In-hospital Growth of Very Low Birth Weight Preterm Infants
Comparative Effectiveness of 2 Human Milk Fortifiers
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
This study received support from the University of Pretoria. No funding received from NIH, Welcome Trust, HHMI or any other.
J.E.K. have in the past received honoraria from the Nestlè Nutrition Institute Africa (NNIA), an affiliation of Nestlè South Africa.
ABSTRACT
Objective:
Amidst a high prevalence of prematurity, limited research on the growth of very low birth weight (VLBW) preterm infants and the availability of a reformulated fortifier c(RF), the study aimed to compare in-hospital growth of such infants receiving exclusively human milk fortified with either of 2 different formulations in a tertiary South African hospital.
Methods:
In a prospective comparative effectiveness design, intakes and growth of VLBW infants on the Original Fortifier (OF; 2016–2017) were compared with those receiving RF (2017–2018). Daily intake was calculated using published composition of preterm and mature milk with fortifier (OF: 0.2 g protein, 3.5kcal/g powder; RF: 0.4 g protein, 4.4 kcal/g powder). Change in z scores from start to end of fortification for weight (WFAZ), length (LFAZ), and head circumference (HCFAZ) for age was the primary outcome. Additionally, weight gain velocity (g · kg−1 · day−1) and gain in length and head circumference (HC) (cm/week) were calculated.
Results:
Fifty-eight infants (52% girls; gestational age: 30 ± 2 weeks; birth weight: 1215 ± 187 g) received OF for 16 days and 59 infants (56% girls; gestational age: 29 ± 2 weeks; birth weight 1202 ± 167 g) received RF for 15 days. Protein intake of RF (3.7 ± 0.4 g · kg−1 · day−1) was significantly higher (P < 0.001) than of OF (3.4 ± 0.2 g · kg−1 · day−1). Protein-to-energy ratio of RF (2.6 ± 0.2 g/100 kcal) was significantly higher (P < 0.001) than of OF (2.3 ± 0.1 g/100 kcal). In both groups, WFAZ and LFAZ decreased; HCFAZ improved slightly. No significant differences (P > 0.05) were noted between the 2 groups for change in z scores, weight gain velocity, length or HC gain.
Conclusions:
Despite a modest increase in protein intake and protein-to-energy ratio, the growth of VLBW infants on RF was not better than on OF during their hospital stay.
What Is Known
- Human milk needs to be fortified to meet the nutritional requirements of very low birth weight preterm infants yet standard fortification may still not meet the protein required for optimal growth in these infants.
- In high-income countries, fortifiers with a higher than standard protein content have been associated with improved growth outcomes in very low birth weight preterm infants.
What Is New
- In a real-life situation in a middle-income country, a reformulated human milk fortifier modestly improved both protein intake and the protein-to-energy ratio but not in-hospital growth over a 15 day period in very low birth weight preterm infants.
Human milk is the feed of choice for all infants, including preterm infants (1., 2.). The advantages of human milk, especially mother's own milk, are numerous and include a reduction in the incidence of necrotizing enterocolitis, late-onset sepsis, and retinopathy, better feeding tolerance and improved neurodevelopmental outcomes (2.-4.). An important limitation is, however, that human milk does not meet the nutritional requirements of most preterm infants, especially those with a very low birth weight (VLBW), with fluid restrictions and comorbidities that increase nutrient requirements (5.-7.). Fortification of human milk to improve the nutrient intake of VLBW preterm infants is practised in most South African neonatal units (8.). Despite fortification and other advances in the nutrition care of preterm infants, poor in-hospital growth remains a problem in South Africa (9., 10.) and in other countries (11., 12.). The composition of the only commercially available fortifier in South Africa (FM85, Nestlè South Africa) has recently been reformulated to include more protein. Liu et al (13.) concluded in a meta-analysis that “human milk fortifiers with a higher-than-standard protein content can improve preterm infant growth.” The growth of preterm infants receiving fortified human milk in low-income and middle-income countries has been under-researched. This study subsequently compared the in-hospital growth of VLBW preterm infants receiving exclusively human milk fortified with the reformulated fortifier (RF) to the original fortifier (OF).
METHODS
Study Design and Setting
In a comparative effectiveness study the in-hospital growth (ie, from start to end of fortification) of VLBW preterm infants was assessed in a real-world setting, namely the 185-bedded neonatal unit of a 3200-bed tertiary academic hospital in a resource-limited part of urban Gauteng, South Africa (see Figure 1, Supplemental Digital Content, for the study design, http://links.lww.com/MPG/C192)
Study Population and Sampling
Fortification was started at the discretion of the hospital dietitians after they identified infants during routine screening or referral from the attending doctor. The hospital dietitians referred all infants for whom fortification was started to the researcher for a fortification audit (from which the OF group originated) and during the study period (RF group).
The study population consisted of all nonsurgical VLBW (birth weight less than 1.5 kg) preterm infants who received exclusively human milk fortified with the OF (September 2016 to March 2017) and those receiving the RF (August 2017 to June 2018). Sampling for the OF group was done from most dated to most recent records of the fortification audit. From the 118 infants in the audit, all those with a birth weight >1.5 kg; major congenital/chromosomal abnormalities; conditions requiring surgery or affecting growth or measurement thereof; receiving formula feeds or being nil per os >24 hours during the study period; weighing >1.6 kg when fortification started, were excluded. The same exclusion criteria were applied in the prospective sampling of the RF group. The sample size calculation modelled change in weight-for-age z score (WFAZ) taking 5 additional parameters into account, namely birth WFAZ; birth weight-for-gestational-age category; birth weight classification; number of days human milk fortifier received; and exposure to human immunodeficiency virus (HIV) for the comparison of the 2 groups. As data analysis employed regression methods, the conventional rule of thumb of 10 to 15 subjects per parameter, was applied, that is, at least 100 (50 per group) infants.
Data Collection
Data for the OF (during the fortification audit) and the RF groups were prospectively collected by the same person (J.E.K.). Infants entered the study on the day that fortification was started. Within 24 hours after fortification was started, biographic and baseline anthropometric (weight, length, and HC) data were collected. For the weight, a calibrated pan-type electronic scale (Seca model 334, Hamburg, Germany), for recumbent length the Seca model 417 (Hamburg, Germany), and for HC, a flexible, nonstretchable measuring tape (Seca model 212, Hamburg, Germany) were used. All measurements were taken by J.E.K. according to standardized techniques. For the length and HC measurements, the infant's mother or nursing staff assisted.
Following baseline assessment, weight, length, and HC were measured at least once a week and as close as possible to the exit from the study. Medical data were documented as they became available, and intake and output data were noted every 24 to 48 hours. Infants exited the study once the discharge weight of 1.65 kg (official hospital policy during the time of data collection) was reached, the infant was transferred to a secondary hospital, took more than 50% of feeds directly from the breast or was started on formula feeds (whichever one occurred first).
Data Management and Statistical Analysis Intake
Intake was calculated for the study period, that is, from the start of fortification to exit from the study. For all calculations, the most recently recorded weight of the infant was used. For each infant, total daily enteral protein (g/day) and energy (kcal/day) intake were calculated in accordance with published human milk composition [first 14 days of life: preterm milk containing 1.5 g protein and 65 kcal per 100 mL; from day 15 of life onwards: mature milk containing 1.2 g protein and 72 kcal per 100 mL (14.)] and fortifier composition.
Mature milk composition was used in the single case of donor milk. The OF contained 0.2 g protein and 3.5 kcal/g powder whereas RF contained 0.4 g protein and 4.4 kcal/g powder. Once added to human milk, the RF had a higher protein content than the OF, that is, 3.1 g protein/100 mL (RF) compared with 2.5 g protein/100 mL (OF) in fortified preterm milk. After addition to human milk, however, the 2 fortifiers yielded similar energy (ie, 82.5 kcal/100 mL in preterm milk) as 1 g OF powder was added to 20 mL human milk, compared with the 25 mL to which 1 g RF powder was added. Other differences in the RF included a lower carbohydrate but higher fat content, and a change in protein hydrolysis from extensively to partially hydrolysed. (see Table, Supplemental Digital Content 2, for the nutritional content of the 2 fortifiers, http://links.lww.com/MPG/C193).
For infants receiving glucose-containing intravenous (IV) fluids and/or parenteral nutrition (PN), the protein and energy contribution was accordingly added to the relevant daily intake. Total daily mean protein (g · kg−1 · day−1), energy (kcal · kg−1 · day−1) intake, and mean protein-to-energy ratio (g/100 kcal) were then calculated for each infant based on the number of days the fortifier was administered.
Growth
In-hospital growth was defined as the growth between the start of fortification and exit from the study. For the exit weight, the weight that was numerically closest to 1.65 kg was used. The WFAZ, LFAZ, and HCFAZ for entrance and exit were calculated for each infant using the Fenton clinical-exact-age-calculator (15.) (postmenstrual age [PMA] in weeks and in days). As primary indices of growth, the respective changes in WFAZ, LFAZ, and HCFAZ were calculated for each infant by subtracting the entrance value from the exit value. Anthropometric gains (g · kg−1 · day−1 and cm/week) were calculated as secondary indices of growth. For each infant, weight gain velocity (g · kg−1 · day−1) from entrance to exit was calculated according to the formula by Patel: growth velocity = [1000 × ln(Wn/W1]/(Dn − D1) where W = weight in grams; D = day; 1 = beginning of time interval; n = end of time interval (16.). Length gain and HC gain (cm/week) from entrance to exit was calculated by using the following formula: exit value − entrance value/days on fortifier × 7.
Statistical analysis included the comparison of baseline information of the infants in the 2 groups to ensure comparability before the intervention in terms of potential confounders. Two-sample t-tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Linear regression controlling for one of the confounding variables, namely HIV exposure, was used to compare the growth outcome of the 2 groups. A P value of 0.05 was regarded as statistically significant. Data were analysed with STATA/IC 15.1 for Windows Revision 15 October 2018 (StataCorp LLC, 4905 Lakeway Drive, College Station, TX 77845, USA) statistical software.
Ethical Considerations
Ethics approval was obtained from the University of Pretoria, Faculty of Health Sciences Research Ethics Committee (Reference no 286/2017) and the University of the Witwatersrand Health Research and Ethics Committee (Clearance certificate no M170546). Institutional permission was granted by the Medical Advisory Committee of the hospital (Letter dated 17 May 2017). The approvals related to the RF study and the use of data previously collected as part of the audit (OF group). Each mother in the RF group provided written informed consent before her infant was included in the study. Participation was voluntary and withdrawal from the study did not affect the routine health care that the infants received.
RESULTS
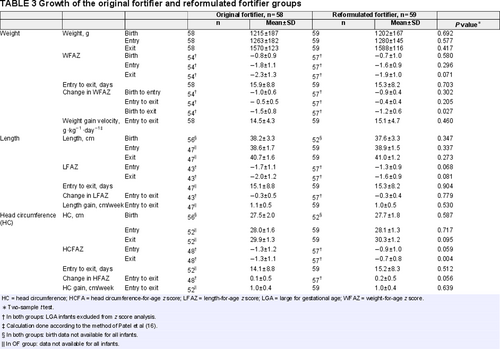
The OF group consisted of 58 eligible VLBW preterm infants who received fortified human milk for a period of 16 days. Fifty-nine VLBW preterm infants who received fortification for 15 days completed the RF-arm of the study (Fig. 1). The 2 groups were comparable for all baseline characteristics (Table 1). Some of the descriptive information was only available for the RF group: the mothers were between 18 and 38 years old (mean (±SD) 27.8 (±6.1)); the majority (91%) received antenatal care and gave birth at the hospital. Seventy percentage of babies were born by Caesarean section and 88% were single births.
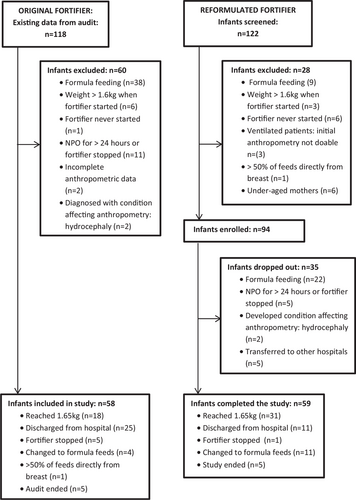
Flow diagram of infants included in the study.
Intake
In both groups, fortification started on a mean day 18 of life at around 32 weeks PMA. The volume of milk at the start of fortification was approximately 165 mL · kg−1 · day−1 with no significant difference between the 2 groups (Table 1). In both groups, fortification was started at half strength and increased to full strength over a few days. The practise at the hospital of adding fortifier per individual feed in increments of 0.5 g meant that in both groups some infants received suboptimal dosages, for example, 1 g fortifier would be added to 28 mL human milk whereas standard fortification should have been 1.4 g (OF) and 1.12 g (RF). All infants received human milk exclusively and were bolus fed via an orogastric tube, a syringe or a feeding cup. Infants in the OF group received only their own mothers’ milk, as there was no donor milk available in the hospital during that period. In the RF group, all infants, except one who received donor milk for a limited number of days, received their own mothers’ milk exclusively. Feeds were well tolerated in both groups.
A few infants in each group (3 in the OF group; 7 in the RF group) received supplementary IV fluids (10% glucose solution) (Neonatalyte; Adcock Ingram, Midrand) for a period between 1 and 3 days. One infant in each group received supplementary PN (Fresenius Kabi, Midrand) containing protein (2.1 g/100 mL), glucose (10.5 g/100 mL), fat (2.1 g/100 mL), and electrolytes. Supplementary glucose (for volumes of less than 100 mL · kg−1 · day−1) or PN (for volumes of more than 100 mL · kg−1 · day−1) were given while infants were not receiving full volumes of enteral feeds yet. All infants in both groups received daily oral vitamin and mineral supplements as per hospital protocol, namely a multivitamin (containing vitamins A [3000 IU/day], B1 [1.15 mg/day], B2 [1.25 mg/day], B3 [10 mg/day], B6 [1 mg/day], C [50 mg/day], D [400 IU/day]); folic acid (0.1 mg/day); iron (3 to 4 mg · kg−1 · day−1), and additional vitamin D (400 IU/day).
There were no differences between the 2 groups in the mean daily volume of milk received. The protein intake in the RF group was significantly higher (P < 0.001) than that of the OF group. The energy intake in the RF group was significantly lower (P = 0.022) than that of the OF group. The protein-to-energy ratio was significantly higher (P < 0.001) in the RF group when compared with the OF group (Table 2).
Growth
A summary of the in-hospital growth in the 2 groups is presented in Table 3. The weight and WFAZ at entry and exit to the study were comparable between the OF and the RF groups. The change in WFAZ between exit and entry as well as the mean weight gain velocities were not significantly different between the 2 groups. Of interest is the significant difference (P = 0.027) between the 2 groups when change in WFAZ between exit and birth was compared: the negative change in WFAZ from birth to exit was less pronounced in the RF.
The lengths at entry and at exit from the study were comparable between the 2 groups. Even though the OF group had a lower LFAZ at the entry and the exit from the study, it did not differ significantly compared with the RF group. The change in LFAZ between exit and entry was not significantly different between the 2 groups. Length gain (cm/week) was similar between the 2 groups.
The HC at entry and exit to the study were comparable between the OF and the RF groups. At entry to the study, there was no significant differences in HCFAZ between the 2 groups, but HCFAZ at exit from the study was significantly higher in the RF group (P = 0.004). In both groups, change in HCFAZ from birth to exit showed slight improvements. The change in HCFAZ between exit and entry did not reach statistical significance. Gain in HC (cm/week) was similar for the 2 groups.
There were no significant differences between the 2 groups with regard to any of the following potentially confounding factors: gestational age; sex; birth weight (ELBW vs VLBW; BWFAZ); birth weight-for-gestational age (SGA vs AGA); birth length; birth HC; HIV-exposure; PMA and day of life on entry to study; and the time fortifier has been received (Tables 1 and 2). Differences in growth outcomes between the 2 groups were hence unlikely to have been influenced by any of these factors. In order to confirm this and because of the importance of HIV exposure in the South African context, the growth outcomes between the 2 groups were adjusted for HIV exposure in a linear regression analysis. Mean predicted effects showed no significant differences between the 2 groups in terms of both primary and secondary growth indices, confirming that HIV exposure did not confound the growth outcome results (see Table, Supplemental Digital Content 3, which reports the linear predicted marginal means adjusted for HIV-exposure, http://links.lww.com/MPG/C194)
DISCUSSION
As the most important difference between the 2 fortifiers was the higher protein content of the RF, better growth was expected in the RF group. Growth in weight, length, and HC in terms of both the primary (change in z scores) and the secondary (anthropometric gains) indices was, however, not statistically different between the 2 groups over the 15 days of fortification in hospital. Rigo et al (17.) compared the same 2 fortifiers in a multicentre randomized controlled trial and reported significantly better in-hospital growth in the “new” group (RF group in our study) for weight gain (WFAZ and g · kg−1 · day−1) but not for length (LFAZ or cm/week) and HC (HCFAZ) only reached significance at 40 weeks corrected age.
Of concern in our study is the drop in z scores (from entry to exit) for weight and length in both groups. To interpret this, the initial drop (from birth to entry into the study) should be noted as well. For WFAZ in both groups, the drop was already close to 1SD on entry to the study, with the recommendation being to not lose more than 1SD from birth to discharge from hospital (6.). As the study was not designed to look at birth to entry, it is difficult to comment on reasons for this and further investigation is warranted. Change in HCFAZ showed slight improvements in both groups and even though it was not compared with birth HCFAZ, can be interpreted positively in view of the aforementioned recommendation (6.). Weight gain velocity in the RF group met the lower end of the 15 g · kg−1 · day−1 to 20 g · kg−1 · day−1 recommended range for weight gain (5., 18.-20.). Similarly, both length and HC gain in both groups approached the recommended 1.1 to 1.4 cm/week and 0.9 to 1.1 cm/week for length and HC, respectively (5., 18.-20.). The “discrepancy” between the negative results (drop in WFAZ and LFAZ) and the positive results (approaching recommendations for weight gain velocity and length gain) should be interpreted with caution. It is well documented that the targets for weight, length, and HC gains/velocities do not “match” growth when evaluated by plotting on growth charts (21.). Both Clark et al (19.) and Fenton et al (21.) warned against using these in isolation and recommended their use in combination with growth charts (z scores).
In both groups, fortification only started when infants were close to full feed volumes, which were higher than the recommended (5., 22., 23.) starting volume of 100 mL · kg−1 · day−1. The reason(s) for the late start is not clear but local fortification practices (adding fortifier per individual feed) and high patient load may have played a role. Whether this late start meant that a “critical period” for protein supply had been missed, may be a consideration in the lack of growth outcome. Apart from the late start, the period of fortification (approximately 15 days) may also have been too short. It is possible that better growth could have been obtained if fortification had lasted longer and all infants had reached the 1650 g exit weight as originally aimed for. Intervention in other fortification studies (17., 24., 25.) where a higher protein intake had a significant effect on in-hospital growth, lasted for at least 21 days. In our study, when exit weight was compared to birth weight (over a 33-day period), the negative change in WFAZ was significantly less pronounced in the RF group (P = 0.027). A 3-week period may still be, however, insufficient to see differences in length, a conclusion also drawn by the Rigo group (17.).
Exposure to the RF led to significant increases in estimated intake of protein. Protein intake in the RF group met published recommendations (3.5--4.5 g · kg−1 · day−1) (26.) for preterm infants but it was still lower than in the Rigo study (17.) (4.5 g · kg−1 · day−1 in the “new” group receiving the RF). A retrospective analysis by Picaud et al (27.) shows that 1 in 3 infants weighing less than 1250 g at birth required protein intakes of approximately 4.2 g · kg−1 · day−1 to achieve satisfactory growth. The protein intake could, therefore, still have been too low in our study. Furthermore, even though the difference in protein intake between the 2 groups was statistically significant, it was only a modest increase of 0.3 g · kg−1 · day−1. This difference may have been even smaller as the protein content of the RF (0.355 g/1 g fortifier) was rounded off to 0.4 g/1 g fortifier when intake was calculated (see Table, Supplemental Digital Content 2, for the nutritional content of the 2 fortifiers, http://links.lww.com/MPG/C193).
In the case of energy, the statistically significant higher intake in the OF group was probably not clinically significant. What is, however, of importance is that 97% of infants in both groups exceeded the Koletzko et al (26.) energy recommendation (110–130 kcal · kg−1 · day−1). This had a negative impact on the protein-to-energy ratio, which could have been too low to impact growth. In a study by Arslanoglu et al (24.) on adjustable fortification, protein intake, but not energy and fat intake, correlated significantly with both weight gain (g · kg−1 · day−1) and HC gain (mm/day). In a similar study by Alan et al (25.), better growth was seen with a higher protein intake (without adjusting energy intake) indicating the importance of the protein-to-energy ratio (3.3 g/100 kcal in the high protein group). In the Rigo et al (17.) study, the protein-to-energy ratio was 3.0 g/100 kcal and 3.6 g/100 kcal in the “old” (ie, OF) and “new” (ie, RF) groups, respectively.
Both the lower protein intake and the lower protein-to-energy ratios in our study in comparison to the Rigo et al (17.) study and to what could theoretically have been achieved, needs further consideration. A plausible explanation could be the extended use of both half strength dosages (eg, in 44 infants [75%] in RF group half strength dosages were not increased after fortifier was tolerated for 24 hours) and suboptimal dosages (46 infants [78%] in the RF group received suboptimal dosages at some point during the study]. It should also be noted that the Rigo et al (17.) study, which lasted 21 days compared with our 15 days, excluded “half strength” fortification days in their estimation of intake. They also used preterm milk composition (even though approximately 50% of their milk was donor milk; usually considered “mature” milk) and acknowledged that this could have led to an overestimation of protein intake in their study (17.).
Another possible explanation for the lack of growth outcome is that the infants may not have received the amount of human milk and fortifier as prescribed. Human milk composition was also not chemically analysed, limiting the information about actual intake. Additional confounding factors that could have influenced growth include comorbidities [eg, patent ductus arteriosus (9.)], micronutrient intake (12., 28., 29.), steroid exposure (30., 31.), and the extent to which kangaroo mother care (32.) was practiced.
A limitation of the comparative effectiveness design of the study lies in its nonrandomization. The similarity of our 2 groups—probably because of “natural selection”—minimizes this potential threat. A strong point of the study is the considerable sample size. In sample size determination for a z score, it is reasonable to assume the standard deviation as 1.0. Therefore, from a planning perspective, the sample size of our study will have had at least 85% power to detect a clinically relevant difference of a 0.5 z score.
The prospective evaluation in a real-life setting of a middle-income country and the exclusive use of human milk are further strengths of the study. The latter making an important contribution to preterm infant growth studies as it is well established that the growth and body composition of such infants differ from those receiving formula feeds (33.-35.).
CONCLUSION
The lack of adequate in-hospital growth of VLBW preterm infants exclusively receiving fortified human milk urgently needs further attention, not only in terms of more studies but also in practical solutions to optimize the nutrition care offered to preterm infants amidst all the challenges facing neonatal units in low- and middle-income countries.