Pomalidomide in Patients With Relapsed and/or Refractory Multiple Myeloma: A Prospective Study Within the Nationwide Netherlands Cancer Registry
Abstract
Patients with relapsed and/or refractory multiple myeloma (RRMM) generally have limited treatment options and a poor prognosis. Previous trials demonstrated that pomalidomide combined with low-dose dexamethasone (Pd) is effective in these patients with significant responses and improved progression-free survival (PFS). Pd has been approved in RRMM patients who received ≥2 prior lines of therapy. Here, we present the results of a population-based study of patients with RRMM treated with Pd in The Netherlands from time of pomalidomide approval. Using the nationwide Netherlands Cancer Registry, data from all nontrial patients with RRMM treated with Pd were collected. Data were analyzed of response, PFS, and overall survival (OS). A total of 237 patients were included in this analysis. Previous treatment consisted of a proteasome inhibitor in 227 patients (96%) and/or an immune-modulating agent in 235 patients (99%). One hundred forty patients (59%) were refractory to an immune-modulating agent in their last line of therapy. Median time from diagnosis to treatment with Pd was 4.9 years (interquartile range, 2.7–7.9), and the median number of prior treatments was 4 (interquartile range, 3–5). Median PFS and OS for all patients were 3.6 months (95% confidence interval [CI], 3.1–3.8) and 7.7 months (95% CI, 5.7–9.7), respectively. For patients achieving ≥PR, median PFS and OS were 10.6 months (95% CI, 8.3–12.9) and 16.3 months (95% CI, 13.6–23.2), respectively. This nationwide, population-based registry study confirms data shown in pivotal clinical trials on Pd. PFS in this analysis is comparable to PFS observed in those clinical trials.
INTRODUCTION
Treatment of patients with refractory and/or relapsed multiple myeloma (RRMM) has improved during the last two decades. However, patients refractory to proteasome inhibitors (PI) and immune-modulating agents (IMiDs) still have a poor prognosis.1, 2 With the treatment of each relapse, progression-free survival (PFS) and overall survival (OS) decrease due to the emergence of drug resistance. Therefore, effective therapeutic strategies to treat RRMM are needed. At first relapse, combinations of carfilzomib/lenalidomide/dexamethasone and daratumumab/dexamethasone with either bortezomib or lenalidomide have proven to be effective and tolerable3-5 and have become the standard of care in many countries. At second and third relapse, it is more challenging to achieve durable remissions. Pomalidomide is a third-generation IMiD with tumoricidal and antiangiogenic activities through binding to cereblon, a protein in the E3 ubiquitin ligase complex. Compared to lenalidomide and thalidomide, pomalidomide has a higher potency toward binding to cereblon and thereby exerts higher antiproliferative activity against myeloma cells.6 Moreover, pomalidomide has been observed to be effective in IMiD and PI refractory patients.7-10 Previous trials showed that pomalidomide combined with low-dose dexamethasone (Pd) in patients with RRMM induces improvement in response, PFS, and OS. In the MM-002 trial, patients were treated with Pd versus pomalidomide alone. PFS was significantly longer in patients treated with Pd than with pomalidomide alone (4.2 versus 2.7 mo, hazard ratio [HR] 0.68, [95% confidence interval (CI), 0.51–0.90], P = 0.003).7 The MM-003 trial randomized patients between treatment with Pd versus high-dose dexamethasone alone. Median PFS was 4.0 months in patients treated with Pd versus 1.9 months in patients treated with dexamethasone alone (HR 0·48 [95% CI, 0.39–0.60]; P < 0.0001).8 This study subsequently led to the approval of Pd in patients with RRMM who received ≥2 prior lines of therapy, including IMiDs, PIs, and alkylating therapy. Moreover, a subanalysis showed that treatment with Pd in patients with renal impairment is well tolerated and leads to comparable efficacy.11 In addition, treatment with Pd improves and prolongs health-related quality of life.12 Pd is reimbursed in most European countries, and currently, it is one of the most used agents in third and further lines of treatment. Kastritis13 performed an analysis of treatment with Pd in the real world. In their cohort, PFS and OS were 5.0 and 12.1 months, respectively, showing that treatment with Pd is an effective regimen in patients in the real world.13 Pd remains an important treatment regimen in elderly and frail patients for whom more intensive treatment with triplets is not an option due to performance status and comorbidity. From the moment of approval and reimbursement of pomalidomide in The Netherlands, we prospectively collected data of patients treated with Pd in a collaborative program of the Haemato Oncology Foundation for Adults in The Netherlands and the nationwide Netherlands Cancer Registry (NCR) from the Netherlands Comprehensive Cancer Organisation. Here, we present an analysis of nationwide, population-based data on the effectiveness of Pd in 237 patients with RRMM in The Netherlands treated between January 2015 and December 2018.
METHODS
Patients and study design
This study is a prospective analysis integrated in the reimbursement program for pomalidomide in The Netherlands. Data of patients with RRMM treated with Pd were collected to analyze this regimen's effectiveness in the real-world and evaluate cost-effectiveness.
The treating physician prospectively enrolled patients in the nationwide NCR via an online registration tool (ALEA). Furthermore, patients not registered by the treating physician were additionally ascertained via the Nationwide Registry of Hospital Discharges (ie, inpatient and outpatient discharges) that hold data on all hospitals’ medical claims in The Netherlands.14 Patients with RRMM treated with Pd according to the label were included; that is, patients with ≥2 previous treatments consisting of at least an IMiD and a PI. According to the cost-effectiveness requirements of the reimbursement program, treatment with Pd was discontinued after three courses if the patient showed no response. Standard baseline characteristics were collected such as age, gender, International Staging System (ISS) stage pretreatment, and cytogenetic data when available. Also, various details about the treatment with Pd were collected, that is, the number of treatment cycles, best response, and whether cyclophosphamide was added to Pd. In addition, data about previous treatments were collected, including response and time to progression. According to the Central Committee on Research involving Human Subjects, this type of observational study does not require approval from an ethics committee in the Netherlands. The Privacy Review Board of the NCR approved the use of anonymous data for this study. Assessments: Symptomatic MM was defined according to the International Myeloma Working Group (IMWG) criteria.15 Treatment responses and disease progression were classified according to IMWG Uniform Response Criteria by the treating physician, with categories for complete response, very good partial response, partial response (PR), and stable disease.15 Refractory disease was defined as progression of disease on treatment or within 60 days after treatment was discontinued according to IMWG criteria. The primary outcome was PFS, defined as the time from Pd initiation until disease progression or death, whichever occurred first. Secondary outcomes included OS (time from pomalidomide initiation to death from any cause), overall response rate (ORR; at least PR), and time to response.
Statistical analysis
Baseline characteristics at the start of Pd were presented using descriptive statistics. The Fisher exact test was applied to compare categorical variables between subgroups, whereas the Kruskal–Wallis test was used for continuous variables. We constructed survival distributions using the Kaplan–Meier method. The survival distributions were compared between subgroups using the log-rank test. Multivariable Cox regression was performed to investigate the association of gender, age, and the number of prior therapy with PFS and OS. Results from the Cox regression produce HRs with associated 95% CIs. Proportional hazard assumptions were tested based on Schoenfeld residuals.
All P values are two-sided, and a significance level α = 0.05 was used. All statistical analyses were performed using STATA Statistical Software Release 16.1 (College Station, TX).
RESULTS
A total of 237 patients (56% males, median age, 67 y; interquartile range [IQR], 60–74 y; 35% >70 y), who started with Pd between January 2015 and December 2018, were included in this analysis. Baseline demographics and disease characteristics are shown in Table 1. FISH data were only known in a minority of patients; therefore, these data are not included in this analysis.
| Patients (n = 237) | Cyclo (n = 72) | No cyclo (n = 165) | |
|---|---|---|---|
| Age (y) | 67 [35–88] | 66 [38–83] | 68 [35–88] |
| >70 | 82 (35) | 20 (28) | 62 (38) |
| Sex | |||
| Male | 133 (56) | 42 (58) | 91 (55) |
| Female | 104 (44) | 30 (42) | 74 (45) |
| WHO performance status | |||
| 0 | 26 (11) | 11 (15) | 15 (9) |
| 1 | 50 (21) | 17 (24) | 33(20) |
| 2 | 22 (9) | 9 (13) | 13(8) |
| 3 | 5 (2) | 0 (0) | 5 (3) |
| Unknown | 134 (57) | 35 (49) | 99 (60) |
| ISS | |||
| 1 | 3 (1) | 0 (0) | 3 (2) |
| 2 | 10 (4) | 3 (40 | 7 (4) |
| 3 | 20 (9) | 7 (10) | 13 (8) |
| Unknown | 204 (86) | 62 (86) | 142 (86) |
| Hemoglobin (mmol/L), median [range] | 6.7 [5.7–7.3] | 6.7 [6.2–7.5] | 6.6 [5.7–7.2] |
| Platelets (109/L), median [range] | 126 [69–190] | 115 [58–175] | 126 [74–192] |
| Creatinin (µmol/L), median [range]a | 92 [73–119] | 89 [73–110] | 92 [73–128] |
| Calcium (mmol/L), median [range]b | 2.4 [2.3–2.5] | 2.4 [2.3–3.5] | 2.4 [2.3–2.5] |
| Albumin (g/L), median [range]c | 35 [31–40] | 35 [31–40] | 36 [31–40] |
| Time from diagnosis, median [range] | 4.9 [1–18] | 4 [1–18] | 5 [1–18] |
| Number of prior treatment, median [range] | 4 [2–10] | 4 [2–9] | 4 [2–10] |
| Previous treatment | |||
| Lenalidomide | 235 (99) | 71 (99) | 164 (99) |
| Thalidomide | 123 (52) | 38 (53) | 85 (52) |
| Bortezomib | 227 (96) | 70 (97) | 157 (95) |
| Carfilzomib | 29 (12) | 15 (21) | 14 (8) |
| Ixazomib | 6 (3) | 4 (6) | 2 (1) |
| Alkylating therapy | 232 (98) | 71 (99) | 161 (98) |
- aUnknown in one patient; bUnknown in 12 patients; cUnknown in 23 patients.
- Cyclo = cyclophosphamide; ISS = International Staging System; WHO = World Health Organization.
Most patients received pomalidomide combined with dexamethasone or prednisolone (179 patients dexamethasone and 40 patients prednisolone). Eighteen patients were treated with pomalidomide monotherapy. The median time from diagnosis to treatment with Pd was 4.9 years (IQR, 2.7–7.9), and the median number of prior treatments was 4 (IQR, 3–5). The vast majority of patients was previously treated with bortezomib (n = 227, 96%) and lenalidomide (n = 235, 99%). One hundred twenty-three patients (52%) were previously treated with thalidomide. One hundred twenty-six patients (53%) received an autologous stem cell transplantation and 29 patients (12%) received an allogeneic stem cell transplantation previously. One hundred forty (59%) patients were refractory to an IMiD in their last line of therapy, 118 patients (50%) were refractory to lenalidomide, and 22 patients (9%) were refractory to thalidomide. The median number of treatment cycles with Pd in all patients was 3 (IQR, 2–7) at the time of database lock (June 12, 2019). Two hundred thirty patients (97%) had discontinued Pd treatment due to progressive disease (n = 118, 51%), unacceptable toxicity (n = 27, 12%), refractory disease (n = 26, 11%), and death of any cause (n = 22, 10%). In 29 patients (13%), the reason for discontinuation of treatment was unknown. Eight patients (3%) stopped Pd treatment to receive a stem cell transplantation, comprising of two patients with an autologous stem cell transplantation, four with an allogeneic stem cell transplantation, and two patients received a donor lymphocyte infusion. ORR was 38%, 15 patients (6%) achieved ≥very good partial response, and 7 patients (3%) achieved complete response (Table 2). The median time to response was 1.6 months (IQR, 0.9–2.8). ORR was not significantly different between age groups, 48 patients (37%) in patients ≤70 years, versus 33 patients (40%) in patients >70 years (P = 0.68). Median PFS and OS for all patients was 3.6 months (95% CI, 3.1–3.8) and 7.7 months (95% CI, 5.7–9.7), respectively (Figure 1). In patients refractory to lenalidomide, median PFS was 3.5 months (95% CI, 2.8–4.3) versus 3.6 months (95% CI, 2.8–4.3) in patients not refractory to lenalidomide (HR 0.96 [95% CI, 0.73–1.26]; P = 0.77). The corresponding median OS was 7.7 months (95% CI, 5.4–10.5) versus 6.8 months (95% CI, 5.2–9.7), respectively (HR 1.04 [95% CI, 0.78–1.38]; P = 0.79). Patients >70 years had a median PFS of 3.7 months (95% CI, 3.4–5.9) versus 3.3 months (95% CI, 2.7–3.8) in patients ≤70 years (HR 0.89 [95% CI, 0.67–1.18]; P = 0.41). The corresponding median OS was 10.3 months (95% CI, 4.7–11.6) versus 6.8 months (95% CI, 5.4–8.8), respectively (HR 1.00 [95% CI, 0.75–1.35]; P = 0.98; Figure 2). Median PFS in patients diagnosed ≥6 years ago and within 3 years before the initiation of treatment with Pd was 5.7 months (95% CI, 3.5–8.0) versus 2.1 months (95% CI, 1.8–3.3), respectively (HR 0.49 [95% CI, 0.36–0.67]; P < 0.001). Median PFS in patients diagnosed between 3 and 6 years was 3.6 months (95% CI, 3.0–4.4) (HR 0.78 [95% CI, 0.55–1.10]; P = 0.15). The corresponding median OS (95% CI) was 14.0 months (95% CI, 7.7–18.0) and 4.0 months (95% CI, 2.6–5.8), respectively, between patients diagnosed ≥6 years and within 3 years before the initiation of treatment with Pd (HR 0.45 [95% CI, 0.32–0.63]; P < 0.001). Median OS in patients diagnosed between 3 and 6 years was 8.5 months (HR 0.71 [95% CI, 0.50–1.01]; P = 0.058). In a multivariable analysis including gender, age, and time from diagnosis, only time from diagnosis was independently associated with survival (Table 3). Median PFS for patients treated with >3 prior lines and patients treated with ≤ 3 prior lines was identical (3.6 mo [95% CI, 3.2–4.3] versus 3.3 mo [95% CI, 2.3–3.9] [adj HR 1.28 (95% CI, 0.90–1.28); P = 0.77]). For patients achieving ≥PR, median PFS and OS were 10.6 months (95% CI, 8.3–12.9) and 16.3 months (95% CI, 13.6–23.2), respectively. In 72 patients (30%), cyclophosphamide was added to treatment with Pd (PCd). Table 1 shows the characteristics of patients treated with and without the addition of cyclophosphamide. ORR in patients treated with PCd was comparable to patients treated without the addition of cyclophosphamide (Table 2), 28 patients (39%) versus 63 patients (38%) (P = 1.00). Median PFS in patients treated with PCd compared to patients treated with Pd was 5.6 months (95% CI, 3.6–7.9) versus 3.6 months (95% CI, 3.1–3.8) (HR 0.74 [95% CI, 0.55–0.99]; P = 0.046). The corresponding median OS was 8.8 months (95% CI, 6.4–13.2) versus 6.1 months (95% CI, 4.7–9.2) (HR 0.78 [95% CI, 0.57–1.07]; P = 0.20).
| Response | All Patients (n = 237), n (%) | Cyclo (n = 72), n (%) | No Cyclo (n = 165), n (%) |
|---|---|---|---|
| CR | 7 (3) | 2 (3) | 5 (3) |
| VGPR | 8 (3) | 0 (0) | 8 (5) |
| PR | 76 (32) | 26 (36) | 50 (30) |
| SD | 66 (28) | 22 (31) | 44 (27) |
| PD | 54 (23) | 18 (25) | 36 (22) |
| Unknown | 26 (11) | 4 (6) | 22 (13) |
- CR = complete respons; Cyclo = cyclophosphamide; PD = progressive disease; PR = partial response; SD = stable disease; VGPR = very good partial response.
| PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univeriable | Multivariable | Univeriable | Multivariable | |||||||||
| Covariate | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| Gender | ||||||||||||
| Male | 1 | 1 | 1 | |||||||||
| Female | 1.05 | 0.80-1.37 | 0.745 | 0.99 | 0.76-1.29 | 0.970 | 0.94 | 0.71-1.26 | 0.691 | 0.92 | 0.70-1.22 | 0.577 |
| Age, y | ||||||||||||
| ≤70 | 1 | 1 | 1 | |||||||||
| >70 | 0.89 | 0.67-1.18 | 0.408 | 0.85 | 0.65-1.13 | 0.266 | 1.00 | 0.75-1.35 | 0.979 | 0.99 | 0.74-1.32 | 0.921 |
| Time from diagnosis to treatment, y | ||||||||||||
| <3 | 1 | 1 | 1 | |||||||||
| 3–6 | 0.78 | 0.55-1.10 | 0.151 | 0.81 | 0.58-1.12 | 0.198 | 0.71 | 0.50-1.01 | 0.058 | 0.73 | 0.52-1.03 | 0.076 |
| ≥6 | 0.49 | 0.36-0.67 | <0.001 | 0.52 | 0.38-0.70 | <0.001 | 0.45 | 0.32-0.63 | <0.001 | 0.46 | 0.33-0.64 | <0.001 |
- CI = confidence interval; HR = hazard ratio; OS = overall survival; PFS = progression-free survival.
DISCUSSION
Previous trials have shown that Pd is an effective treatment regimen in patients with RRMM, including patients with lenalidomide refractory disease.7-10, 13 This analysis presents real-world data from patients treated with Pd with or without cyclophosphamide in The Netherlands. ORR in our real-world data was 42% which is slightly higher than observed in the MM-002 (ORR = 32.8%), MM-003 (31.4%), and the STRATUS trials (32.6%).7-9 The observed median PFS of 3.6 months was comparable to these trials, while the median OS of 7.7 months was inferior compared to these trials. However, cross-comparison between trials should be interpreted with caution (Table 4).
| Column 1 | ORR (%) | PFS (mo) | OS (mo) |
|---|---|---|---|
| Real-world data | 42 | 3.6 | 7.0 |
| MM-0027 | 32.78 | 4.2 | 16.5 |
| MM-0038 | 31.4 | 4.0 | 12.7 |
| STRATUS9 | 32.6 | 4.6 | 11.9 |
| Kastritis13 | 33 | 5 | 12.1 |
- ORR = overall response rate; OS = overall survival; PFS = progression free survival.
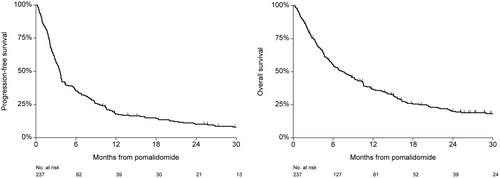
PFS and OS in all patients. OS = overall survival; PFS = progression-free survival.
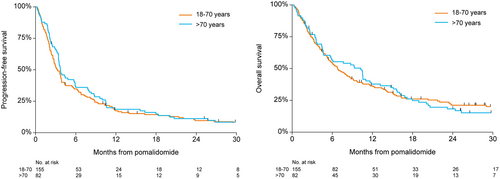
PFS and OS based on age. OS = overall survival; PFS = progression-free survival.
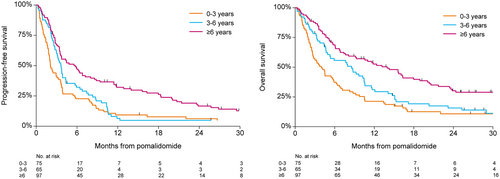
PFS and OS based on time from diagnosis. OS = overall survival; PFS = progression-free survival.
As previously mentioned, most patients were refractory to an IMiD before the start of Pd. Therefore, the question arises if Pd is an effective treatment regimen in IMiD refractory patients. Siegel et al10 showed that treatment with Pd is an effective regimen directly after failure on treatment with lenalidomide. Kastritis13 performed an analysis in patients from Greece who were treated with Pd in the real world to evaluate the impact of the last lenalidomide treatment. In their cohort, PFS and OS were 5.0 and 12.1 months, respectively, including patients who received lenalidomide just before treatment with Pd. However, PFS and OS improved to 10.3 and 27.1 months, respectively, in patients with an IMiD-free interval of ≥18 months. Nonetheless, these data confirm that Pd is an effective treatment in patients previously treated with an IMiD. Our data are similar to these results with no difference in PFS between IMiD refractory patients versus IMiD nonrefractory patients.
In our cohort, we attempted to identify subpopulations who would benefit most from treatment with Pd. We looked at age and duration of the disease. Cytogenetic evaluation was available in only a small subset of patients and therefore not included in the analysis. Time from diagnosis was significantly associated with PFS and OS, in favor of patients diagnosed ≥6 years before initiation of Pd compared to patients treated within 3 years from diagnosis (Figure 3). Probably, these latter patients had a more aggressive MM with short responses to different treatment modalities. This analysis suggests that patients with a longer interval from diagnosis are more likely to benefit from treatment with Pd.
Previous trials showed that the addition of a third agent improves response and survival.16-21 The REPEAT trial showed that the addition of cyclophosphamide to lenalidomide and dexamethasone improves response and survival.21 Baz et al16 performed a phase 2 study randomizing patients with lenalidomide refractory disease to treatment with Pd or PCd, which showed a difference in PFS of 4.4 versus 9.5 months. Therefore, it is an attractive option to add cyclophosphamide to treatment with Pd to improve efficacy. In our real-world data, a subset of patients was treated with PCd. ORR was not different in patients treated with Pd or PCd. However, PFS and OS improved by adding cyclophosphamide to treatment with Pd, which we expected based on data from previous trials. It should be kept in mind that this analysis was not designed to retrieve information to answer this specific question. From our series, we could not extract data to explain why cyclophosphamide was added to Pd. Data concerning the extent and severity of other comorbidities were not available in our database. Generally, the decision to add cyclophosphamide in individual patients usually is based on expectations regarding disease activity, tolerance, and other patient-related factors.
Recently, several phase 3 trials showed an improvement in response and survival by adding a third treatment modality to Pd, other than cyclophosphamide.18-20 In the ICARIA trial, isatuximab was added to treatment with Pd and showed an improvement in PFS of 6.5–11.5 months compared to patients treated with Pd.18 The OPTIMISMM trial showed a comparable improvement in response by adding bortezomib to Pd. PFS was improved from 7.1 to 11.2 months.19 In the APOLLO trial, daratumumab was added to Pd, which also improved PFS of 6.9–12.4 months compared to treatment with Pd.20 These data show that, concerning PFS, patients should preferably be treated with a third agent added to the backbone Pd. In pretreated patients refractory to a PI or anti-CD38 treatment, the addition of cyclophosphamide may be an attractive option. In conclusion, this nationwide, population-based study confirms data observed in key clinical trials. The lower OS probably reflects the heterogeneity of patients treated in the ‘real-world’ versus patients included in “clinical trials.” Therefore, it is reasonable to assume that survival rates in these real-world patients present a more realistic view.22 The addition of cyclophosphamide did improve PFS and OS, as shown in previous trials.
This analysis confirms the effectiveness of treatment with Pd or PCd in heavily pretreated patients considered not eligible for inclusion in clinical trials. Moreover, it is an affordable and available treatment modality in many countries.
DISCLOSURES
RW received honoraria from Sanofi and Janssen. SZ received advisory from Takeda, Janssen, BMS-Celgene, Sanofi, Oncopeptides, and Amgen; and research funding from Takeda and Janssen. MM received consultancy from Janssen, Cilag, Gilead, Alnylam; and speaker of BMS, Hospitality BMS. EdW received honoraria from Celgene en Janssen. PS received research support from Amgen, BMS/Celgene, Janssen, Karyopharm, and SkylineDx; and advisory/honoraria from Amgen, BMS/Celgene, Carsgen, GSK, Janssen, Karyopharm, Seagen, and SkylineDx. AB received honoraria/advisory from BMS, Sanofi, Janssen, and Amgen. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
Support by Netherlands Comprehensive Cancer Organisation, The Haemato Oncology Foundation for Adults in the Netherlands (HOVON), and Dutch Cancer Foundation.