Survival in Children Below the Age of 15 Years With Leukemia: Temporal Patterns in Eastern and Western Germany Since German Reunification
Supplemental digital content is available for this article.
After German reunification in 1990, the former German Democratic Republic (GDR) underwent substantial political, (socio-)economic and societal changes, including healthcare reform aimed at assimilating to the Western system.1 Adult mortality rates in Eastern Germany were higher compared with those in Western Germany around reunification, but the gap has narrowed rapidly. However, while cancer mortality rates for women have decreased in Eastern Germany to a similar level as in Western Germany, rates for men remained higher in Eastern Germany.2
Leukemia is the most frequent cancer diagnosis in children, accounting for about one-third of all cancers diagnosed in 0- to 14-year olds, with incidence rates ranging between 42 and 59 cases per million in Europe.3 Acute lymphoblastic leukemia (ALL) is the most common type of childhood leukemia, representing >75% of all cases, followed by acute myeloid leukemia (AML), accounting for about 15%.4, 5 Notably, differences between Eastern and Western Germany were also evident for the incidence of childhood leukemia. An approximately 20% lower incidence rate of lymphoblastic leukemia in Eastern Germany compared to Western Germany was observed at the time of reunification, followed by a remarkable increase in Eastern Germany until around 2000, when incidence rates reached the same level as those in Western Germany.6
Advances in tumor biology, diagnostics, pharmacology, risk grouping, and treatment combinations have led to considerable improvements in childhood cancer survival, with 5-year survival now exceeding 80% in most high-income countries.4, 7 Treatment according to standardized protocols is considered as the standard of pediatric leukemia care with 5-year ALL survival exceeding 90% in some European and North American countries today.8 However, despite past advancements, reported population-based 5-year AML survival still rarely exceeds 75%.4, 9-11 Furthermore, survival has not improved similarly across countries and is still inferior in most Eastern European countries compared to other parts of Europe.9, 12 Notably, evidence is accumulating that not only clinical factors but also social and socioeconomic conditions impact childhood cancer prognosis, even within European countries with presumably equal access to cancer care irrespective of social background.13 The reunification of Germany in 1990 offers the unique opportunity to examine childhood leukemia survival trends in 2 merging countries with homogeneous genetic make-ups but remarkably different initial social conditions and welfare and healthcare systems.
In the following report, we use “Eastern Germany” and “Western Germany” to refer to the former states of the GDR and West Germany, respectively.
The study population comprised all children in Germany with a first primary diagnosis of ALL (defined according to the diagnostic subgroup I(a) of the International Classification of Childhood Cancer—third edition (ICCC-3)14; but excluding diagnoses of non-acute lymphoblastic leukemia (N = 5; ICD-O-3 morphology codes 9823/3, 9834/3, and 9948/3) or AML (ICCC-3 group I(b)) at ages 0–14 years between 1991 and 2015. Cases were identified from the nationwide, population-based German Childhood Cancer Registry (GCCR), which collects data on pediatric cancer diagnoses since 1980 (since 1991 also for Eastern Germany) with excellent data quality and high completeness for both Eastern and Western Germany.4 Due to ambiguities in assignment of districts, cases from Berlin were excluded from this study (N = 523).
We used the most up-to-date follow-up information on vital status from the GCCR database as of November 30, 2021. The GCCR conducts passive and active follow-up regularly (at least every 2 y) using information from the relevant therapy trials, treating hospitals and population registries. Of the 13,251 leukemia cases, follow-up information was available for 13,239 children (99.9%). Of those, 99.2% had complete follow-up information for at least 3 years after diagnosis and 98.0% for at least 5 years.
We used univariable and multivariable (adjusting for sex and age at diagnosis) Cox proportional hazards models, estimating hazard ratios (HR) and 95% confidence intervals (CI), as well as Kaplan-Meier curves and log-rank tests to compare 5-year overall ALL and AML survival (with time since diagnosis as underlying time scale) between children residing in Eastern and Western Germany at diagnosis. Analyses were stratified by leukemia type and diagnostic period, and, for some analyses, additionally by age group and sex. We censored at 5 years of follow-up, as few directly disease-related deaths occur after this time, whereas the incidence of competing causes of death increases. We performed trend analyses to assess the statistical significance of temporal changes in the comparison of survival estimates. Analyses were performed using SAS Software 9.4.
Our study population included 11,353 (86%) children diagnosed with ALL and 1886 (14%) with AML (Table 1). One thousand eight hundred five children died within 5 years of diagnosis. Demographic characteristics by sex and age per diagnostic period are given in Suppl. Table S1. Survival estimates and Cox model results revealed poorer ALL survival in Eastern compared to Western Germany at reunification (Table 1). Comparing survival between Eastern and Western Germany by diagnostic period, the steadily decreasing HRs (from a HR of 1.28, 95% CI, 0.98-1.66; for 1991–1995 to 0.59, 95% CI, 0.34-1.05; for the most recent diagnostic period of 2011–2015; P for trend = 0.002) indicated larger improvements in ALL survival in Eastern Germany with survival probabilities in recent years even exceeding those observed in Western Germany (95.2% versus 92.1%) (Table 1). Survival differences for AML have also diminished in recent years, but survival probabilities in Western Germany still exceed those in Eastern Germany (84.4% versus 81.3%) (Table 1).
| Diagnostic period | No. of cases/no. of deathsb | Person-years of follow-upb | 5-y overall survival (95% CI) Western Germany | 5-y overall survival (95% CI) Eastern Germany | Crude HRc (95% CI) (Ref: Western Germany) | Adjusted HRc,d (95% CI) (Ref: Western Germany) |
|---|---|---|---|---|---|---|
| Acute lymphoblastic leukemia | ||||||
| All diagnostic periods | 11,353/1,205 | 52,349.29 | 89.32% (88.69-89.92) | 88.98% (87.34-90.43) | 1.04 (0.89-1.22) | 1.03 (0.87-1.20) |
| 1991–1995 | 2,351/ 346 | 10,561.28 | 85.68% (84.04-87.17) | 83.05% (79.03-86.36) | 1.22 (0.94-1.59) | 1.28 (0.98-1.66) |
| 1996–2000 | 2,393/ 309 | 10,924.59 | 87.31% (85.80-88.68) | 85.05% (80.65-88.51) | 1.21 (0.89-1.65) | 1.12 (0.82-1.52) |
| 2001–2005 | 2,352/ 215 | 10,978.54 | 90.86% (89.53-92.03) | 90.41% (86.41-93.28) | 1.06 (0.71-1.58) | 0.98 (0.66-1.46) |
| 2006–2010 | 2,234/ 189 | 10,497.85 | 91.06% (89.69-92.25) | 94.32% (91.03-96.43) | 0.62 (0.38-1.03) | 0.64 (0.39-1.06) |
| 2011–2015 | 2,023/ 146 | 9,387.02 | 92.11% (90.72-93.30) | 95.17% (91.82-97.16) | 0.60 (0.34-1.06) | 0.59 (0.34-1.05) |
| P = 0.003 | P = 0.002 | |||||
| Acute myeloid leukemia | ||||||
| All diagnostic periods | 1,886/ 600 | 7,000.69 | 68.70% (66.36-70.92) | 63.15% (57.20-68.50) | 1.26 (1.02-1.56) | 1.25 (1.01-1.55) |
| 1991–1995 | 401/ 188 | 1,226.74 | 55.35% (49.79-60.55) | 42.07% (30.64-53.06) | 1.57 (1.11-2.21) | 1.58 (1.12-2.23) |
| 1996–2000 | 433/ 167 | 1,527.44 | 60.60% (55.41-65.38) | 65.35% (52.31-75.64) | 0.85 (0.54-1.33) | 0.85 (0.54-1.34) |
| 2001–2005 | 370/ 110 | 1,421.53 | 70.71% (65.37-75.38) | 66.67% (51.98-77.78) | 1.20 (0.71-2.01) | 1.18 (0.70-1.99) |
| 2006–2010 | 357/ 85 | 1,454.88 | 76.59% (71.36-80.99) | 72.73% (58.90-82.57) | 1.21 (0.69-2.11) | 1.22 (0.70-2.14) |
| 2011–2015 | 325/ 50 | 1,370.10 | 84.44% (79.61-88.22) | 81.33% (64.79-90.63) | 1.25 (0.56-2.77) | 1.23 (0.55-2.73) |
| P = 0.54 | P = 0.51 | |||||
- aClassified according to the International Classification of Childhood Cancer—third edition (ICCC-3). Acute lymphoblastic leukemia was defined according to the ICCC-3 diagnostic subgroup I(a) excluding non-acute lymphoblastic leukemia.
- bRefers to 5 y after diagnosis.
- cHRs refer to 5y overall survival. Western Germany was used as the reference.
- dHR adjusted for sex and age at diagnosis (modeled categorically) and corresponding confidence interval.
- CI = confidence intervals; HR = hazard ratio; N = number.
Additional analyses by age at diagnosis revealed that the temporal change from lower to higher ALL survival in Eastern compared with Western Germany was solely driven by children diagnosed at ages 5 years and above, most pronounced for the oldest age group studied (10–14 y) (Suppl. Figure S1, Suppl. Table S2), and evident for both boys and girls (Figure 1, Suppl. Table S3). HRs for the 5- to 14-year olds decreased remarkably from 1.46 (95% CI, 1.06-1.99) for 1991–1995 to 0.45 (95% CI, 0.18-1.12) for 2011–2015 (P for trend < 0.001) (Figure 1, Suppl. Table S3). Kaplan-Meier curves suggested that the ALL survival differences between Eastern and Western Germany for the diagnostic period 2006–2015 emerged around 8–9 months after diagnosis (Suppl. Figure S2).
This unique register-based study with minimal potential for bias showed poorer ALL survival in Eastern compared to Western Germany at reunification, followed by a steeper increase in the east, resulting in survival estimates exceeding those in Western Germany. This temporal pattern was particularly pronounced for children aged 5–14 years at diagnosis. Contrarily, AML survival in Western Germany still somewhat exceeds that in Eastern Germany, although survival has improved remarkably in both geographic areas and the gap has narrowed in recent years.
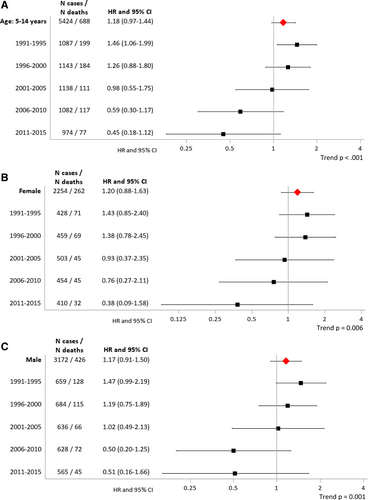
Hazard ratios of the association between 5-year overall acute lymphoblastic leukemia survival and place of residence (Eastern Germany versus Western Germany) by diagnostic period for (A) 5- to 14-year olds and for (B) females and (C) males. HRs refer to 5-year overall survival. Western Germany was used as the reference. CI = confidence intervals; HR = hazard ratio; N = number.
We consider clinical, healthcare-related, social and socioeconomic factors and their interplay to be probable contributing factors to the observed temporal survival patterns.
Treatment of childhood leukemia has been highly standardized over the past decades. In the GDR, treatment was performed according to protocols only slightly modified from those in Western Germany, with all drugs available (in principle).15 The standardized treatment protocols from Western Germany were implemented in all of Germany immediately after reunification. The lower population density in the former GDR combined with lacking information technology and poorer infrastructure and socioeconomic conditions may have played an important role in healthcare provision.16 Evidence from Eastern and parts of Southern Europe indicated that residence in a rural area elevates the risk of poor childhood leukemia survival, due to limited access to specialized healthcare.12, 17
Why present-day ALL survival appears superior in Eastern compared with Western Germany, where survival probabilities have improved to a somewhat lesser degree, remains a puzzling question. Improvements in socioeconomic conditions and healthcare in Eastern Germany may be underlying causes, but the fact that socioeconomic conditions are still inferior in large parts of Eastern compared to Western Germany actually contradicts this supposition.
Another explanation may relate to differences in treatment adherence. ALL treatment usually lasts 2 years and involves a long period of home-administered maintenance therapy, starting after about 8 months of hospital treatment, when parents become responsible for complying with medical recommendations for the continuation of therapy, including daily drug administration and frequent outpatient appointments. Poor adherence to oral maintenance therapy may lead to poor outcome. A study from the United Kingdom revealed that survival differences by socioeconomic status emerge about the time when treatment management requires parental/child adherence, that is, from the time of outpatient oral treatment.18 Kaplan-Meier curves for the diagnostic period 2006–2015 of this study showed similar patterns.
There are various determinants influencing treatment adherence, including socioeconomic background and the organization of healthcare.19 It remains speculative whether the interplay between improving infrastructure, healthcare provision, and behavioral factors influencing compliance and treatment adherence contributed to the remarkable survival improvements in Eastern Germany. Communication barriers between families and healthcare professionals might have also played a central role, by, for instance, possibly adversely affecting treatment adherence in non-German speaking families and thus worsen prognosis for children in Western Germany where the proportion of families with a migration background is substantially higher compared to Eastern Germany.
The validity of our findings is underlined by the use of high-quality data from the GCCR, with excellent nationwide coverage of all diagnosed leukemia cases for 25 years, validated residential address information, virtually no loss to follow-up and no nonparticipation. Nevertheless, some results from subgroup-analyses involve a large degree of estimation uncertainty due to small numbers and should be interpreted with caution.
Older children diagnosed with ALL in Eastern Germany may have benefited most from improvements in socioeconomic conditions and healthcare. Why ALL survival in Eastern Germany surpasses survival in Western Germany today remains speculative. Further research considering social conditions in more detail is warranted to further unravel the mechanisms underlying the observed survival inequalities.
ACKNOWLEDGMENTS
We are grateful to Claudia Trübenbach, Melanie Kaiser, Irene Jung, and Claudia Bremensdorfer (data manager at the German Childhood Cancer Registry) for their technical support with data preparation for this article. Further, we wish to thank the German Society for Paediatric Oncology and Haematology (GPOH) and the pediatric hematology-oncology units for their data contributions to the German Childhood Cancer Registry.
AUTHOR CONTRIBUTIONS
MW, FE, AB, and HZ were responsible for the conceptualization and design of the study. MW conducted the statistical analyses. MW was responsible for the visualization. MW, FE, and AB drafted the article. FE, CS, and DG were responsible for the data acquisition. FE, AB, and HZ supervised this work. All authors interpreted the data. All authors read, revised, and approved the final version of the article.
COMPLIANCE
No ethics approval or consent was required for this study, as no active participation of patients was required. This research was carried out in compliance with the requirements of the General Data Protection Regulation and in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Information on previous presentation of the information reported in the article.
DISCLOSURES
Prof Arndt Borkhardt is a member of the HemaSphere Editorial Board. All other authors declare no conflicts of interest to disclose.
SOURCES OF FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The work of the authors was supported by core funds from their respective institutions, namely the Institute of Medical Biostatistics, Epidemiology and Informatics at the University Medical Center of the Johannes Gutenberg University, Mainz, Germany, the Leibniz Institute for Prevention Research and Epidemiology—BIPS, Bremen, Germany and the Heinrich Heine University, Medical Faculty, Düsseldorf, Germany. The German Childhood Cancer Registry is part of the Division of Childhood Cancer Epidemiology at the Institute of Medical Biostatistics, Epidemiology and Informatics and is funded by the Federal Ministry of Health and the Health Ministries of the 16 federal states of Germany. The funding sources were not involved in the conceptualization, design, content or preparation of the manuscript, or the decision to submit for publication.