Tixagevimab/Cilgavimab Pre-exposure Prophylaxis in Patients With Lymphoproliferative Disorders on BTKi
Food and Drug Administration (FDA), on December 8, 2021, issued an emergency use authorization (EUA) of tixagevimab/cilgavimab (AZD7442) for pre-exposure coronavirus disease 2019 (COVID-19) prophylaxis in immunocompromised patients, based on phase III placebo-controlled PROVENT study.1, 2 In Italy, tixagevimab/cilgavimab was first approved only for seronegative immunocompromised patients, then extended independently from antibody titers, to all subjects not expected to have an immune response from vaccination. Results from the pivotal trial clearly support the use of this monoclonal antibody combination (MoAbC) for the prevention of symptomatic and severe COVID-19 in adults with an increased risk of inadequate response to vaccination and/or progression to severe infection.
However, in this trial, immunocompromised represented only 3.8% of the population with no details regarding the condition determining the immunodeficiency.2
It is well known that patients with lymphoproliferative disorders are the most vulnerable to COVID-19, being those with the highest mortality (up to 33%),3, 4 and the poorest response to vaccination, even more so if on active therapy (on treatment, 0%–16%).5 Patients receiving Bruton tyrosine kinase inhibitors (BTKi) were reported to show even lower serological conversion.6
To assess the efficacy of tixagevimab/cilgavimab in preventing breakthrough and severe COVID infections, we analyzed a series of consecutive patients with indolent B-cell lymphoproliferative disorders on covalent BTKi monotherapy treated in 4 Italian centers. The study was performed with institutional review board approval. All patients received tixagevimab/cilgavimab given as intramuscular injections administered in 2 different sites at the dose of 150 mg each. No additional doses were planned according to European Medicines Agency (EMA) drug approval.
Demographic and clinical data, including vaccination and previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection history at the time of tixagevimab/cilgavimab administration, were collected. Concomitant conditions considered detrimental for severe COVID-19 in the PROVENT study were also recorded. Anti-SARS-CoV-2 receptor-binding domain (RBD) of the spike subunit 1 protein (anti-S1-RBD) IgG levels (Abbott Diagnostics) were measured before MoAbC administration and after 1, 3, and 6 months. The minimal threshold for seroconversion was defined as anti-S1-RBD IgG ≥50 AU/mL.
All patients were asked to report a diagnosis of COVID-19 as soon as this was made, by a positive SARS-CoV-real time reverse transcriptase–polymerase chain reaction and/or rapid antigen test on nasal swabs. Infection was classified as asymptomatic, mild (any various signs or symptoms without need of hospitalization), and severe (defined by the need of hospitalization requiring oxygen therapy, noninvasive or invasive mechanical ventilation). The incidence of infections at different timepoints was calculated with 95% confidence interval according to Clopper-Pearson. The association between categorical variables was analyzed using Fisher's exact test. All the statistical tests were 2-tailed; the statistical significance was assumed for P < 0.05, unless in the case of multiple comparisons where the Bonferroni correction was used.
From March to September 2022, overall 139 patients were analyzed. Table 1 summarizes patients and disease characteristics. The majority (82%) had a diagnosis of chronic lymphocytic leukemia (CLL). Half of the patients were receiving BTKi as salvage therapy, most commonly ibrutinib. All patients were in disease response after a median time on therapy of 25.6 months (range, 1–113). The whole population was vaccinated with full cycle (2 doses of mRNA-1273 or BNT162b2), with all but 4 having received at least 1 booster dose. The time from the last vaccine to tixagevimab/cilgavimab was 6.1 months (range, 0.5–14.5). Prior history of infection was reported in 28% with a median time from the infection to prophylaxis of 4.9 months. Before MoAbC, all patients had anti-S1-RBD measurement with no seroconversion after vaccination/previous infection in 69 (49.6%). In the remaining patients, the median antibody titer was 855 AU/mL (range, 52 to >40,000).
| Characteristic | All Patients | Not Infected After Tixagevimab/Cilgavimab | Infected After Tixagevimab/ Cilgavimab | P |
|---|---|---|---|---|
| n = 139, Value n (%) | n = 118, Value n (%) | n = 21, Value n (%) | ||
| Median age, y (range) | 72 (40–87) | 72 (40–87) | 73 (48–86) | 0.463 |
| Sex: male/female | 89(64)/50(36) | 76 (64.4)/42(35.6) | 13 (61.9)/8(38.1) | 0.754 |
| Diagnosis (CLL/WM/MZL) | 114(82)/14(10.1)11(7.9) | 97(82.2)/10(8.5)/11(9.3) | 17(80.9)/3(14.3)/1(4.8) | 0.527 |
| Prior Tx | ||||
| Median (range) | 0 (0–4) | 0 (0–4) | 0 (0–3) | 0.815 |
| 0–1 | 75(53.9) | 63(53.4) | 12 (57.9) | |
| >1 | 64(46) | 55(46.6) | 9 (42.9) | |
| Ibrutinib/acalabrutinib/zanubrutinib | 114(82)/8 (5.8)/17 (12.2) | 99 (83.9)/7 (5.9)/12 (10.2) | 15 (71.4)/1 (4.8)/5 (23.8) | 0.576 |
| At least PR (yes/no) | 134 (96.4) | 115 (97.5) | 19(90.5)/2(9.5) | 0.216 |
| Current steroid therapy | 5 (3.6) | 4 (3.4) | 1 (4.8) | 0.790 |
| Current neutropenia | 4 (2.9) | 4 (3.4) | 0 (0) | 1.000 |
| Hypogammaglubulinemia | ||||
| IgG < 700 | 72 (51.8) | 72 (62.6) | 10 (47.6) | 0.940 |
| IgG < 500 | 38 (27.3) | 32 (27.1) | 6 (28.6) | 0.802 |
| IgA < 70 | 64 (46) | 54 (45.8) | 10 (47.6) | 0.825 |
| ECOG-PS | ||||
| 0/≥1 | 118 (84.9)/23 (16.5) | 98(83.1)/20(16.9) | 19 (90.5)/2(9.5) | – |
| CIRS mediana (range) | 4 (0–13) | 4 (0–13) | 4 (0–7) | 0.337 |
| CIRS > 6 | 24 (17.3) | 22 (18.6) | 2 (9.5) | |
| CrCl mL/min | ||||
| ≥50/<50 | 101(72.6)/38(27.3) | 85(72)/33(29) | 16(76.2)/5(23.8) | 0.653 |
| Pts with: | ||||
| Cardio-comorbidity | 29 (20.9) | 22 (18.6) | 7 (33.3) | 0.060 |
| Hypertension | 69 (46.6) | 60 (50.8) | 9 (42.9) | 0.492 |
| Obesity | 16 (11.5) | 13 (11) | 3 (14.3) | 0.841 |
| Diabetes | 15 (10.8) | 14 (11.9) | 1 (4.8) | 0.367 |
| Active smokers | 13 (9.4) | 11 (9.3) | 2 (9.5) | 0.867 |
| Asthma/COPD | 8 (5.8) | 8 (6.8) | 0 (0) | 1.000 |
| Autoimmune disease | 8 (5.8) | 7 (5.9) | 1 (4.8) | 0.749 |
| Second cancer | 24 (17.3) | 19 (16.1) | 2 (9.5) | 0.408 |
| Second active cancer | 15 (10.8) | 7 (5.9) | 3 (14.3) | – |
| Liver disease | 9 (6.5) | 6 (5.1) | 3 (14.3) | 0.172 |
| Vaccinated | 139 (100) | 118 (100) | 21 (100) | – |
| Complete vaccine cycle (2 doses) | 4 (2.9) | 3 (2.5) | 1 (4.8) | |
| ≥1 booster doses | 135 (97.1) | 115 (97.5) | 20 (95.2) | |
| Infection before tixagevimab/cilgavimab | 39 (28.1) | 37 (31.4) | 2 (9.5) | 0.094 |
| Severe infectionb | 10 (7.2) | 10 (8.5) | 0 (0) | – |
| Negative serology before tixagevimab/cilgavimab | 66 (56.9)/116 | 52 (44.1) | 14 (66.7) | 0.547 |
| Positive serology before tixagevimab/cilgavimab | 50 (43.1)/116 | 66 (55.9) | 7 (33.3) | |
- aMedical conditions that are deemed to be complications of CLL are not included as part of the total CIRS score.
- bSevere infection: requiring hospitalization.
- CLL = chronic lymphocytic leukemia; COPD = chronic obstructive pulmonary disease; CIRS = cumulative illness rating scale; CrCL = creatinine clearance; ECOG-PS = Eastern Cooperative Oncology Group performance status; MZL = marginal zone lymphoma; PR = partial response; Tx = therapy.
Grade 1-2 injection-site reactions were the only adverse events recorded, occurring in 5.8% of patients. None of the patients developed early or late-onset grade ≥3 adverse events after the administration of the MoAbC.
Figure 1 presents antibody titer kinetics after tixagevimab/cilgavimab. All patients developed a serologic response after MoAbC administration that increased from a median title of 62 AU/mL (on 139 tested patients, range: <50 to >40,000) to 13,578 AU/mL at 1 month (on 112, range: 980 to >40,000); 7530 at 3 months (on 60, range: 2378 to >40,000), and 3261 at 6 months (on 25, range: 995–6575). After a median observation of 4.4 months (range, 1.2–7.8) from MoAbC prophylaxis, 21 patients (15.1%) developed COVID-19 with a median time to infection of 52 days (range, 6–101). The incidence of COVID-19 infection was evaluated monthly (from months 1 to 6) with respect to the population at risk, excluding from the cohorts patients who were tested positive (Figure 1). All patients were infected with Omicron variant of concern (VOC). Two patients (1.4%) were asymptomatic, 16 (11.5%) had only mild symptoms, whereas 3 (2.2%) developed a severe disease. Among the 3 hospitalized patients, 1 was admitted to intensive care unit and died due to acute respiratory distress syndrome. Overall, 13 patients did not receive any COVID-19 therapy; antivirals were administered within 5 days from symptoms-start in 8 cases (remdesivir 2, nirmatrelvir/ritonavir 3, and molnupinavir 3).
Among all patients’ disease and health factors analyzed and listed in Table 1, history of prior COVID-19 infection was the only variable showing a trend (P = 0.056) toward a reduced risk of infection with an odds of 77%.
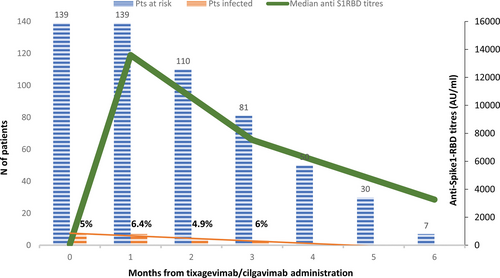
Anti-spike1-RBD kinetic and infections. Green line, antibody titers kinetic (median) measured at baseline and at 30, 90, and 180 days after tixagevimab/cilgavimb administration. Blue columns, number of patients at risk. Orange columns, number of patients with COVID-19 infection at 0–1; 1–2; 2–3; 3–4; 4–5; and 5–6 months after tixagevimb/cilgavimab administration. RBD = receptor-binding domain.
In a bivariate model, history of prior COVID-19 adjusted for a cumulative illness rating scale (CIRS) >6 resulted to be independently associated with a lower risk of breakthrough infection (P = 0.049). Tixagevimab/cilgavimab EUA for pre-exposure prophylaxis in immunocompromised patients was based on a randomized control trial that excluded those who had been vaccinated or had a prior COVID-19 infection.2 Furthermore, it should be highlighted that it was carried out when different and no longer circulating SARS-CoV-2 VOC were predominant.
Given the continuous evolution of the virus, the efficacy of the MoAbC against Omicron variant is still not fully understood. However, preclinical pseudovirus assay data and retrospective studies,7-10 conducted during Omicron-predominant outbreak, seemed to confirm the protective role of pre-exposure prophylaxis against the infection (rates, 3.5%–3.9%) and severe illnesses (rates, 0.1%–0.5%).
Although these experiences included a significant proportion of hematological patients, their specific outcome was not outlined, and no stratification according to disease type and treatment status was present. To our knowledge, ours is the first study specifically addressed to a homogeneous population for disease diagnosis and indolent lymphoproliferative disorders while on active treatment with BTKi monotherapy. Moreover, the observation period of 4.4 months was longer than other retrospective series (53–99 days).8-10 Of note, during the study period, Omicron was the only circulating variant in Italy, with a predominance of BA.2 sublineage from March to July and BA.5 from August to September 2022.11
In our experience, rate of breakthrough and severe infections was higher than in literature (breakthrough infections, 1.6%–4.4%; severe infections, 0%–0.5%; and mortality, 0%–0.2%).8-10, 12
Nevertheless, any indirect comparison between studies has a strong limitation, because no similar homogeneous populations have ever been described. Rates of infections and severe illnesses may be explained by the elevated vulnerability of this selected population, which is well known to have the highest COVID-19 mortality due to both disease-related B-cell dysfunction and BTK-induced immunosuppression.13 In our cohort, the presence of anti-S1-RBD, either after vaccination or previous exposure, before tixagevimab/cilgavimab did not exert protection over subsequent infection.
Interestingly, history of previous COVID-19 resulted as the only independent protective factor toward breakthrough infection; as previously reported, this may be related to the presence of SARS-CoV-2 S specific mucosal neutralizing antibodies.14
Notably, all our patients developed an antibody titer after tixagevimab/cilgavimab, reaching a peak after 1 month, with a median of 218-fold rise in level. Moreover, in all those analyzed in the 6th month, despite showing 76% decrease, antibody titer remained significantly above the lower reference limit.
FDA, but not EMA, revised and doubled the authorized tixagevimab/cilgavimab dosage based on data showing its reduced efficacy to achieve a meaningful neutralization of Omicron-RBD in patients with hematologic malignancies treated with a 150 + 150 mg single dose.8 As in our series, all patients received the lower MoAbC dosage, and we might speculate that this could have been an additional reason, other than the vulnerability of this population, for a high incidence of infections.
In the PROVENT study, a higher incidence of serious cardiovascular adverse events was reported in the experimental arm compared with placebo. Considering that cardiological toxicity is a known BTKi-related off-target effect,15 the absence of cardiovascular events in our population demonstrates the feasibility of tixagevimab/cilgavimab even in this setting.
Our study has several limitations: bias due to different baseline serological statuses, and the absence of a comparative population, as the MoAbC was proposed to all identified eligible patients. Moreover, COVID-19 infection results in increased expansion of antigen-specific CD4+ and CD8+ T-cell subsets that play a key role in protecting against severe infection.14 Considering the descriptive purpose of this analysis, no data were collected on T-cell expansion. We cannot, therefore, draw conclusions on the role of T-cell immunity in our population.
In addition, our rate of COVID-19 infection may be underestimated by having missed some asymptomatic patients.
Finally, as most of the patients received early antiviral treatment, it is not possible to determine how tixagevimab/cilgavimab accounted alone on the SARS-CoV-2 clinical evolution.
Despite the availability of new drugs for the prevention and treatment of COVID-19, patients with lymphoproliferative disorders under ongoing treatment remain those more at risk of developing a serious disease. Therefore, in these patients, it is essential to continue administering all possible preventive strategies including vaccines and monoclonal antibodies.
Considering that previous published experiences on pre-exposure prophylaxis included heterogeneous cohorts for diseases and grade of immunosuppression, no comparison can be made on the incidence and severity of COVID-19 infection between different studies.
Therefore, it is not possible to give a concluding judgment on the protective role of tixagevimab/cilgavimab in our specific population.
Taking into account all these considerations, in a situation in which the epidemiological scenario is so dynamic, real-world data are crucial and may help to implement the timely medical update of the prescribers.
ACKNOWLEDGMENTS
The authors acknowledge Michele Nichelatti, PhD, for statistical analyses.
AUTHOR CONTRIBUTIONS
GZ, AMF, GT, and AT conceived and designed the study, analyzed data, and wrote the manuscript; CB, GR, MM, MD, EB, VM, MBF, GC, CGP, RC, and MP collected the data; and all the authors critically reviewed the manuscript and approved the final version.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
The authors have no sources of funding to declare.