Ex Vivo Production of Platelets From iPSCs: The iPLAT1 Study and Beyond
The COVID-19 pandemic has had an enormous impact on multiple facets of human society.1 Although lockdown and similar measures have slowed and limited laboratory research, the shortage of blood products has also drawn much closer attention to the ex vivo production of transfusable blood cells.2, 3 Aside from the pandemic, inclement weather or long holidays could also reduce blood donations. Meanwhile, there had already been concerns about platelet product supplies because of their short shelf life (up to 7 days), supply-demand imbalance in aging societies of developed countries, and alloimmune-mediated platelet transfusion refractoriness (allo-PTR).4, 5 As such, there are high expectations for ex vivo products to solve these aforementioned issues. One approach is using artificial components such as liposomes to create particles that mimic the function of erythrocytes or platelets.6, 7 Another is differentiating blood cells from stem cells, especially induced pluripotent stem cells (iPSCs),8, 9 in conjunction with ongoing attempts to integrate gene-editing technology into their applications.
Ever since the initial reporting of human iPSCs in 2007,8, 9 there has been great interest to use iPSCs as an optimal source for cell therapy, owing to their versatile stemness (self-renewal and pluripotency), and the potential for personalization and amenability to gene editing to provide products that are patient-specific or customized for desired phenotypic properties. Now, following the first clinical trial of iPSC-derived cells, transplantation of autologous retinal pigment epithelium for age-related macular degeneration,10 clinical trials including allogeneic products have followed,11-14 such as mesenchymal stromal cells for acute graft-versus-host disease (GVHD).15
As for blood products, the clinical trial of autologous iPSC-derived platelet products (iPSC-PLTs), iPLAT1, was conducted from 2019 to 2022.16, 17 The clinical application of iPSC-derived red blood cell (RBC) products requires more time because of several obstacles.18-21 For instance, a much greater number of RBCs is needed for standard transfusion than platelets, whereas 1 erythroblast can produce only 1 RBC through enucleation. Adding to that difficulty, the enucleation rate remains low and the desired level of conversion to adult hemoglobin A is yet to be realized. In this article, we will provide an overview of the iPLAT1 trial and discuss the implications it has on allogeneic iPSC-PLTs, other iPSC-derived blood cell products, and the regenerative medicine field as a whole.
ACHIEVEMENTS AND LIMITATIONS OF THE IPLAT1 CLINICAL TRIAL
The iPLAT1 trial was designed as a single-center, open-label, uncontrolled dose-escalation study of autologous iPSC-PLTs (Figure 1).16 The subject was an aplastic anemia patient with allo-PTR caused by anti-human platelet antigen (HPA)-1a antibodies. The multiparous female patient in her 50s, with the HPA-1b/1b phenotype, had not been previously transfused, suggesting the sensitization to HPA-1a had occurred at the time of pregnancy. Although allo-PTR, a significant clinical issue in which the platelet count does not show a significant increase even by 1 hour after transfusion, are typically treated with blood products with HLA class I or HPA compatibility, they may not be readily available for rare blood types or emergencies.5, 22 In this case, the frequency of the patient's HPA-1b/1b phenotype in Japan is estimated to be <0.002%,23 and such blood donors were not available in the repository of Japanese Red Cross Society (JRC), the only organization that provides blood transfusion products in Japan.
After establishing a patient-specific immortalized megakaryocyte progenitor cell line (imMKCL) master cell bank (MCB) and good manufacturing practice (GMP)-based iPSC-PLT production system,17 the clinical trial was approved under the Act on the Safety of Regenerative Medicine by Kyoto University and subsequently by the Ministry of Health, Labor and Welfare, and registered at the Japan Registry of Clinical Trials (jRCTa050190117). During the trial, iPSC-PLT doses of 1 × 1010, 3 × 1010, and 1 × 1011 (corresponds to 5 Japan units, the half of regular dose) were sequentially administered. The primary end point was safety (frequency and severity of adverse events), for which no clinically significant symptoms or findings were observed following all dose applications. At the end of the 1-year follow-up period after administrating the final dose, the external Efficacy and Safety Evaluation Committee concluded that the administration of autologous iPSC-PLTs was safe for the patient.16
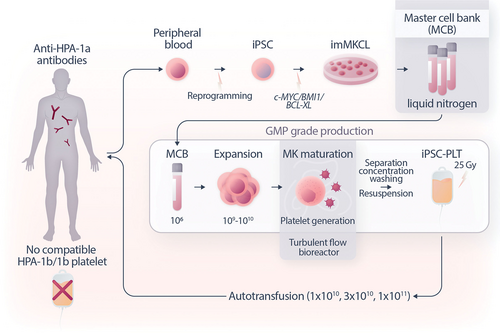
An overview of the iPLAT1 trial. iPSCs were manufactured from blood samples, with which imMKCLs were established by sequential introduction of transgenes encoding c-MYC and BMI1 followed by the transgene encoding BCL-XL and stocked frozen as the master cell bank. Whenever necessary, a vial of the MCB was thawed and, after sufficient expansion in DOX-ON culture, transferred to a bioreactor to produce platelets under the DOX-Off condition. The platelets, including media, were concentrated, separated, washed, and packaged as the transfusion product, followed by radiation to eliminate potential tumorigenicity arising from any remaining imMKCLs. In the trial, iPSC-PLTs were transfused in a dose-escalation manner. iPSC = induced pluripotent stem cells; iPSC-PLTs = iPSC-derived platelet products; imMKCL = immortalized megakaryocyte progenitor cell line; MCB = master cell bank.
As for the secondary end point of efficacy, there was no significant increase in the patient's platelet count, even after the transfusion of the final maximal dose. However, flow cytometry analysis showed the existence of larger-sized platelets, indicative of iPSC-PLTs in circulation.16 It is possible that the cell counter used by the hospital was unable to detect the larger-sized iPSC-PLTs. Another possibility is that, unlike the 1-hour peak for donor-derived platelets, iPSC-PLTs may have peaked later at 2–6 hours posttransfusion as evidenced by some animal circulation studies.16, 17 On the contrary, a slight increase in a coagulation marker, d-dimer, was observed after transfusion in the third cohort,16 raising the possibility that unexpected coagulation of iPSC-PLTs had occurred soon after transfusion. The potential causes of this suspected compromise in iPSC-PLT circulation will be discussed in the sections below.
EXPANDABLE MKS AS MASTER CELLS FOR PLATELET PRODUCTION
To produce the huge number of platelets (1011-order) for transfusion, a method that uses iPSCs as master cells—expanding designated iPSCs and differentiating them into MKs that yield platelets—was a long and inefficient process, associated with high costs and the risk of a highly variable product. The establishment of expandable MKs by introducing specific gene sets into iPSCs or iPSC-derived cells differentiating to MKs instead would shorten this process. Such expandable MKs include imMKCLs from our group, obtained by introducing c-MYC/BMI1/BCL-XL transgenes in a doxycycline-dependent manner,24 and forward programmed MKs generated by the Ghevaert group through the introduction of GATA1/FLI1/TAL1 transgenes.25 imMKCLs proliferate in the presence of doxycycline in the culture media, through the induced expression of the 3 transgenes (DOX-ON stage), and stop to proliferate, mature, and eventually release iPSC-PLTs after doxycycline removal (DOX-OFF stage).
For the iPLAT1 study, we established imMKCLs from the patient's iPSCs from 2015 to 2017.17 An imMKCL clone, M35-1, was selected for the MCB after an extensive evaluation of proliferation and platelet-producing capacity, quality of the produced iPSC-PLTs, and absence of microbial pathogens. Although autologous products are essentially nonrejectable and invaluable products unique to iPSC technology, the current costs of time, money, and labor necessary for establishing and evaluating autologous products remain prohibitively high for universal application. Meanwhile, to improve the efficacy of establishing imMKCLs, additional measures such as the knockdown of CDKN1A and p53 could potentially expedite the process.26
Another consideration is the intrinsic properties of imMKCLs that may affect iPSC-PLT generation and the functional characteristics of iPSC-PLTs. As is known with erythrocyte hemoglobin,21 iPSC-derived cells may exhibit nonadult features. Compared with adult counterparts, fetal MKs are more proliferative but generate fewer platelets, and neonatal platelets are also known to be less responsive.27, 28 Although this may explain the lower platelet productivity of imMKCLs, up to 100 platelets/MK, compared with the assumed 800–2000 of endogenous MKs,29 it does not, however, account for the supposed compromised circulation of iPSC-PLTs. Meanwhile, immune phenotypes of MKs and platelets have been recently reported,30 which may cause immuno-thrombosis and explain the slight increase in d-dimer and leucocytes after the third transfusion dose in iPLAT1.16
GENERATING IPSC-PLTS USING A TURBULENT FLOW BIOREACTOR, NOVEL REAGENTS, AND A CLOSED PACKAGING PROCESS
After 21 days of DOX-ON expansion culture with doxycycline, stem cell factor, and a thrombopoietin mimetic, TA-316,31 imMKCLs were resuspended into a DOX-OFF media,17 which does not include doxycycline but includes a Rho-associated kinase inhibitor and an arylhydrocarbon receptor antagonist, which together enables maturation without feeder cells,29 and an ADAM17 inhibitor, KP-457, which blocks GPIbα (CD42b) shedding.32 The 6-day DOX-OFF culture is performed in a turbulent flow-type reciprocal vertical motion bioreactor, VerMES, based on the discovery that turbulence enhances thrombopoiesis.29
After this DOX-OFF maturation process, iPSC-PLTs in the 32 L of media are separated, washed, and concentrated using hollow fibers, ACP215 centrifugation system, and leukocyte-depleting filters. This seamless closed-system process is followed by packaging in a blood bag with approximately 10 × 1011 iPSC-PLTs in 200 mL bicarbonated Ringer's solution with 20% acid citrate dextrose and 2.5% human serum albumin.33 Finally, the bag is irradiated with 25 Gy to eliminate the tumorigenicity of any remaining nucleated cells.17 The DOX-OFF maturation-to-packaging process mentioned here delicately handles the generated iPSC-PLTs. However, the evaluation of iPSC-PLT activation by CD62P expression and PAC-1 binding suggests the possibility of low-grade activation during some process (eg, centrifugation and radiation).17 In addition, iPSC-PLTs may have different glycosylation or sialylation status that, together with preactivation, could significantly compromise iPSC-PLT circulation capacity.34, 35
NONCLINICAL STUDIES OF IPSC-PLATELETS DEMONSTRATE SAFETY, EFFICACY, AND QUALITY
To ensure the safety of iPSC-PLTs, comprehensive nonclinical studies have been conducted.17 First, it is a prerequisite that the production system to be based on GMP standards. The starting material, imMKCL MCB, was certified as free of pathogenic microorganisms. As a feature of regenerative medicine products, this certification includes a long list of viruses that could potentially exist in allo- and xeno-components of the raw materials. From imMKCL master cells, iPSC-PLTs were aseptically manufactured using materials that meet the standards for biological raw materials. In addition, novel additives in the cell culture manufacturing process were confirmed to be nongenotoxic. Regarding the safety of iPSC-PLTs, general toxicity was assessed using experimental animals through single-dose tests in mice and single/multiple-dose tests in rats. An important safety point to consider in regenerative medicine using iPSCs is tumorigenicity. As such, it was confirmed that iPSCs, imMKCLs under DOX-ON conditions, and the final iPSC-PLT preparation were not tumorigenic upon 25 Gy of irradiation, the usual dose used for blood transfusion products to prevent GVHD.
Regarding quality and efficacy,17 the diameter of iPSC-PLTs was about 4 μm, which is larger than that of the donor-derived platelets, 2 μm, but they were concluded to have similar internal structures by electron microscopy. Surface molecules indispensable for platelet function such as CD41a and CD42b were highly expressed. In vitro functional tests included upregulation of activation markers such as CD62P expression and PAC-1 binding upon stimulation, aggregation test, and clot retraction test. The in vivo circulation and hemostasis functions of iPSC-PLTs were confirmed using rabbit models.29, 36 After the iPLAT1 trial, we performed an in vivo imaging system study in mice and confirmed the systemic circulation and distribution of iPSC-PLTs to be similar to those of general platelet transfusions.37 However, the fact that competent circulation was not similarly observed in the iPLAT1 study suggests that animal models that better reflect transfusion in humans are needed.
DEVELOPMENT OF ALLOGENEIC PRODUCTS AND NONTRANSFUSION APPLICATIONS OF IPSC-PLTS
For wider use of iPSC-PLTs, one strategy being considered is to stock imMKCLs, which are capable of stable production of high-quality platelets with high efficiency. Given that most allo-PTR are predominantly caused by anti-HLA class I antibodies, HLA homozygous iPSCs with wide HLA compatibility stocked for regenerative medicine will serve as a suitable source. Theoretically, 10 of the most frequent HLA haplotypes can cover about 50% of the Japanese population.38 In this regard, a clinical trial of iPSC-PLTs with the most common HLA haplotype in the Japanese population was started by a company recently.39
Alternatively, based on the amenability of iPSCs to gene editing, HLA class I-depleted iPSC-PLTs are being developed.40-43 This will be a universal product that should, in theory, largely avoid rejection by allo-PTR and prevent its development. We previously generated HLA class I-depleted iPSC-PLTs as experimental clones by knocking out the beta-2 microglobulin gene and evaluated their immunogenicity using mice reconstituted with a humanized immune system. They were confirmed to circulate equivalently to that of wild type iPSC-PLTs even in the presence of natural killer (NK) cells.43 As NK cells are known to attack cells downregulated for HLA class I molecules,44 it is of tremendous research interest to determine why platelets could evade such NK cell missing self immunity.
Ultimately, a single preparation using 1 excellent HLA-I-deficient O-type imMKCL clone could serve as the basis for the industrial production of iPSC-PLTs, thereby significantly cutting production costs. Such products could be used as starting material for HPA-compatible platelets through further gene modification of HPA genes.45 Panels of such HPA-edited iPSC-derived MKs and platelets could also serve as a standard for identifying anti-HPA antibodies for allo-PTR patients.46 HLA-knockout iPSC-PLTs would also be suitable as base materials for drug delivery systems, platelet-rich plasma (PRP) therapy, or other nontransfusion purposes.
CONCLUSION
The derivation of MKs and platelets from pluripotent stem cells has led to many basic research advances in hematopoiesis and now nearly reaching clinical applications. As summarized in Figure 2, from basic laboratory-based developments, the establishment of a GMP-based production system, the fulfillment of an extensive list of nonclinical evaluations required, and finally thorough consultations with regulatory authorities, the iPLAT1 study was executed. The trial confirmed safety in an allo-PTR patient for whom no compatible practical platelet products were available. At the same time, it revealed uncertainties in circulatory capacity and in vivo animal models, as well as limitations in cost and efficacy associated with establishing imMKCLs. The study is an important milestone in the clinical application of iPSC-PLTs, lighting the path for allogeneic products and, based on their potential advantages, making blood transfusion medicine more complete. It will also contribute to the field of regenerative medicine by providing the blueprints to production systems of progenitor cells as master cells and pioneering novel platelet products targeted for various nontransfusion purposes.
ACKNOWLEDGMENTS
The authors thank Dr Kelvin Hui (CiRA) for the critical reading of this article.
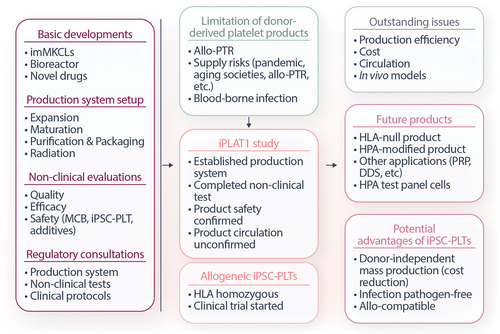
Mapping of the iPLAT1 study. Based on the basic developments, production system setup, nonclinical evaluations and regulatory consultations, and with background of limitation of donor-derived platelet products, the iPLAT1 study was conducted followed by clinical trial of allogeneic iPSC-PLTs. Although the iPLAT1 trial confirmed the safety, outstanding issues were revealed. At the same time, it raised expectations for realizing future products with potential advantages of iPSC-PLTs. iPSC = induced pluripotent stem cells; iPSC-PLTs = iPSC-derived platelet products; MCB = master cell bank; PTR = platelet transfusion refractoriness; HPA = human platelet antigen; PRP = platelet-rich plasma; DDS = drug delivery system.
AUTHOR CONTRIBUTIONS
NS and KE wrote the manuscript.
DISCLOSURES
NS and KE applied for patents related to this article. KE is a founder of Megakaryon and was a member of its scientific advisory board without salary; NS served as an advisor for Megakaryon. KE is receiving funding from Megakaryon, Otsuka Pharmaceuticals, and Kyoto Seisakusho. The interests of NS and KE were reviewed and are managed by Kyoto University in accordance with its conflict of interest policies.
SOURCES OF FUNDING
This work was supported by the Program for Technological Innovation of Regenerative Medicine (22bm0704051) and Core Center for iPS Cell Research (22bm0104001) from the Japan Agency for Medical Research and Development (AMED), by Grant-in-Aid for scientific research (C) (21K08413, NS) and Grant-in-Aid for scientific research (S) (21H05047, NS and KE) from the Japan Society for the Promotion of Science (JSPS).