Cow's Milk Allergic Infants on Elemental Formula Maintain Adequate Mineral Status Despite Using Acid-suppressive Drugs
To the Editor: We recently presented data in this Journal showing that cow's milk allergic infants who received an amino acid–based formula (AAF) for 16 weeks as oral feeding had adequate mineral status (1.). One factor that may negatively affect mineral solubility and bioavailability and hence mineral status, is a high gastric pH (2.). Therefore, we currently present data on mineral status of a subgroup of infants on AAF receiving acid-suppressive drugs.
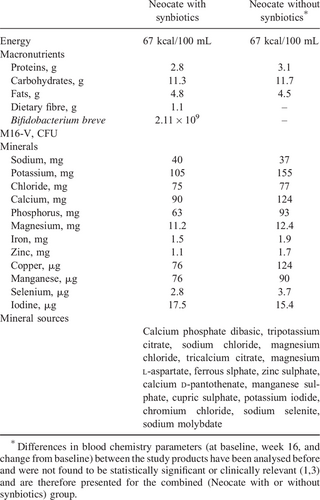
We analysed retrospectively the data of infants (0–8 months) with confirmed immunoglobulin E or non-immunoglobulin E–mediated cow's milk allergy who were randomized between 2008 and 2012 in a double-blind fashion to either an AAF with or without synbiotics (3.). In- and exclusion criteria of the study and baseline characteristics of the enrolled infants have been reported before in our original paper (1.). Details about the composition of the study formulae (Neocate; SHS International Ltd, Nutricia Advanced Medical Nutrition, Liverpool, UK) can be found in Table 1.
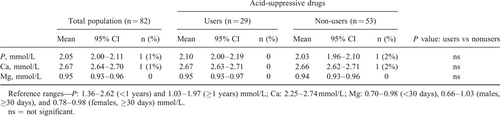
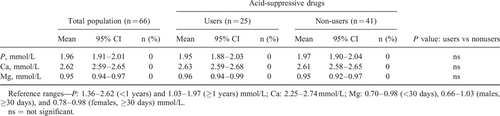
Serum concentrations of phosphorus, calcium, and magnesium were determined at baseline (n = 82) and after 16 weeks (n = 66) on AAF and compared to age-specific reference ranges. Subgroup analysis was performed for infants who were receiving acid-suppressive drugs (proton-pump-inhibitors/H2-antagonists), that is, approximately one-third (35%) of our sample. Between-group comparisons were made by 2-tailed Student t tests. P values >0.05 were considered as not significant.
Serum concentrations of phosphorus, calcium, and magnesium for the total population and for the subgroups of infants receiving or not receiving acid-suppressive drugs are presented in Tables 2 and 3. After 16 weeks, mineral concentrations of all infants were within the reference range.
Our data show that, although doses, compliance, and the neutralizing effect of the acid-suppressive drugs were not measured and infants were not randomized for acid-suppressive drug use, cow's milk allergic infants orally fed with AAF for 16 weeks maintain target serum concentrations of phosphorus, calcium, and magnesium even when receiving acid-suppressive drugs. Regular review of the ongoing need for acid-suppressive drugs remains recommended.