: Very Low, Agreement: 95%
Summary of Evidence
There is limited evidence on whether GI symptoms can reflect disease activity, as the published literature is very heterogeneous, especially regarding the completeness of follow-up (clinical only, endoscopic, histologic). A validated symptom assessment questionnaire for EoG and EoN has only very recently been published for patients aged 12 years and above and awaits application in clinical studies in children with non-EoE EGIDs (50). A multicenter retrospective study of children and adult patients with non-EoE EGIDs assessed response to treatment in those patients who had documented follow-up within 6 months. The study suggested that a similar proportion of patients responded clinically and histologically, but this was not confirmed in the analysis (7). One phase 2 clinical trial provided evidence that both symptoms and mucosal eosinophilia improved with treatment with AK002, a drug that selectively targets both mast cells and eosinophils (33), but the results were not confirmed by a 24-week, Phase 3, randomized, double-blind, placebo-controlled study of lirentelimab (AK002) in patients with biopsy confirmed EoD as reported on September 9, 2022 on the website (https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-eodyssey-study-patients/) of Allakos, the biotechnology company developing lirentelimab. In a retrospective multicenter study from the United States of 108 EoC patients, diet and corticosteroids were used in 31% of patients with EC. Multiple concomitant treatments were used in 41%. This resulted in overall clinical improvement in n = 14 (54%) of patients with any treatment at 6 months follow-up, endoscopic improvement was noted in 6 of 13 (46%) of patients, and colonic mucosal eosinophil counts were reduced in 8 of 9 (89%) (7).
3. What is the impact of non-EoE EGIDs on quality of life?
Statement 4 Studies assessing the impact of non-EoE EGIDs on quality of life of children and their families are lacking.
QoE: Very low, Agreement: 95%
Summary of Evidence
Similar to children with eosinophilic esophagitis (51), the quality of life of children with non-EoE EGIDs should be assessed by validated questionnaires. Two studies have directly examined the impact of health-related quality of life (HRQOL) that may be unique to non-EoE EGIDs. Using semi-structured interviews with 7 adult patients with EoG and/or EoN, Bedell et al identified common HRQOL themes, including the psychological impact of diagnosis, impact on social relationships, financial impact, and impact on the body. These issues generally improved over time and with effective treatment (52). In a follow-up study, Guadagnoli et al examined quality of life and internalized stigma in adult patients with non-EoE EGIDs using a validated EoE quality of life scale (53). Thirty-four of the 149 total participants had non-EoE EGIDs. The results provided evidence that decreased quality of life was associated with increased internalized stigma and that more outpatient visits and endoscopies were associated with increased internalized stigma, such as alienation (53). Bedell et al (52) reported an impact of EoG on HRQOL, with reduced scores for the domains on psychological impact of diagnosis, impact on social relationships, financial impact, and impact on the body. In EoC, there is insufficient information on HRQOL in pediatric patients. It is thought that those patients who are more affected by inflammation-related symptoms such as dysmotility or who require dietary interventions or immunosuppressive treatment to control symptoms may have a greater impact on quality of life. To date, no studies have addressed this question in pediatric patients.
4. Is peripheral eosinophilia helpful in making the diagnosis of non-EoE EGIDs or for monitoring disease activity?
Statement 5 Peripheral blood eosinophilia may occur in patients with non-EoE EGIDs but is neither a specific nor a sensitive indicator for non-EoE EGIDs.
QoE: Moderate, Agreement: 95%
Statement 6 The available data do not allow conclusions to be drawn regarding the use of peripheral eosinophilia as a marker for the resolution of tissue inflammation.
QoE: Very low, Agreement: 95%
Summary of Evidence
The differential diagnosis of peripheral eosinophilia is broad. Increased eosinophilia in peripheral blood is commonly seen in patients with EoG. This may be related to active EoG or to other conditions such as comorbid allergies.
The majority of patients with EoN have elevated peripheral blood eosinophil counts, and even total white blood cell counts (43, 54-68). In EoC, peripheral eosinophilia may occur in a proportion of patients (13, 20), as well as iron deficiency anemia and hypoalbuminemia (20).
The percentage of eosinophils in leukocytes may exceed 50% in some cases but is usually lower and may even be within the normal range (69-73).
The diagnostic characteristics of peripheral eosinophilia have not been studied, but because other causes can lead to increased numbers of eosinophils in the peripheral blood, it is unlikely that peripheral eosinophilia can be used as a diagnostic marker of non-EoE EGIDs. Although the number of eosinophils in peripheral blood usually decreases during treatment of EoN, this is not an indicator that can be relied upon to assess the success of therapy (33).
Peripheral eosinophilia does not reflect disease severity, and even patients with very severe disease may have normal peripheral blood eosinophil counts.
Recommendation 5 We recommend that peripheral blood eosinophilia in the clinical context not be used as the sole criterion to make the diagnosis of non-EoE EGIDs.
SoR: Strong, Agreement: 95%
Recommendation 6 We conditionally recommend that when consistently associated with mucosal eosinophilia in an individual patient, peripheral eosinophilia may be considered as an adjunct to monitor non-EoE EGIDs disease activity.
SoR: Weak, Agreement: 91%
5. Is fecal calprotectin helpful in making the diagnosis of non-EoE EGIDs or in monitoring disease activity?
Statement 7 There is lack of evidence on the usefulness of fecal calprotectin for diagnosing or monitoring non-EoE EGIDs.
QoE: Very low, Agreement: 100%
Summary of Evidence
Fecal calprotectin (FCP) is a marker of neutrophilic inflammation. It is not present in eosinophils and is not a marker of eosinophilic disease activity. Nonspecific tests for inflammation such as FCP may also be elevated in a proportion of pediatric patients with non-EoE EGIDs (74), indicating the presence of other inflammatory cell populations but may be useful in cases where IBD is considered in the differential diagnosis. The limited utility of FCP in diagnosing or monitoring non-EoE EGIDs is based on the following: (1) limited studies examining the sensitivity and sensitivity of FCP in non-EoE EGIDs; (2) the amount of non-neutrophil associated FCP (monocytes, epithelial cells, others) has been considered limited in non-EoE EGIDs; (3) the ample evidence to support FCP in the evaluation and monitoring of IBD; (4) the risk of misdiagnosis or mismanagement of a patient based on FCP, that is, diagnosis of non-EoE EGIDs in a patient who actually has IBD.
Recommendation 7 We recommend that fecal calprotectin concentrations not be used to make the diagnosis of non-EoE EGIDs or to monitor non-EoE EGIDs disease activity.
SoR: Strong, Agreement: 95%
6. What endoscopic findings are associated with non-EoE EGIDs and what is the appropriate biopsy protocol for diagnosing non-EoE EGIDs?
Statement 8 Various non-specific endoscopic findings have been described in patients with EoG/EoN (See Table 4).
| Types of non-EoE EGIDs | Endoscopic findings | References |
|---|---|---|
| EoG/EoD/EoN (mucosal disease; Figures 1–3, Supplemental Digital Content 1, http://links.lww.com/MPG/D223) |
|
(7, 14, 18, 21, 56-59, 61, 64-70, 78-88, 92) |
| EoG/EoD/EoN (muscular disease) | Narrowing of the lumen, pyloric stenosis | (57, 78, 89) |
| EoC |
|
(11, 46, 93) |
- EoC = eosinophilic colitis; EoD = eosinophilic duodenitis; EoG = eosinophilic gastritis; EoI = eosinophilic ileitis; EoJ = eosinophilic jejunitis; EoN = a term that can be further subdivided into EoD, EoJ, and EoI.
QoE: Moderate, Agreement: 95%
Statement 9 In many patients with EoG/EoN, the GI mucosa looks macroscopically normal.
QoE: Low, Agreement: 100%
Statement 10 In patients with EoC, the colonoscopy findings include mucosal nodularity, edema and mucosal friability but many patients may have normal macroscopic appearance of the colonic mucosa.
QoE: Low, Agreement: 100%
Summary of Evidence
A number of adult studies and a few pediatric studies describe endoscopic findings in EoG and EoN. Patients with EoG may have nodular, erythematous gastric mucosa, with linear hemorrhage, polyps, erosions, or ulcers, or their gastric mucosa may appear macroscopically normal (20, 30, 66, 75). Two recent studies identified and graded key features of EoG in order to provide clinical outcome metrics (32, 76). In a study of 16 patients, gastroduodenal ulcers were noted in 3 patients (12.5%) and nonspecific findings, including gastritis and duodenitis, were noted in 13 and 11 patients, respectively (43). In a large study of 142 patients with EoG, a normal endoscopic appearance of the stomach was the most common finding and was noted in 62% of patients. Erythema (24%), ulceration (8%), nodularity (8%), and friability of the mucosa (6%) were also commonly reported (14). Fewer endoscopic findings may occur in children, as demonstrated by Lwin et al (77) in a study of 10 children and 50 adults with EoG. Several cases of gastric outlet obstruction mimicking pyloric stenosis have been reported in children (57, 78). The authors reported a normal appearance more frequently in children than in adults (60% vs 22%) (77).
Endoscopic findings associated with non-EoE EGIDs include shallow mucosal erosions but also deep ulcers, perforated ulcers, diffuse friability, thickened folds, nodularity or granularity, mucosal edema and redness, as well as normal-appearing mucosa (7, 14, 18, 21, 56, 58, 59, 61, 64-70, 78-88). In a multicenter retrospective cohort of 317 children and 56 adult patients with non-EoE EGIDs, the most common finding was normal appearance of the mucosa in the stomach (66%), duodenum (83%), jejunum (67%), and ileum (81%) (7). Less common findings were ulceration (6%), nodularity (3%), erythema (2%), and friability of the mucosa (2%). Colonoscopy findings may include a nodular or polypoid appearance, as well as overt hyperemia, nodularity, edema, and friability (14). Raffaele et al studied the endoscopic findings in 50 patients with EoC (46). The majority of patients had normal endoscopy (74%). Nodular lymphoid hyperplasia was present in 26%. Other pathologic findings included mucosal bleeding in 2, edema of the ileocecal valve in 2, inflammatory polyps in 2, and mild erosions of the mucosa in 1.
In patients with involvement of the muscular layer, narrowing of the lumen can sometimes be seen on endoscopy (89). Capsule endoscopy can also be used for more detailed examination of mucosal changes in the small bowel in case of mucosal disease (90, 91) but should not be used if narrowing of the GI lumen is suspected.
Recommendation 8 We recommend that assessment of the gross appearance of the mucosa be documented during endoscopic assessment.
SoR: Strong, Agreement: 95%.
Recommendation 9 We recommend multiple biopsies including gastric antrum, gastric body and duodenum to be obtained in case of symptoms suggestive of EoG/EoD, taken from the involved segments of the GI tract, from normal and abnormal appearing areas of the mucosa.
SoR: Strong, Agreement: 95%
Recommendation 10 We conditionally recommend multiple biopsies from terminal ileum and from at least three sites (cecum/ascending colon, transverse/descending colon, and sigmoid/rectum) in case of symptoms suggestive of EoC, to be obtained from both normal and abnormal appearing areas of the mucosa.
SoR: Weak, Agreement: 100%
Recommendation 11 We conditionally recommend biopsies be labeled as such in separate containers to help interpret eosinophil numbers based on threshold diagnostic numbers (See Table 6).
SoR: Weak, Agreement: 95%
7. Are any imaging studies helpful in the evaluation of patients with non-EoE EGIDs?
Statement 11 Imaging studies such as abdominal ultrasound, computed tomography, magnetic resonance imaging and contrast series do not directly contribute to the diagnosis of non-EoE EGIDs.
QoE: Low, Agreement: 100%
Statement 12 Imaging studies such as abdominal ultrasound, computed tomography, magnetic resonance imaging and contrast series give important additional information about the depth of inflammation through the bowel wall (muscular, serosal layers), the extent of involvement, and the presence of complications.
QoE: Low, Agreement: 100%
Summary of Evidence
Imaging studies such as abdominal ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and contrast series do not directly contribute to the diagnosis of non-EoE EGIDs but can provide data on structural involvement (muscular, serosal) and follow disease status during treatment. Findings include thickening of the GI wall, gastric or intestinal folds, hypertrophy of the pylorus, thickening of the mesentery, dilated bowel loops or narrowing of the bowel, enlarged inflammatory mesenteric lymph nodes, and ascites (54, 57-59, 61, 62, 65, 67, 82, 94).
Endoscopic ultrasonography can be used to observe local changes in detail or to measure wall thickness (82). Radiographic studies may reveal normal imaging despite the findings of histologic inflammation (55, 95).
Recommendation 12 We recommend that imaging studies be considered in selected cases for providing information on the depth of bowel wall inflammation and disease extent.
SoR: Strong, Agreement: 95%
Recommendation 13 We recommend that imaging studies be considered in selected cases to localize involved areas for targeted tissue diagnosis.
SoR: Strong, Agreement: 95%
8. Are any other tests helpful in making the diagnosis of non-EoE EGIDs or monitoring disease activity?
Statement 13 Complete blood count with differential, hemoglobin, ferritin, serum albumin, immunoglobulin G concentrations and total IgE levels may be abnormal in selected patients with non-EoE EGIDs, but these abnormalities are not specific for non-EoE EGIDs and may be secondary to other diseases that need to be excluded.
QoE: Low, Agreement: 100%
Statement 14 Assessment of complete blood count with differential, hemoglobin, ferritin, serum albumin and immunoglobulin G as well as fecal a1-antitrypsin concentrations may be helpful to monitor non-EoE EGIDs response to treatment if they were abnormal at diagnosis.
QoE: Low, Agreement: 95%
Statement 15 When analyzing ascitic fluid, a predominance of eosinophils amongst inflammatory cells is highly suggestive of the serosal form of non-EoE EGIDs.
QoE: Very low, Agreement: 100%
Summary of Evidence
Non-EoE EGIDs are often associated with iron deficiency anemia due to impaired iron absorption and/or occult GI bleeding, especially in the mucosal subtype of the disease (43). Hypoalbuminemia may occur due to the increased mucosal permeability, and protein-losing enteropathy can be assessed measuring fecal alpha 1-antitrypsin in a 24-hour feces collection (43, 96). Low levels of immunoglobulins can present consequently to the protein loss. The erythrocyte sedimentation rate is usually normal or modesty elevated in some patients (97). In contrast to the low sensitivity and specificity of peripheral eosinophilia, the finding of leukocytosis with a preponderance of eosinophil granulocytes (usually >50% but as high as 100%) in the ascitic fluid is a marker of serosal involvement by EGE (43, 58, 87). IgE levels are usually normal in patients with non-EoE EGIDs (43, 55, 57, 58, 66, 72, 82, 98, 99).
Currently, no peripheral biomarkers exist to diagnose non-EoE EGIDs or monitor disease activity, but promising results are emerging. Two multicenter studies (30, 100) present an EoG gene panel-based biomarker panel that may provide diagnostic clarity in the future with further validation. Shoda et al (100) found that serum levels of both TSLP and IL-33 were specifically elevated and the genes for them were upregulated in the inflamed mucosa of patients with infantile EGE, compared with results from healthy controls and from children with asymptomatic immediate-type food allergies, active atopic dermatitis, and active ulcerative colitis. Among the 36 serum cytokines and chemokines analyzed, CCL3 and CCL21 were also specifically elevated in children with EGE. However, when they examined the diagnostic utility of these cytokines/chemokines by establishing optimal cutoff values, only TSLP and IL-33 showed both sensitivity (78.8%) and specificity (TSLP, 97.6%; IL-33, 95.2%) for EGE. In addition, the authors discovered that serum levels of TSLP and IL-33 decreased with clinical improvement of symptoms and signs (100).
Recently, a multicenter CEGIR study identified endoscopic, histologic, molecular, and blood biomarkers for EoG (32). A total of 185 patients were included (EoG, 74; non EoG controls, 111), two-thirds of whom were children and one-third adults. The control group included patients with non-EoG chronic gastritis, EoE, or atopic comorbidities. A resultant EoG diagnostic panel consisted of 18 genes that are upregulated or downregulated in patients with active EoG, completely distinguishing patients with EoG from control subjects. After this EGDP 18 score was generated based on a study with a smaller number of patients, it was validated on a larger cohort. The EGDP 18 score was significantly decreased in patients with active EoG compared with patients without EoG (P < 0.0001). In addition, the EGDP 18 score inversely correlated with disease activity as defined by the number of eosinophils in gastric biopsies (P < 0.0001). Significant correlations were found between specific genes within EGDP 18 and gastric eosinophil peak counts, as well as histologic and endoscopic disease severity. The authors also developed a blood-based immunoassay platform with 10 cytokines/chemokines to differentiate patients with EoG from those without EoG. They found significantly higher levels of 3 cytokines in plasma (eotaxin-3/CCL26, IL-5, and TARC/CCL17) and 3 cytokines in serum (eotaxin-3/CCL26, IL -5, and TSLP) in patients with EoG. Based on these results, they developed an EoG biomarker scoring system for plasma and serum. This blood based EoG score distinguished patients with active EoG from controls without EoG (P < 0.001), and patients with active EoG had significantly higher scores than patients with inactive disease.
Recommendation 14 We conditionally recommend that blood tests not be used to make the diagnosis of non-EoE EGIDs but may be useful to monitor treatment responses in selected cases.
SoR: Weak, Agreement: 95%
Recommendation 15 We recommend that in the appropriate clinical context, ascitic fluid should be assessed and the finding of eosinophilic predominant inflammation will support the non-EoE EGIDs diagnosis.
SoR: Strong, Agreement: 100%
Section D. Histology
1. What is the peak eosinophil mucosa density of “healthy” childhood GI mucosa?
Statement 16 Only few studies have investigated the eosinophilic infiltration of the GI mucosa in children with no organic diseases reporting the area of high-power field (Table 1, Supplemental Digital Content 2, http://links.lww.com/MPG/D224).
QoE: Low Agreement: 100%
Summary of Evidence
To define pathologic eosinophil densities in pediatric GI tract, normal peak eosinophil levels were first examined. In contrast to the esophagus, which is normally devoid of eosinophils, eosinophils in the rest of the GI tract are resident mucosal cells. Attempts have been made over several decades to determine eosinophil density in general and peak eosinophil counts in the normal pediatric GI tract (25-28, 30, 32, 47, 49, 74, 77, 101-108). However, the studies were subject to several significant limitations. First, since it would be unethical to subject perfectly healthy children to endoscopy under sedation/anesthesia solely for mucosal biopsies, studies have used data from children with various nonspecific GI symptoms. While most of these studies used cross-sectional cohorts of children with obvious functional complaints, in most cases the children were not followed up to ensure that no organic disease developed after endoscopy or that they had no evidence of bacterial (H pylori infection), parasitic, or systemic disease that might have contributed to the mucosal eosinophilia. Two studies by Hoofien et al (104) and Koutri et al (28) compared children with resolving symptoms with those who had FGIDs, and found no significant differences in peak eosinophil counts. It should be noted however, that other studies have reported higher GI eosinophil counts in the gastric antrum and body of children with FGIDs compared to normal pediatric reference values (24) and in gastric antrum of children with FGIDs compared to healthy controls (25). The limitation remains that asymptomatic children were not included for ethical reasons. Both studies (28, 104), which were international cohorts from countries in Europe and Israel, but did not include cohorts from North America, found geographic variance in peak eosinophil counts. The geographic differences noted within Europe mean that further differences around the world cannot be ruled out which may be another limitation in setting universal standards. Geographic differences in eosinophil density may be due to genetic, infectious, environmental, or other causes.
Another limitation noted in many studies is the reporting of eosinophil density in terms of non-standardized eosinophils/high-power field (HPF) without providing a method that allowed comparison between fields from different microscopes (eg, either reporting eosinophils/mm2 or reporting the area of the specific HPF used, which could allow conversion to eos/mm2). Kiss et al (105) and others have highlighted the variability and sometimes large errors that arise when attempting to compare studies that lack these details because the HPF areas of the various commercially available microscopes vary widely (105), which however, may be less of a consideration now that lenses in microscope objectives are made by machines and not by hand. An additional methodological error noted was the calculation of mean values (± standard deviations) instead of median values (interquartile ranges; IQR), which are appropriate for the non-normal distributions of peak eosinophil counts in healthy mucosa. However, in a few studies, the highest single peak value in the cohort per anatomic site (the upper limit of the range) was included, which is independent of the calculation of the mean or median.
Articles reporting peak mucosal eosinophil density were reviewed if the following criteria were met: (a) Mucosal GI biopsies from sites other than the esophagus were analyzed for peak eosinophil counts from endoscopically healthy-appearing endoscopies; (b) Biopsies were obtained from children; if both adult and pediatric specimens were included, articles were assessed if the counts from children were reported separately or if combined values were reported but there was a specific statement that the counts from children were not different from adult counts; (c) Results were either presented as eosinophils/mm2 or the HPF area used was reported to allow conversion to a standardized field size. We excluded articles that did not clearly state that the biopsies from which eosinophil counts were obtained were considered normal. In the final analysis, we also excluded manuscripts in which only the mean eosinophil counts in each biopsy were assessed rather than the peak eosinophil counts because we wanted to determine the upper limit of normal eosinophil density.
The reported sizes of the HPFs in manuscripts meeting all criteria ranged from 0.196 to 0.55 mm2. To allow for comparisons between studies, values were mathematically converted to a standardized HPF area, 0.27 mm2, which was chosen because it is the standardized area used in a large multi-institutional study of non-EoE EGIDs in children by the CEGIR.
We were able to obtain raw data from two large (28, 104) and one small (108) study that included pediatric patients who underwent endoscopy for nonspecific functional complaints and were later found to have no organic disease on follow-up (28, 104, 108). Using individual patient-data, we created a single large cohort (n = 143–314 at various anatomic sites) from which the median, IQR, outliers [defined as (upper limit of IQR + 1.5 × IQR) − (upper limit of IQR + 3 × IQR)], and extreme outliers [defined as >(upper limit of IQR + 3 × IQR)] were calculated. In our analysis, we defined “likely normal” as values below the upper limit of IQR + 1.5 × IQR (where the outliers begin), “possibly abnormal” as values between the likely normal and the upper limit of IQR + 3 × IQR (where the extreme outliers begin), and “probably abnormal” for values above the upper limit of this interval. In contrast to the calculation of the upper limit of normal values, the eosinophil cutoffs used to define non-EoE EGIDs considered both “probably abnormal” data and the clinical experience of pathologists and clinicians, and recommendations were made on the basis of expert opinion (Figures 4–6, Supplemental Digital Content 1, http://links.lww.com/MPG/D223). In the statistical analysis performed using IBM SPSS Statistics for Windows (Version 27; IBM Corp, Armonk NY), we found that the peak eosinophil counts in GI tissues did not have normal distributions, therefore, results were reported as medians with IQRs and ranges as appropriate, while Tukey fences were applied to describe outliers; graphical presentations (Figure 6, Supplemental Digital Content 1, http://links.lww.com/MPG/D223) show outlying values in addition to the medians and IQRs. As noted above, these data are derived from studies performed in Europe and Israel, and therefore may not be universally applicable.
Some patients with concomitant atopic diseases, may harbor elevated eosinophil counts in their GI mucosa (19). In light of this, in order to avoid over-diagnosis of non-EoE EGIDs, the authors decided to choose higher rather than lower cut-off numbers of eosinophilic densities. Furthermore, considering that eosinophils are normally found in the GI mucosa distal to the esophagus in a relatively broad range of concentrations, some children may have counts that overlap with pathologic values. Therefore, a simple cut-off value may lead to over- or underdiagnosis of non-EoE EGIDs. Therefore, both clinical and pathologic considerations must be taken into account in addition to peak mucosal eosinophil density, and the lower the eosinophil count, the more one should evaluate for additional pathologic findings in the clinical context before making a non-EoE EGIDs diagnosis. With regards to normal eosinophil density of the muscular or serosal layers of the pediatric GI tract there are currently no peer-reviewed published data for sites other than the esophagus.
Currently, pathologists and clinicians are accustomed to using eos/hpf. However, as centers gradually transition from standard microscopes to digitized slide analysis and automated systems, Eos/mm2 will become more common practice. We therefore reported our analysis in both forms using the standardized 0.27 mm2 CEGIR HPF. Reporting counts per HPF and counts per unit area provide an ideal form of reporting that may not be practical for all, but it is helpful for comparing studies as well as histologic reports on patient referrals more accurately.
2. What are the current diagnostic thresholds for eosinophil mucosal density used to make the diagnosis of non-EoE EGIDs?
Statement 17 Non-EoE EGIDs are clinicopathological entities, therefore, histology alone is not enough to diagnose them without compatible symptoms and signs.
QoE: Low, Agreement: 95%
Summary of Evidence
Because there were no consensus recommendations for thresholds of eosinophil counts in the GI tract to define non-EoE EGIDs, several studies reported on non-EoE EGIDs using empirical values and/or values from the literature. Studies using peak eosinophil counts in a study of non-EoE EGIDs, as opposed to simply recommending threshold values, are listed in Table 5. They represent varied populations, including studies from Korea (43, 66), Japan (19, 109), Malaysia (73), Australia (70), Italy (46), Turkey (11), Iran (45), Jordan (101), and the United States (14, 30, 32, 36, 74, 77, 110, 111). Studies that reported on both children and adults without distinguishing between them were included (14, 19, 32, 70, 73, 74, 77, 101). Some studies provided the size of the HPF used, and to aid interstudy comparisons for those studies the reported eosinophil value was converted to a value in a standardized HPF size and also expressed as a number/mm2 (Table 5). Most studies used a peak count in 1 HPF as the threshold value for diagnosis, but several HPFs were also used to define the non-EoE EGIDs being evaluated (30, 32, 36, 74, 77). One study of colonic eosinophilia determined that counting eosinophils in multiple segments did not confer an advantage over a peak count in a single segment (46). One study used 2 eosinophil values, the lower value representing moderate disease and the higher value representing severe disease, for all non-EoE EGIDs (43).
| Site | Reported threshold converted to peak eosinophil count/0.27 mm2 HPF | Reported threshold converted to peak eosinophil count/mm2 | Unconverted reported threshold values/hpf (N of patients) |
|---|---|---|---|
| Stomach | 23 (74), 27 (30), 34 (77), 34 (19), 80 (36) | 86 (74),102 (30), 126 (77), 126 (19), 298 (36) | 10 (66), 20 (45, 70, 74, 109, 110), 30 (11, 14, 19, 30, 32, 77, 111), 30 (moderate)/50 (severe) (66), 70 (36) (pediatric = 102, adult = 122) |
| Duodenum | |||
| Bulb | Not reported | Not reported | Not reported |
| 2nd portion | 34 (74) | 126 (111) | 20 (11, 66, 70, 109), 30 (66, 74), 30 (moderate)/50 (severe) (66), 50 (110) (pediatric = 39, adult = 9) |
| Terminal ileum | * | * |
(pediatric = 5, adult = 69) |
| Small intestine | 57 (19) | 212 (19) |
(pediatric = 32, adult = 75) |
| Cecum and ascending colon | * | * | 50 (11), 100† (46) |
| Transverse and descending colon | * | * | 35 (11), 84† (46) |
| Rectum and sigmoid colon | * | * | 64† (46) |
| Colon not specified | 34 (101), 68 (19) | 126 (101), 253 (19) | 20 (45, 70, 73, 109), 30 (101), 30 (moderate)/50 (severe) (66), 60 (14, 19) (pediatric = 76, adult = 287) |
- * In order to convert reported numbers in the last column to either an eosinophil count in a standardized hpf or a count per mm2 the size of the high-power field used in the study was required and was not reported in all studies, prohibiting the conversion of some reported threshold values.
- † The total number of patients was reported but not the number who had non-EoE EGIDs at a particular site in the colon and therefore the number of patients at those sites could not be calculated. Numbers in brackets represent the relevant references.
Recommendation 16 We recommend that GI segment specific threshold peak eosinophil counts (See Table 6) be considered prior to making a non-EoE EGIDs diagnosis (expert opinion).
| Site | Consensus threshold peak eos/0.27 mm2 HPF | Consensus threshold peak eos/mm2 |
|---|---|---|
| Stomach | ≥30 | ≥110 |
| Duodenum | ≥50 | ≥185 |
| Terminal ileum | ≥60 | ≥220 |
| Cecum and ascending colon | ≥100 | ≥370 |
| Transverse and descending colon | ≥80 | ≥300 |
| Rectum and sigmoid colon | ≥60 | ≥220 |
SoR: Strong, Agreement: 91%
3. What other histological features help to characterize GI mucosal tissue affected by non-EoE EGIDs?
Statement 18 Some pathologic features are not normally associated with non-EoE EGIDs but do not necessarily rule out that diagnosis. Such features include acute neutrophilic inflammation, neutrophilic glandulitis/cryptitis and granulomas that are characteristic of inflammatory bowel disease but may be also seen in biopsies taken from non-EoE EGIDs-related ulcers/erosions or from patients with parasitic infection.
QoE: Low, Agreement: 95%
Statement 19 Histological features in favor of non-EoE EGIDs in the presence of eosinophilic infiltration of the GI mucosa are eosinophil glandulitis/cryptitis, eosinophils in muscularis mucosa/submucosa, fibrosis/fibroplasia of the lamina propria, degranulation of eosinophils and lymphoid aggregates (See Table 7). However, the diagnostic/prognostic value of these ancillary findings remains unclear.
| Anatomic site/cohort | Features | References |
|---|---|---|
| Stomach/children |
|
(32) |
| Stomach/children |
|
(30) |
| Stomach/children-adults |
|
(77) |
| Features cited in more than one report |
|
|
| Ileum or colon/children |
|
(73) |
| Colon/children |
|
(46) |
| Colon/adults |
|
(101) |
| Features cited in more than one report |
|
QoE: Low, Agreement: 100%
Statement 20 In the presence of eosinophilic infiltration of the GI mucosa, the presence of signs of chronicity (such as atrophy, fibrosis and smooth muscle hyperplasia in the stomach and duodenum and architectural abnormalities such as villous blunting in the small intestine, and crypt elongation/branching/distortion in the small and large intestines) are helpful features to confirm the histological part of the diagnosis, especially if the endoscopic appearance is normal.
QoE: Low, Agreement: 82%
Summary of Evidence
Only articles that reported histologic features that were used in a study, and not merely mentioned in a review article for example, were analyzed. Features in addition to increased eosinophils that have been evaluated in studies of EoG in children were the following: eosinophilic glandulitis, eosinophilic abscess, eosinophils in muscularis mucosa, lamina propria fibroplasia, lamina propria smooth muscle hyperplasia, reactive epithelial changes, acute inflammation, erosion/ulcer (32), coiled glands, eosinophils in the superficial rather than in the deep lamina propria, eosinophils in submucosa, lamina propria fibrosis, and acute and chronic inflammation (30); in EoI or EoC, eosinophilic cryptitis, degranulation, eosinophilic microabscesses, and eosinophil extension into muscularis mucosa and submucosa (73); and in EoC chronic inflammation with lymphoid aggregates and microabscesses (46). Eosinophil clusters were reported in adults with non-ulcer dyspepsia and duodenal eosinophilia (112), and degranulation, eosinophilic cryptitis, lymphoid aggregates, and eosinophil crypt abscesses in EoC (101). Features evaluated in a study of EoG reporting on both children and adults were clusters/sheets of eosinophils, eosinophil glandulitis, infiltration of muscularis mucosa/submucosa, and intestinal metaplasia (77). A summary of the changes observed in more than 1 study is also presented in Table 7. Analyses of the frequency of additional histological features with increasing eosinophil counts for example were not reported in the studies reviewed. One study reported that periglandular circumferential collars of eosinophils, lamina propria eosinophil sheets, and eosinophil glandulitis showed the highest correlation with dysregulated genes in EoG (32).
Recommendation 17 We conditionally recommend that evaluation of acute and chronic features of mucosal inflammation should be recorded as these can be supportive of the non-EoE EGIDs diagnosis (See Table 7).
SoR: Weak, Agreement: 100%
Section E. Differential Diagnosis
1. What is the differential diagnosis for mucosal eosinophilia?
Statement 21 Differential diagnosis of eosinophilic inflammation of the GI tract occurring as segmental disease or as part of more diffuse involvement of the GI tract, includes a wide range of conditions (See Table 8).
| EoG |
|
| EoD/EoJ/EoN/EoI |
|
| EoC |
|
- EoC = eosinophilic colitis; EoD = eosinophilic duodenitis; EoG = eosinophilic gastritis; EoI = eosinophilic ileitis; EoJ = eosinophilic jejunitis; EoN = a term that can be further subdivided into EoD, EoJ, and EoI; hyper-IgE syndrome = hyper-immunoglobulin E syndrome; PTEN = phosphatase and tensin homolog.
QoE: Low, Agreement: 100%
Summary of Evidence
There is a broad differential diagnosis for mucosal eosinophilia affecting the stomach either in isolation or as part of a more diffuse non-EoE EGIDs. The following should be considered in the differential diagnosis of gastric mucosal eosinophilia.
Parasitic Infections
These represent the archetypal condition associated with GI mucosal eosinophilia, although isolated gastric eosinophilia is generally not reported, and these conditions are rare in developed countries. Characteristically, intestinal helminths are associated with mucosal eosinophilia. Examples of associated parasitic infections include tapeworms, hookworms, and Strongyloides species (113, 114). Esteve et al (115) reported the case of a 14-year-old boy diagnosed with EoG, which proved to be a type I hypersensitivity mechanism to the helminth parasite Anisakis simplex. The same parasite was detected in a 49-year-old man with EoG (116). Basidiobolomycosis may also be associated with severe GI eosinophilia (117). Kurteva et al (117) reported a 22-month-old boy with confirmed colonic basidiobolomycosis, presented with severe eosinophilic inflammation of the GI tract. Panfungal PCR performed on DNA extracted directly from a tissue sample confirmed the presence of Basidiobolus. He made a full recovery with a combination of surgery and prolonged targeted antifungal medication. Anisakiasis is an infectious disease caused by a roundworm found primarily in Japan and associated with the consumption of raw fish (sushi or sashimi) or undercooked seafood. Basidiobolomycosis is an infectious disease caused by the fungus Basidiobolus ranarum and occurs in tropical/subtropical Africa, Latin America, the Middle East, and Asia, but also rarely in the United States and Europe. GI symptoms are vague, can be severe, and may occur in immunocompetent children and adults.
Food Allergy and Atopy
Coexisting allergic conditions are reported more frequently in patients with EoG (7, 9, 14). In a retrospective observational study of children aged 0–12 years from Colombia, gastric mucosal eosinophilia was thought to be secondary to food allergy, with the most sensitizing foods being egg, milk, shrimp, wheat, and chicken (13). Caldwell et al found that 7 of 14 patients diagnosed with EoG tested positive for foods or aeroallergens with skin prick tests (30).
Cow's milk allergy was found in 76.9% of 13 Korean pediatric patients [6 boys, 7 girls; mean age 2.8 years (0–12 years)] diagnosed with histologic EGE, with 8 infants having significant eosinophilic infiltration (32.6 ± 16.3/hpf) in the gastric mucosa. Clinical improvement occurred in all infants when cow's milk formula was changed to extensively hydrolyzed or amino acid-containing formula or when nursing mothers restricted cow's milk (66). It should be noted however, that it is not clear if this was EoG responding to milk elimination or an IgE/non IgE mediated allergy. Furthermore, there are a number of case reports suggesting that other allergies may underlie individuals with symptomatic EoG, including contact allergy to components of dental prostheses (118).
Helicobacter pylori Infection
Helicobacter pylori (H pylori) infection is well recognized as a cause of gastritis and ulcer disease. Du et al consecutively recruited newly diagnosed patients with functional dyspepsia. H pylori infection was determined by both a positive C13 breath test and gastric histology. Although there was no significant difference in the number of eosinophils in the gastric antrum or body between patients with functional dyspepsia and controls, the number of eosinophils in the stomach appeared to be related to H pylori infection (119). This is supported by Papadopoulos et al (120) who describe a symptomatic 44-year-old woman in whom gastric biopsies showed both H pylori infection and dense eosinophil infiltration (29 eosinophils/hpf) of the gastric mucosa. Treatment of erosive H pylori gastritis resulted in resolution of symptoms and normal repeat gastroscopy and biopsies 2 months later (120). In the study by Lee et al (24), eosinophil counts in the gastric antrum and body were significantly higher in children with H pylori infection than in children with AP-FGID (P < 0.001 in the antrum, P = 0.002 in the gastric body). However, other case reports have not demonstrated a clear association between EoG and H pylori infection (121).
Inflammatory Bowel Disease
Although the exact relationship has not yet been established, non-EoE EGIDs have been described in association with IBD (122, 123). Gastric eosinophil counts (both gastric antrum and gastric body) were significantly higher in children with Crohn's disease than in children with FAPD or normal controls, although in the latter comparison, eosinophil counts were higher at almost all levels of the GI tract from stomach to rectum (25).
Connective Tissue Diseases with Vasculitis and Collagenous GI Disorders
Gastric eosinophil infiltration has been described in several connective tissue diseases (CTD) including Churg-Strauss syndrome (124-127), as well as in collagenous colitis (128). Benchimol et al (128) reported a 4-year-old girl with EoG associated with collagenous colitis. Arnason et al (129) performed a multi-institutional series of 40 patients [26 females, 14 males; mean age 16 years (range 3–89)], including 24 patients (60%) younger than 18 years. Twelve patients (30%) had celiac disease, collagenous sprue, or collagenous colitis. An eosinophil-rich pattern (≥30 eos/hpf) was noted in 21/40 (52%) patients.
Lecouffe-Desprets et al (130) performed a systematic literature review and identified 20 cases of autoimmune CTD associated with non-EoE EGIDs, specifically systemic lupus erythematosus (35%), followed by rheumatoid arthritis (20%), systemic sclerosis or inflammatory myopathies (15% each), and Sjögren syndrome, scleromyositis, or other overlapping CTD (5% each). No patient had a history of atopy. In contrast to classic non-EoE EGIDs, EoG and/or EGE occurred in 95% of cases. GI symptoms were often nonspecific. Peripheral eosinophilia was noted in 67% of cases. Upper and lower GI endoscopy showed abnormal findings in only 40% and 30% of cases, respectively. The diagnosis of non-EoE EGIDs was confirmed by evidence of eosinophilic infiltration mainly in the mucosa or submucosal layer.
Idiopathic Hypereosinophilic Syndrome (HES)
HES is a term to indicate a more aggressive form of hypereosinophilia (absolute eosinophil count of over 1500/mm3) resulting in complications such as cardia morbidity which is not the case in primary non-EoE EGIDs. HES has been described in a number of case reports in both children and adults aged 8–71 years with eosinophilic infiltration and wall thickening of the stomach (131-134). Kuang et al (135) reported 56 patients with non-EoE EGIDs and HES, of whom 34 were categorized as HES/non-EoE EGIDs overlap and 22 as multisystem HES. Eosinophilia in multiple GI segments was present in 20 of 30 (67%) patients in whom tissue samples were obtained from all 4 (esophagus, stomach, small bowel, and colon) GI segments. Tissue eosinophilia in all 4 GI segments was found in 5 of 30 (17%) patients. Isolated eosinophilia of the gastric mucosa was not described. Based on the findings of the above study there are certain similarities between the 2 entities, but more studies are needed to draw strict conclusions.
Other
Other entities which should be considered in the differential diagnosis of EoG include autoimmune gastritis (136), invasive gastric adenocarcinoma (137), congenital GI obstruction (79), and adrenal insufficiency (138). When evaluating for EoC, one should consider the above mentioned conditions as well as allergic conditions, infections, hypereosinophilic syndrome, IBD, autoimmune diseases, malignancy, chronic graft-versus-host disease, immune dysregulation such as polyendocrinopathy enteropathy X-linked syndrome and other immunodeficiencies (5, 139-143), as well as mastocytic enterocolitis (144, 145) or systemic mastocytosis (146, 147). Oral immunotherapy (OIT) is currently used to treat food allergies. During desensitization, many patients develop GI symptoms and few develop non-EoE EGIDs (148), although this is not universally the case. It is possible that in such cases, unmasking of preexisting non-EoE EGIDs may occur, and the occurrence is likely multifactorial. Thus, additional data are clearly needed to draw conclusions about the associations between OIT and non-EoE EGIDs.
Recommendation 18 We recommend that other clinically relevant diseases associated with mucosal eosinophilia be evaluated prior to making a non-EoE EGIDs diagnosis (See Table 8).
SoR: Strong, Agreement: 100%
2. What are the key aspects of an initial evaluation of a patient with mucosal eosinophilia and what are the diagnostic criteria for non-EoE EGIDs in children?
Statement 22 The initial evaluation of a patient with mucosal eosinophilia depends on the presenting symptoms, history, physical examination, laboratory findings as well as the involved GI segment(s) and may include a combination of tests (See Table 9).
| In all patients | Only if clinically indicated |
|---|---|
|
|
- ACTH = adrenocorticotropic hormone; GI = gastrointestinal; HES = hypereosinophilic syndrome; IBD = inflammatory bowel disease.
- * Patients who have recently travelled to or stayed in endemic areas should also be screened for antibodies to fungi and parasites such as Coccidioides, Echinococcus, Schistosoma, and Trichinella spiralis.
QoE: Low, Agreement: 100%
Summary of Evidence
With regard to the diagnosis of EoG, it seems reasonable to adopt a structured approach to assessment that ensures the following: (1) the final diagnosis of EoG is confirmed and an assessment of severity and associated clinical presentation is made; (2) differential diagnoses are considered and systematically ruled out; and (3) an assessment of comorbidities is made, which may help determine the optimal therapeutic approach. Jensen et al (9) identified the main symptoms for EoG in their large US study and found that dysphagia, weight loss, and GI obstruction, which were seen in EGE, were absent in their cohort. In most cases, eosinophilia is mucosal inflammation, but if it is not, other investigations may be considered, including imaging and surgical exploration and biopsies.
A careful history should capture the onset and nature of symptoms, including exacerbating factors such as food and the presence of fever. A family history should reveal the presence of atopy, autoimmune disease, and IBD. In a small study of 14 patients with EoG (30) it was found that half of the patients tested positive for food or aeroallergens with a skin prick test, although the utility of this test as a routine test is unclear. With regard to parasite infections, a detailed travel history, including time spent abroad, should be obtained, keeping in mind that some helminth infections can persist decades after leaving endemic areas (eg, filariae, schistosomes) (114). Routine blood tests could include a complete blood count, looking for the presence of anemia and peripheral eosinophilia, which is documented in EoG but may also indicate allergy, parasitic infection, and in rare cases, HES. Acute phase reactants (CRP, ESR) and a chemical pathology panel may indicate the presence of systemic or multiorgan involvement and protein leakage. A FCP test is useful to rule out IBD.
In small bowel involvement, the initial workup depends on the individual and the presenting symptoms, as well as the segments involved and whether peripheral eosinophilia is present. A thorough history and physical examination should be performed in all patients, with particular emphasis on identifying other causes of symptoms (including those mentioned in Section E, Q1). Subsequently, a series of laboratory tests should first be performed in most patients. These usually include a complete blood count and differential, electrolytes, renal function, liver tests, serum albumin, and total protein, but should be based on clinical picture. Additional or special laboratory tests may include pancreatic tests, measurements of coagulation profile, iron and ferritin, nutrition panel, celiac serology, thyroid tests, autoimmune panel, adrenocorticotropic hormone stimulation tests, stool for ova and parasites, parasite/worm serology, stool for protein (alpha-1-antitrypsin) and/or stool steatocrit, fecal elastase and/or tryptase, or other, depending on clinical presentation. If ascites is noted on physical examination or imaging, diagnostic paracentesis is required to determine the presence of eosinophils in the fluid and other causes. Endoscopy with biopsy is critical for the diagnosis of non-EoE EGIDs. Upper endoscopy is almost always performed, but whether colonoscopy, enteroscopy, or video capsule endoscopy is performed depends on the clinical picture. Thorough biopsies are required during endoscopy. Recent data suggest that similar to EoE, increasing the number of biopsies (perhaps with up to 8 fragments from the stomach and 4 fragments from the duodenum) increases diagnostic sensitivity (149). In addition to taking the biopsies, communication with the pathologist about the suspicion of non-EoE EGIDs and the need for quantification of peak eosinophil levels is critical. Finally, referral to hematology for evaluation of HES would be necessary if peripheral absolute eosinophil counts are significantly elevated (>1500 cells per microliter of blood), and referral to rheumatology or allergology/immunology if autoimmune or immunologic processes are suspected. It is beyond the scope of this article to list all possible clinical evaluations for persistent and chronic GI symptoms. Therefore, the physician should proceed by clinical indication until either an alternative diagnosis for secondary eosinophilia is made or until the other conditions are considered and excluded and the diagnosis of non-EoE EGIDs is confirmed.
For colonic symptoms, the initial evaluation of a patient with mucosal eosinophilia should include a history of allergic disease, parasitic infections, and medications (especially immunosuppressants); the examination should include signs of IBD, and the initial evaluation should include blood tests for IBD and stool tests for parasitic infections. A detailed history should assess for allergic conditions such as asthma, atopic dermatitis, and food allergies. The most important examination is endoscopy with biopsies to make the definitive diagnosis and confirm the location and severity of mucosal inflammation. IBD must be excluded in all patients, while in infants and young children, very early onset IBD must be excluded and when clinical suspicion is strong, screening for monogenic diseases may require specific genetic analysis. Microscopic examination of stool for ova and parasites and serologic testing for Strongyloides and Toxocara species are required to exclude parasitic infections. Patients who have recently traveled to or stayed in endemic areas should also be screened for antibodies to fungi and parasites such as Coccidioides, Echinococcus, Schistosoma, and Trichinella spiralis. Mucosal eosinophilia is frequently observed in patients with primary or secondary immunodeficiency in association with the changes in bacterial colonization (139, 140). Tissue eosinophilia can also be found in mucosal biopsies from the GI tract of patients with immunosuppression after transplantation, especially in patients receiving tacrolimus (150-152). Therefore, patients with GI tissue eosinophilia post-transplant should be evaluated for symptoms suggestive of non-EoE EGIDs, and if present, they should be evaluated appropriately.
Eosinophilia of the mucous membranes is often found in early infancy. Allergic proctocolitis in infancy may also be associated with >20 eos/hpf in rectal biopsies (153) with patchy distribution (154) and the disease regresses clinically and histologically after the triggering antigens (typically cow's milk) are removed from the affected infant's diet. Similarly, there are some infants with massive mucosal eosinophilia with lymphoid hyperplasia who show bloody stools shortly after birth but before feeding (155, 156). In systemic disease with colonic involvement suggestive of EoC, CT/MRI endoscopy may be indicated to assess inflammation in the small and large bowel in patients who are not candidates for colonoscopy. Ultrasound, CT, and/or MRI scanning are useful to confirm mucosal edema and ascites.
Recommendation 19 We conditionally recommend the initial evaluation of a patient with symptoms suggestive of non-EoE EGIDs be individualized based on history and clinical examination and associated laboratory testing (See Table 9).
SoR: Weak, Agreement: 100%
Recommendation 20 We conditionally recommend that during the initial evaluation of a patient with GI mucosal eosinophilia one should consider allergic diseases, parasite infections, drug administration (especially immunosuppressants), inflammatory bowel diseases, and malignancy as a part of the differential diagnosis (See Table 8).
SoR: Weak, Agreement: 95%
Recommendation 21 We recommend that the choice of endoscopic examination(s) of the gastrointestinal tract should be guided by symptoms, laboratory and radiographic findings.
SoR: Strong, Agreement: 95%
Recommendation 22 We recommend that the diagnosis of non-EoE EGIDs in children and adolescents must include all three of the following: (a) Symptoms and/or signs of GI dysfunction including but not limited to vomiting, abdominal pain/cramping, bloating, anorexia, weight loss, early satiety, hematemesis, heartburn, dyspepsia, tenesmus, diarrhea or constipation, hematochezia or melena, abdominal distention, ascites, iron deficiency, protein loss. (b) Dense eosinophilic infiltrates found in mucosal or full thickness biopsies above organ specific threshold values (See Table 6). (c) Absence of other diseases associated with GI mucosal eosinophilic inflammation (See Table 8)
SoR: Strong, Agreement: 91%
Recommendation 23 We conditionally recommend using an Algorithm to guide in the diagnostic approach of children and adolescents with symptoms suggestive of non-EoE EGIDs (See diagnostic Algorithm).
SoR: Weak, Agreement: 100%
Diagnostic Algorithm: Diagnostic algorithm for Pediatric non-EoE EGIDs
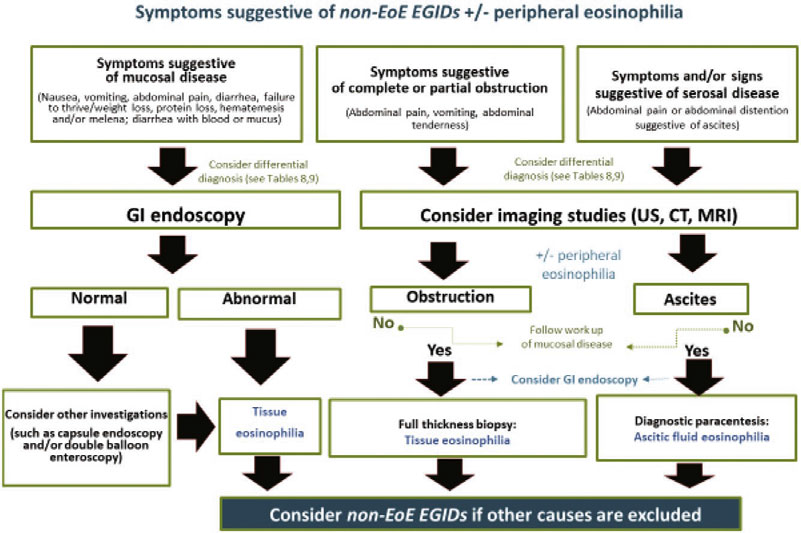
Section F: Treatment
1. What is the goal of treatment in non-EoE EGIDs?
Summary of Evidence
Non-EoE EGIDs-specific guidelines or consensus recommendations to clarify specific treatment goals are currently lacking but based on clinical experience and review of the literature, these include improving symptoms, promoting growth and development, and preventing complications. Four features make these goals challenging: (1) the lack of correlation of symptoms with histologic and endoscopic findings, requiring endoscopic/histologic assessments to determine response to treatment; (2) the current lack of knowledge about the natural history of non-EoE EGIDs and associated complications, making therapeutic timing and selection difficult; (3) the lack of validated biomarkers to guide treatment and response; and (4) the challenge of accessing the specific part of the GI tract affected by a non-EoE EGIDs.
Patients with EoG may develop erosions and ulcers (36, 157), gastric outlet obstruction (78), bleeding (36), and/or protein loss (36, 65, 110). Similar features may be observed in EoN, including salmon-colored erosions and marked anemia and hypoalbuminemia. To date, no premalignant associations have been noted with any of the non-EoE EGIDs, including EoC. Improvement and/or resolution of these features should remain a high priority.
The choice of treatment should be individualized based on the affected segment, depth of inflammation, patient characteristics (age, clinical presentation, nutritional status, and comorbidities), and family resources and capabilities. Most of the available literature consists of case reports and small patient series, with a lack of randomized, placebo-controlled trials. A limited body of data suggests that effective therapy may improve symptoms, endoscopy, histology, and associated abnormalities in blood tests in children with non-EoE EGIDs (110).
Recommendation 24 We conditionally recommend that the goals of treatment in non-EoE EGIDs include achieving resolution of symptoms, improving gross endoscopic and histological abnormalities, promoting normal childhood growth and development, and preventing disease complications.
SoR: Weak, Agreement: 100%
Recommendation 25 We conditionally recommend that the timing of endoscopic and histological re-assessment should be decided on an individualized basis.
SoR: Weak, Agreement: 100%
2. Are systemic oral steroids effective in inducing remission for a non-EoE-EGIDs?
Statement 23 In limited numbers of case series, systemic oral steroids have been effective in inducing clinical and histological remission in non-EoE EGIDs.
QoE: Low, Agreement: 95%
Statement 24 There are no data on the selection criteria of which patients with non-EoE EGIDs should be treated with oral steroids, nor on the optimal dose or duration of treatment.
LE: Very low, Agreement: 95%
Summary of Evidence
In a limited number of studies in children with non-EoE EGIDs, the majority of patients respond rapidly to short-term treatment with oral systemic corticosteroids (158, 159). When treatment is discontinued however, relapses may occur, indicating the need for long-term maintenance therapy (43, 75). Evidence of its efficacy in EoG is currently limited to case reports in infants with gastric outlet obstruction (57, 78, 160) and a young child with associated protein losing enteropathy (65). In EoC, systemic corticosteroids have been shown to induce remission in a proportion of children. In a retrospective multicenter study from the United States of 108 patients (predominantly children) with EoC and EoN, 19% of children were treated with systemic corticosteroids (7). Overall, clinical improvement was noted in 14 (54%) of treated patients at 6-month follow-up, endoscopic improvement in 6 of 13 (46%), and a reduction in mucosal eosinophilia in 8 of 9 (89%) of patients reexamined. In one case series, 1 of 3 children with EoC achieved remission with systemic corticosteroids (11). In a single center study in Colombia, systemic corticosteroids were used to treat EoC in 13 of 65 children. The authors also reported the use of immunosuppressants in 4 children, possibly indicating the need for maintenance therapy (13). Several individual case reports of adult patients with EoC have reported that remission of EoC was achieved with systemic steroids.
Therapy with oral prednisone at a dose of 0.5–1 mg/kg with a maximum dose of 40 mg for 2 weeks is recommended to induce remission (161). Once clinical improvement is achieved, the prednisone dose is reduced over 2–8 weeks. However, some patients may relapse and maintenance treatment is given at a minimum dose required to maintain clinical response (43, 161-163).
Recommendation 26 We recommend that the use of oral systemic steroids be considered to induce remission in individual patients with non-EoE EGIDs and that their use should be undertaken after thorough discussion with the patient and parents about their benefits and risk (expert opinion).
SoR: Strong, Agreement: 100%
3. Are topical steroids effective in inducing and maintaining remission in non-EoE EGIDs?
Summary of Evidence
Topical steroids in the form of metered-dose inhalers (fluticasone, beclomethasone, ciclesonide) or in the form of solutions or tablets (budesonide) are effective in the treatment of EoE because they deliver a local anti-inflammatory agent to the mucosal surface. Their efficacy in the treatment of distal non-EoE EGIDs has shown positive results, but studies are limited to retrospective reviews.
Enteric-coated budesonide capsules, used to treat ileocolonic Crohn's disease, have been offered in a variety of preparations to treat different regions of the GI tract in patients with non-EoE EGIDs. Capsules have been left intact to reach the colon and ileum, opened, and placed in a vehicle to deliver granules to the small intestine, and opened and crushed and placed in a vehicle to reach the stomach and proximal small intestine. In a small case series including 8 children with non-EoE EGIDs, targeted combinations of crushed, opened, and intact budesonide capsules were associated with significant improvement in gastric eosinophil counts (164). In the above study, 3 patients showed resolution of clinical symptoms following treatment, 1 of whom developed a relapse following drug titration, while in the rest of patients, clinical symptoms following treatment were not recorded. In the treatment of other diseases, budesonide has similar efficacy and even a better safety profile compared to prednisone (163, 165, 166).
In a multicenter study of the natural history of 142 children and adults with EoG conducted as part of the CEGIR (7) it was observed that treatments, including topical steroids and food elimination diets, improved clinical symptoms in 75%, 65%, and 54% of patients with EoG, EGE, and EoC, respectively and decreased the number of eosinophils in the tissues within 6 months of starting the first treatment. Topical steroids significantly decreased gastric eosinophil counts (from 145.4 eosinophils/hpf before therapy to 50.8 eosinophils/hpf after therapy). In the CEGIR study, crushed enteric-coated budesonide granules reduced peak gastric eosinophil counts from 235/hpf before therapy to 12.5 eosinophils/hpf. In another study (167) a significant decrease in gastric eosinophils was observed in a limited number of pediatric EoE patients treated with fluticasone who also had elevated gastric eosinophils. This suggests that higher doses of fluticasone may be helpful, particularly in patients with predominant gastric involvement. In a retrospective multicenter study from the United States of 108 patients with EoC, 19% of children were treated with topical corticosteroids in the form of enteric-coated budesonide capsules (7). After 6 months of treatment, patients who underwent repeat colonoscopy showed a decrease in mucosal eosinophilia with crushed corticosteroids, but the changes did not reach statistical significance.
The optimal dosage and timing of use have yet to be determined, and current practice is largely based on experience with patients with IBD. Budesonide is a synthetic steroid with low systemic bioavailability due to its hepatic first-pass metabolism, resulting in a lower risk of long-term side effects (166, 168, 169). The suggested dose of budesonide is 9 mg/day (164), then it can be reduced to 6 mg/day and finally to 3 mg/day for maintenance therapy. It is also suggested that the drug be taken orally on an empty stomach before bedtime for maximum duration of action.
Recommendation 27 We conditionally recommend that topical steroids may be considered in selected patients with non-EoE EGIDs (expert opinion). See General approach to treatment.
SoR: Weak, Agreement: 96%
4. Are food allergen elimination diets effective in inducing and maintaining clinical and histological remission in non-EoE EGIDs?
Statement 25 Elimination diets may induce clinical improvement or remission in a proportion of children with non-EoE EGIDs but there are very limited data on histological response.
QoE: Very low, Agreement: 95%
Statement 26 There is insufficient data on which foods should be eliminated, but case series suggest that avoidance of cow's milk may be effective in some children.
QoE: Very low, Agreement: 91%
Statement 27 There is no evidence to support the use of food allergy tests to guide dietary restriction therapy.
QoE: Very low, Agreement: 100%
Summary of Evidence
As with EoE, there are no standardized approaches to identify food triggers for non-EoE EGIDs. While it is known that dietary elimination therapy can effectively induce disease remission in children with non-EoE EGIDs, there is no evidence to support the routine use of standard food allergy testing to identify allergens associated with non-EoE EGIDs. There are no data on the role of dietary elimination therapy in maintaining disease remission in children with non-EoE EGIDs. Care should be taken to differentiate between IgE-mediated food allergy and food triggers for non-EoE EGIDs. Consultation with an allergist may be helpful in this regard.
In a retrospective study by Ko et al (36), a total of 17 children with EoG received dietary restriction therapy, 3 of whom were concomitantly treated with a proton pump inhibitor (PPI). Dietary restrictions consisted of a modified elemental diet (exclusive feeding with an amino acid-based formula along with 1–12 foods), an empiric elimination diet with elimination of 6 foods in addition to avoiding beef, or an empiric avoidance of 1–3 common food allergens (milk, egg, soy, and/or wheat). Clinical improvement of symptoms occurred in 82% of children. The majority of these patients also underwent histological follow-up and 78% of them showed a histologic response, defined by a decrease in gastric eosinophilia to less than 10 eos/hpf. Response to dietary therapy did not correlate with patients’ total serum IgE levels or food sensitization profile, the latter determined by prick skin tests or food-specific serum IgE levels. Some of the children with EoG in this report had concomitant esophageal eosinophilia, which was more resistant to dietary restriction therapy than gastric eosinophilia and occurred de novo even in patients with EoG who did not respond to therapy. In a retrospective multicenter study conducted by CEGIR (7), a significant decrease in gastric eosinophil counts was observed with dietary therapy that included an elemental diet, an allergen test-based elimination diet, and a 6-food empiric elimination diet. The number of peak gastric eosinophils decreased significantly from 183/hpf before therapy to 53.1/hpf after therapy. No further information was available on the comparative efficacy of the different diets.
In a limited number of case reports and small case series, dietary restriction (elemental, empiric) in the treatment of non-EoE EGIDs resulted in symptomatic improvement and, in some cases, histological remission (110, 158, 170-172). Effective dietary treatment is administered under the supervision of a trained dietitian to ensure adherence to the diet and avoidance of nutritional deficiencies. Once clinical and/or histological remission is achieved, food reintroduction is gradually started to identify causative foods.
In a retrospective multicenter study from the United States, 58% of children with EoC were treated with an elimination diet (7), most commonly, an allergen-specific targeted elimination diet (40%), which included milk in 32%, soy in 16%, egg in 15%, and peanut in 12%, and less commonly, an elemental diet (11%), or a 6-food elimination diet (6%). All types of elimination diets were associated with clinical improvement, but direct comparisons between the different forms of dietary therapy were not reported. Confounding factors included concomitant use of corticosteroids or other treatments in some patients. A reduction in colonic eosinophilia and endoscopic abnormalities was noted in 14 patients studied 6 months after initiation of dietary treatment, but this was not statistically significant because of the small number of patients. Because no placebo-controlled intervention or control cohort of patients was reported, treatment effects must be interpreted with caution. In a meta-analysis of 30 studies (86 patients) examining the efficacy of nutritional therapy in EGE and EoC, clinical improvement occurred in 87% of children and 88% of adults (171). Only 20 patients from these studies underwent repeat histologic examination, but 80% of these patients experienced improvement or disappearance of tissue eosinophilia. An elemental diet with an amino acid-based formula was used in 29 children and resulted in clinical remission in 75.8%, but histologic remission was not assessed. A semi-elemental diet (extensively hydrolyzed formulas) was used in 2 children, and histologic remission was documented in 1 child. A dairy elimination diet was used in 16 patients and resulted in clinical improvement in 62.5%. A gluten-free diet for EGE was unsuccessful in 2 patients. A 6-food elimination diet was used in 34 patients with EGE and EoC and resulted in symptom improvement in 85.3%. In a single-center study, an elimination diet was used in 65 children with EoC, using empiric food restriction (6-food elimination), an allergy test-based elimination diet, and an elemental diet. The most sensitizing foods were reported to be egg, milk, shrimp, wheat, and chicken (13). In a case report of one child with EoC, elimination diet was used successfully to achieve clinical and histologic remission for 7 weeks and for 2 weeks if a flare-up occurred, but adherence was problematic for longer treatment periods (98). In patients with EoN or EoC therefore, although some studies suggest that dietary treatment may be an option to induce symptomatic remission, there are limited data on histologic response and long-term outcome (171).
Recommendation 28 We conditionally recommend that empiric elimination diets may be considered in selected patients with non-EoE EGIDs (expert opinion). See General approach to treatment.
SoR: Weak, Agreement: 100%
Recommendation 29 We conditionally recommend not using food allergy tests to guide dietary restriction therapy for the treatment of non-EoE EGIDs.
SoR: Weak, Agreement: 100%
5. Do proton pump inhibitors or histamine-2 receptor antagonists have a role in inducing and maintaining remission of EoG/EoD?
Statement 28 Evidence supporting the use of proton pump inhibitors or histamine-2 receptor antagonists in the treatment of children with EoG/EoD is lacking.
QoE: Very low, Agreement: 95%
Summary of Evidence
The antisecretory agents, PPIs, and histamine-2 receptor antagonists are known to reduce gastric acid production and treat peptic esophagitis (173). Esophageal eosinophils were first identified as a histologic marker for reflux esophagitis in 1982 (174). Since then, PPIs have been associated with successful therapy in some patients with various degrees of esophageal eosinophilia (175, 176) and anti-inflammatory effects related to inhibition of TH2 cytokines have been demonstrated for PPIs. Their clinical use to date in the direct treatment of non-EoE EGIDs is uncertain, as noted in a few reports, but is likely related to their traditional use in comorbid peptic disease, although several case reports have described the use of these drugs in patients with EoG (36) In a recent retrospective multicenter study of children with EoC and EGE, PPIs were used in 30% of patients in addition to other treatments, including elimination diet, diet plus medication, systemic steroids, topical steroids/crushed enteric budesonide, and 5-ASA (7).
Recommendation 30 There is insufficient data to make a recommendation for or against the use of proton pump inhibitors or histamine-2 receptor antagonists for treating childhood EoG/EoD.
Agreement: 100%
Recommendation 31 We conditionally recommend that proton pump inhibitors may be considered for treating upper GI ulcerations in children with EoG/EoD.
SoR: Weak, Agreement: 90%
6. Do antihistamines, leukotriene inhibitors or mast cell stabilizers have a role in the treatment in non-EoE EGIDs?
Summary of Evidence
The use of sodium cromoglycate, a mast cell membrane stabilizer, in children with non-EoE EGIDs is limited to a few case series (36, 75, 111, 177, 178), which showed some (75, 177, 178) or no (36, 111) improvement in symptoms but no assessment of mucosal eosinophilia (75, 111).
Competitive leukotriene receptor antagonists have been shown to generally reverse the leukotriene-mediated inflammatory process. Few case reports showed improvement of EGE with administration of leukotriene receptor antagonists (43, 59). However, there is no evidence that leukotriene receptor antagonists improve tissue eosinophilia.
Ketotifen, an H1 antihistamine and mast cell stabilizer, has also been used to treat EGE in case reports (66, 179). Similar to sodium cromoglycate and leukotriene receptor antagonists, ketotifen has not shown a significant effect on tissue eosinophilia in EGE (180). Although a few case reports or series have reported that these anti-allergic drugs improve the clinical symptoms of some patients with EGE, almost all of these reports did not accurately determine the disease state or perform follow-up biopsies to determine the effect of these drugs on tissue eosinophilia. In a retrospective multicenter study from the United States of 108 patients (predominantly children) with EoC, 4 patients were treated with a mast cell stabilizing agent but did not undergo re-evaluation within 6 months to assess treatment response (7). Currently, there is no evidence that sodium cromoglycate, leukotriene receptor antagonists, or ketotifen should be used to treat tissue eosinophilia in patients with non-EoE EGIDs.
Recommendation 32 There is insufficient data to make a recommendation for or against the use of antihistamines, leukotriene inhibitors or mast cell stabilizers as a sole treatment of non-EoE EGIDs.
Agreement: 90%
7. Do immunomodulators have a role in the treatment of non-EoE EGIDs?
Summary of Evidence
Immunomodulators are drugs that alter immune function to treat an inflammatory process. Azathioprine and 6-mercaptopurine are the preferred immunomodulators for GI disorders. Scientific studies have shown that immunomodulators are effective in certain GI diseases such as Crohn's disease and ulcerative colitis (181). Although few case reports suggest that immunomodulators may be beneficial in the treatment of EGE (182, 183), other patient series have not demonstrated significant beneficial use of immunomodulators in EGE (43, 45). More detailed studies are needed to evaluate the potential benefit of immunomodulator therapy for patients with EGE. In a retrospective multicenter study from the United States of 142 patients with EoG, 123 with EGE and 108 with EoC, only 5(4%), 4(3%) and 8(7%), respectively, were treated with an immunomodulatory drug but there was no information on treatment response (7).
Recommendation 33 There is insufficient data to make a recommendation for or against the use of immunomodulating drugs for the treatment of non-EoE EGIDs.
Agreement: 100%
8. Do biologic drugs have a role in the treatment of non-EoE EGIDs?
Summary of Evidence
Biological drugs, almost always monoclonal antibodies, are either made from living organisms or contain components of them. In the last 2 decades, many of these drugs have been approved and have been scientifically shown to significantly treat a variety of diseases. More specifically, with regard to eosinophilic disorders (eosinophilic asthma, dermatitis, HES, nasal polyposis, etc), several biologic therapies have been approved (184, 185).
Mepolizumab and reslizumab are monoclonal biologic anti IL-5 antibodies that have been studied and approved for the treatment of eosinophilic asthma. Both drugs have been shown to significantly reduce tissue eosinophilia in patients with EoE but their efficacy in treating non-EoE EGIDs in adults and in adolescents is currently under investigation (186).
A few case studies have suggested that infliximab/adalimumab, anti-tumor necrosis factor alpha agents, and vedolizumab, a humanized monoclonal antibody targeting the α4β7-integrin heterodimer, may be useful in the treatment of EGE. However, these studies did not accurately describe how EGE was diagnosed or perform repeated histologic follow-up in the patients studied (12, 111).
Omalizumab is an anti-IgE monoclonal antibody that has been associated with significant improvement in symptoms and disease parameters in patients with IgE-mediated allergy. In a case series that included 9 patients with various localizations of tissue eosinophils in the GI tract, the decrease in tissue eosinophilia was not statistically significant when compared before and after treatment (187).
Recently, dupilumab, a monoclonal antibody that blocks the downstream signaling of Il-4 and IL-13, has been associated with clinicohistologic remission in 3 pediatric patients with refractory non-EoE EGIDs (EoG, EoD, EoJ), and concomitant severe atopic diseases such as atopic dermatitis or asthma (188).
Another recent phase 2 study in patients with EoG or EoD using an anti-Siglec-8 antibody (AK002), which depletes eosinophils and inhibits mast cells and has shown potential in animal models to treat EoG and EoD, reported a reduction in GI eosinophils and symptoms in patients receiving AK002 compared with placebo (33). It should be noted however, that Allakos, a biotechnology company developing lirentelimab (AK002) reported on September 9, 2022 on its website (https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-eodyssey-study-patients/) data from EoDyssey, a 24-week, Phase 3, randomized, double-blind, placebo-controlled study of lirentelimab in patients with biopsy confirmed EoD claiming that AK002 Lirentelimab met histologic co-primary endpoint but it did not achieve statistical significance on the patient reported symptomatic co-primary endpoint, in both the intent to treat population and in a prespecified subpopulation.
Pesek et al (7), in a retrospective multicenter study of 142 patients with EoG, 123 with EGE, and 108 with EoC, reported that only 2 (1%), 4 (3%), and 1 (1%), respectively, were treated with a monoclonal antibody, but there was no information on disease outcome. There is therefore, insufficient evidence to recommend the use of biologics for maintenance therapy of non-EoE EGIDs.
Recommendation 34 There is insufficient data to make a recommendation for or against the use of biological drugs in treating childhood non-EoE EGIDs.
Agreement: 90%
9. Is there a role for endoscopic dilation or surgery in the management of non-EoE EGIDs?
Statement 29 Limited number of case series describe the use of endoscopic dilation to manage partial obstruction in adults with non-EoE EGIDs
QoE: Very low, Agreement: 95%
Statement 30 Surgical intervention is used for non-EoE EGIDs-related clinically significant bowel obstruction that does not respond to treatment with systemic steroids, whereas pyloromyotomy has rarely been shown to be effective in children with EoG and associated pyloric stenosis.
QoE: Low, Agreement: 91%
Summary of Evidence
There is minimal to no evidence to date for endoscopic dilation in children with non-EoE EGIDs. Pyloromyotomy with clinical response has been reported in rare cases of children with EoG and associated pyloric stenosis but it is not always successful (57). If the small or large intestine is involved, there is no evidence for surgical treatment of mucosal non-EoE EGIDs. Surgical intervention is performed only when there is non-EoE EGIDs-related bowel obstruction that does not respond to treatment with systemic steroids or in serosal EoC in which ascites or peritonitis require escalated medical and surgical management.
Recommendation 35 We conditionally recommend that in addition to medical/dietary treatment, endoscopic dilation may be considered in selected cases with significant objective signs of obstruction.
SoR: Weak, Agreement: 91%
Recommendation 36 We conditionally recommend that surgical treatment of non-EoE EGIDs may be useful for patients with refractory ulcers, intestinal perforation or bowel obstruction which cannot be controlled otherwise.
SoR: Weak, Agreement: 100%
10. In which situations may combination therapy have a place in the treatment of non-EoE EGIDs?
Statement 31 Combination therapy has been used in a proportion of patients with non-EoE EGIDs with variable effects.
QoE: Low, Agreement: 82%
Summary of Evidence
Apart from case reports, no studies have investigated whether combination therapy plays a role in children with EoG. Combination therapy could be used as a bridge in the transition from systemic steroid therapy to dietary therapy to see if remission can be maintained by diet alone. In addition, if the established threshold dose for maintenance therapy with systemic steroids is higher than desired, it is reasonable to investigate whether elimination of food could lower this threshold dose. Corticosteroids are the most effective and widely used therapeutic option for the treatment of EGE (158, 159). Dietary therapy (elemental or 6-food elimination diet) has been shown to be effective in selected patients, but further studies are needed to establish guidelines for dietary management of EGE and identify patients most likely to respond. It is reasonable to assume that selected patients may benefit from initial treatment with systemic steroids to achieve more rapid remission while transitioning to an elimination diet under the supervision of an experienced dietitian to avoid relapse upon discontinuation of systemic steroids or to determine whether a lower total dose of systemic steroid can be used to maintain remission. Studies to date do not support the use of leukotriene receptor antagonists, cromolyn, PPIs, or ketotifen as monotherapy in EGE. Additional benefit when combining these agents with systemic steroids or dietary therapy is unlikely unless the patient has a concomitant disease for which these agents are specifically indicated.
In patients with EoC, combination therapy may be tried if multiple severe or refractory symptoms (abdominal pain, vomiting, diarrhea, rectal bleeding, nausea) occur. In a retrospective multicenter study from the United States of 108 patients, diet and corticosteroids were used in 31% of patients with EoC. Multiple concomitant treatments were used in 41%. Fourteen (15%) patients had a 6-month follow-up. Clinical improvement was reported in 7 of 13 (54%), endoscopic improvement in 6 of 13 (46%), and histological improvement in 8 of 9 (89%) (7).
Recommendation 37 There is insufficient data to make a recommendation for or against the use of combination therapy for treating non-EoE EGIDs.
Agreement: 90%
Recommendation 38 We conditionally recommend that combination therapy may be useful for treating concomitant allergic diseases.
SoR: Weak, Agreement: 85%
11. What is a suggested approach to initiating treatment for non-EoE EGIDs?
Statement 32 There is lack of randomized controlled trials assessing the efficacy of the available treatment options of non-EoE EGIDs.
QoE: Low, Agreement: 100%
Statement 33 Treatment with systemic steroids at appropriate doses followed by timely tapering is an effective initial approach to the treatment of most patients with non-EoE EGIDs.
QoE: Very low, Agreement: 100%
Summary of Evidence
Treatment with systemic steroids at appropriate doses, followed by timely tapering, is currently the recommended initial approach for the management of most patients with non-EoE EGIDs. Elimination diets can be tried in highly motivated patients with less acute disease, as there are currently no established guidelines for dietary management.
Since the goals of non-EoE EGIDs treatment are to reduce symptoms and GI eosinophilic inflammation while preventing disease complications, initial treatment with systemic steroids at an appropriate dose followed by tapering is warranted because of the higher rate of rapid initial remission in response to this approach. As relapses are common during systemic steroid reduction and prolonged use of systemic steroids is associated with adverse side effects, switching to a topical steroid preparation such as budesonide with lower systemic bioavailability is recommended if steroid maintenance therapy is required to avoid relapses. Limited reports on the success of elimination diets (elemental formula and 6-food elimination diet) suggest that they could initially be tried in motivated symptomatic patients, in patients with malabsorption, and in patients with less acute disease, although efficacy has not been fully established. Although it is expected that biologics will eventually be successfully incorporated into the treatment of non-EoE EGIDs, none is currently approved for their treatment.
In symptomatic patients with active EoC, there is insufficient evidence to support diet or drug treatment, therefore, individual circumstances should be reviewed and discussed with the patient to consider preferences, adherence to therapy, nutritional status, tolerance of medications, and strategies for weaning from medications. The main treatment options are elimination diets and systemic steroids. It is currently unclear what histologic criteria indicate sustained remission. In retrospective studies without controls, all types of elimination diets, systemic and topical steroids were associated with clinical improvement in the majority of patients (7). This was also evident in several case reports of children treated with elimination diets or systemic steroids (11, 13, 98). When symptoms are suggestive of upper GI tract involvement, such as vomiting and nausea, treatment options include PPIs (7, 13). When symptoms are characterized by diarrhea and blood in the stool, treatment options include 5-ASA (7). In general, treatment plans should consider strategies that optimize the balance of desired treatment effects and minimize cumulative steroid exposure.
Recommendation 39 We conditionally recommend that the initial treatment of children with non-EoE EGIDs be individualized based on the symptoms, impact on growth and development and other co-morbid features with an attempt to involve patients and parents/caregivers in shared decision making.
SoR: Weak, Agreement: 95%
Recommendation 40 We conditionally recommend that changes in symptoms and histology should be monitored, preferably with objective tools to allow meaningful conclusions on treatment effects.
SoR: Weak, Agreement: 100%
12. What is a suggested approach for maintenance of remission in non-EoE EGIDs?
Statement 34 There are no studies that have examined the role of maintenance treatment in patients with non-EoE EGIDs.
QoE: Very low, Agreement: 100%
Recommendation 41 Since the natural history of non-EoE EGIDs is uncertain, we conditionally recommend that the long-term treatment should be discussed with patients and parents/caregivers and include the benefits and risks of long-term treatments as well as their impact on health-related quality of life and financial costs.
SoR: Weak, Agreement: 100%
A comprehensive synthesis of the knowledge accumulated during the development of this consensus document and the clinical experience of the consensus group follows below. It is intended to serve as a general guide to the basic elements of therapy for pediatric patients with non-EoE EGIDs and will be further refined with additional research and clinical experience.
CONCLUSIONS
General Approach to Treatment of non-EoE EGIDs (See Guidelines for more details)
Goals
Because there is not yet a well-developed literature on the treatment of children with non-EoE EGIDs, specific therapeutic guidelines are lacking. As with EoE, the goals of treatment include maximizing growth and development, improving quality of life, balancing the risks and benefits of treatment with potential side effects, and improving gross and histological evidence of inflammation. This last goal necessitates a repeat endoscopic examination and is based on several items listed in the last part of this section. Regardless of the treatment used, approaches should always consider the patient's developmental stage, family resources, access to follow-up, availability of supportive resources, and the ability of the patient and family to adhere to treatment. In the coming years, different approaches to EoG, EoN, and EoC are likely to emerge based on the mechanisms involved.
Remission induction
As mentioned in the guideline, several small case series with antigen exclusion or drug treatment have been published. Although no prospective, placebo-controlled studies have shown symptomatic, endoscopic, and histological outcomes to improve in children with non-EoE EGIDs, the use of systemic or topical steroids and antigen exclusion may play a role and provide an approach to induce remission. The timing, duration of treatment, and specific doses/eliminations should be decided based on the severity of the patient's disease. For example, (a) a 17-year-old patient with EoG hospitalized for life-threatening bleeding from an antral ulcer may require hospitalization and intravenous and then oral prednisone; (b) a 12-year-old patient with EoG presenting with mild abdominal pain and gastric nodularity may benefit from topical budesonide; and (c) a 3-year-old patient with EoN presenting with diarrhea and mild abdominal pain may improve with elimination of milk. Proton pump inhibitors may help acid-related comorbidity associated with gastric and duodenal mucosal ulcers. The risks and benefits of each of these approaches should be discussed with patients and their families, and supportive care should be provided by a pediatric dietitian when available. Biologics targeting TH2-type inflammation may also be beneficial in other comorbid systemic diseases such as asthma and atopic dermatitis, and their role in non-EoE EGIDs-related inflammation is the focus of ongoing prospective studies.
Maintenance treatment
Since no pediatric study has determined the natural history of non-EoE EGIDs and no study of maintenance therapy has been conducted, the consensus group advocates maintenance therapy until more data are available. The clinical decision to continue chronic treatment should be made in collaboration with the patient and family and should be based on the above goals. If the decision is made not to continue treatment, mandatory follow-up is recommended because the natural history and full list of complications are unknown. If the decision is made to treat only during periods of symptomatic recurrence, a repeat endoscopic examination should be considered before resuming treatment to ensure that eosinophilic inflammation is present. Long-term treatment options include topical steroids and dietary exclusion of potential allergens. The risks and benefits of chronic use of these treatments should be discussed with patients and their families, and supportive care should be provided by a pediatric dietitian, if available.
Treatment of recalcitrant disease
In some circumstances, patients do not respond to steroids or dietary exclusions. In this case, the recommended next steps are re-evaluation of current disease activity, re-evaluation of the development of another disease process, review of treatment instructions, evaluation of treatment adherence, revision of treatment plans to modify dosage or dietary exclusion, and finally, discussion of therapeutic alternatives. When treatment with immunomodulators, biologics, or other approaches is considered, careful discussions with patients and their families should include a plan for close monitoring and observation of the clinical course and side effects of treatment.
Assessment of inflammation
After a diagnostic endoscopic examination, endoscopic follow-up for signs of mucosal inflammation is recommended in patients with non-EoE EGIDs at some point in the clinical course under the clinical circumstances described below. The timing, number, and frequency of endoscopic examinations should be based on the physician's clinical judgment after speaking with the patient and parents to answer clinically relevant questions. These decisions are complicated by the fact that the natural history is not yet fully understood in terms of clinical course and complications, that the long-term preventive aspects of treatment are unknown, and that the correlation of symptoms with mucosal inflammation is uncertain. Since none of these issues has yet been resolved, the pediatric gastroenterologist must weigh the decision between obtaining more clinical data from an endoscopic procedure versus continuing observation without knowledge of these clinical inflammatory endpoints. The advantages of the former option include the certainty that the inflammation has resolved. The advantages of the latter option include sparing the patient and parents the physical, psychological, and financial costs of a time-consuming intervention. When procedures are performed, both endoscopic and histological measurements should be obtained to track them over time. The reasons for intervention in a child with non-EoE EGIDs are listed below:
- 1.
Acutely ill (based on symptoms or associated laboratory abnormalities) with treatment – reassess disease activity and determine the need to modify the treatment plan or perform further investigations.
- 2.
Acutely ill (based on symptoms or associated laboratory abnormalities) without treatment – determine if non-EoE EGIDs are the cause of symptoms.
- 3.
After treatment change – determine if there is evidence of changes in inflammatory indices.
- 4.
After initial treatment – determine disappearance of inflammation.
Acknowledgments
The authors thank Ruth Suhami (Librarian at Tel Aviv University, Israel) for the literature search and Rachel Andrews (Senior Clinical Research Coordinator, Gastrointestinal Eosinophilic Diseases Program, Children's Clinical Research Organization, Research Institute, Children's Hospital Colorado, Aurora, CO, USA) for her assistance in retrieving the full papers indicated by the author group leaders and linking the references to EndNote.
REFERENCES
Disclaimer
ESPGHAN is not responsible for the practices of physicians and provides guidelines and position papers as indicators of best practice only. Diagnosis and treatment is at the discretion of physicians.
The NASPGHAN clinical practice guidelines and position papers are evidence-based decision- making tools for managing health conditions. This document is not a disease management requirement or rule and should not be construed as establishing a legal standard of care, or as encouraging, advocating for, mandating or discouraging any particular diagnostic methodology or treatment. Our clinical practice guidelines and position papers should also not be used in support of medical complaints, legal proceedings, and/or litigation, as they were not designed for this purpose. The NASPGHAN clinical practice guidelines and position papers should also not be utilized by insurance companies or pharmacy benefit managers to deny treatment that is deemed medically necessary by a patient's physician. The health care team, patient, and family should make all decisions regarding the care of a patient, after consideration of individual specific medical circumstances. While NASPGHAN makes every effort to present accurate and reliable evidence-based information, these clinical practice guidelines and position papers are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the clinical practice guidelines and/or position papers or reliance on the information presented.