Germ-Line TP53 Mutation in an Adolescent With CMML/Atypical CML and Familiar Cancer Predisposition
The authors declare no conflicts of interest relevant to this study.
Supplemental Digital Content is available for this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.hemaspherejournal.com).
Abstract
Supplemental Digital Content is available in the text
Atypical chronic myeloid leukemia (aCML) and chronic myelomonocytic leukemia (CMML) are rare hematological malignancies, belonging to the group of translocation t(9;22) negative myelodysplastic syndromes/myeloproliferative neoplasms (MDS/MPN). In adult patients, aCML incidence is around 1% to 2% of total CML cases (the vast majority carrying t(9;22)); whereas it is less common in childhood.1 CMML incidence is 4/100,000 with median age at diagnosis of 75 years and extremely rare in pediatric patients.2 In the 2016 WHO classification, aCML is defined by persistent leukocytosis (≥13 × 109/L) with immature circulating myeloid precursors (≥10% leukocytes), marked dysgranulopoiesis, and absent/minimal monocytosis (<10% leukocytes) or basophilia (often <2%).3 In the same classification, CMML is defined a clonal hematopoietic stem cell disorder characterized by persistent peripheral blood monocytosis (>1 × 109/L) with monocytes accounting for >10% of WBC, with normal or abnormal morphology, circulating promonocytes being the blast equivalent.4, 5 The distinction between the aCML and CMML is not always obvious, both from clinical and biological standpoints, as they share several pro-oncogenic pathways (eg, RAS/MEK signaling, ASXL1, TET2, and EZH2 epigenetic pathways).6
We present here the case of a 15-year old boy of Filipinos origin who came to our attention for diffuse and persistent lymphadenopathy and referred to as patient #1 henceforward.
Physical examination revealed diffuse palpable stiff lymphadenopathy, mainly cervical and inguinal (maximum size 5 cm), hepatomegaly (7 cm below costal margin) and splenomegaly (6 cm below costal margin) with night sweats but without fever. Hyperleukocytosis (white blood cell count –WBC- 110 × 109/L) with 16% monocytes, 17% metamyelocytes and 5% blasts on peripheral blood smear (Fig. 1A), mild anemia (Hb 11.2 g/dl), thrombocytopenia (75 × 109/L), and consistent biochemistry with hyperuricemia (9.2 mg/dL) and elevated LDH (1199U/L) were detected, in the absence of fetal hemoglobin (<1%).
Bone marrow showed myeloid hyperplasia, with left-shifted myeloid maturation, compatible with CML diagnosis. It also showed monocytic hyperplasia (immunohistochemistry CD68+, CD34−). No blasts were detected in bone marrow. Cytogenetics revealed the presence of t(1;4)(q21;q31) and t(6;17)(q23;q25). The absence of t(9;22) rearrangements and BCR-ABL fusion transcript (by FISH and RT-qPCR) allowed to exclude the diagnosis of typical CML. Moreover, the absence of PDGFR-A or -B mutations and STAT5 hyper-phosphorylation ruled out other myelodysplastic or myeloproliferative disorders. On these bases, according to the WHO 2016 criteria, the patient was diagnosed with CMML/aCML spectrum disease, more likely a form of pediatric CMML-2.1
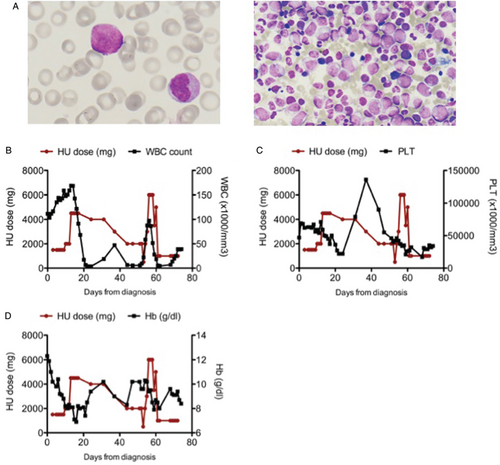
(A) Blast morphology on peripheral blood smear (left) and bone marrow (right). May Grunvald Giemsa staining. 100× magnification (B) WBC count in relation to HU dosage. The graph shows a partial resistance of the disease to HU and explains the need to increase HU dosage. (C) HU dosage in relation to PLT count. (D) HU dosage in relation to Hb levels. Toxicity of high dose of HU can be appreciated on both PLT and Hb.
The patient was initially treated with hydroxyurea (HU), 20 mg/kg, subsequently increased up to 85 mg/kg with good, though transient, response (Fig. 1B). The onset of severe thrombocytopenia (<20 × 109/L) led to HU discontinuation, with rapid WBC increase (Fig. 1B–D). HU treatment was resumed, at the dose of 33 mg/kg, subsequently increased up to 99 mg/kg, with a clinical benefit7 in terms of hematological cell counts and patient symptoms. However, bone marrow biopsy showed persistence of myeloid hyperplasia. In order to achieve control of the disease, the patient underwent hematopoietic stem cell transplantation (HSCT) from his HLA-identical brother after fully myeloablative conditioning. After transplant, he developed severe hepatic graft-vs-host-disease, which required prolonged immunosuppressive treatment, and died 7 months after transplantation from infectious complications (cerebral aspergilloma).
No mutation was detected in the most frequently associated genes with CMML or aCML (i.e., csf3r, etnk1, c-kit, setbp1).8, 9 Therefore, in the attempt of better classifying the disease and understanding its biology, we performed targeted RNA sequencing on the diagnostic sample by means of the Trusight PanCancer Next Generation Sequencing panel (Illumina), composed of 1385 genes frequently involved in cancer and leukemia. We detected a novel fusion transcript involving MCL1-GCFC2 genes, involving chromosome 1 and 2, different from the one identified by cytogenetic analysis. However, we hypothesize the existence of a complex fusion involving chromosome 1, 2 (2 (likely an insertion of a small region) and 4 which is not detectable with conventional cytogenetic analysis.
Among variants in the target genes, only the TP53 variant c.344A>G, p.His115Arg (rs730881996) was reported as exceedingly rare in population databases (no minor allele frequency reported in dbSNP nor in ExAC. Variant allele frequency is 0.36). The presence of the p.His115Arg variant, located in the DNA binding domain of TP53, was confirmed by PCR and Sanger sequencing on the diagnostic DNA of our patient (Fig. 2A). Notably, mutation p.His115Arg causes a significant decrease in TP53 DNA binding affinity, affecting in particular p21 activation.10 TP53 p.His115Arg, though currently considered as a variant of uncertain significance (ClinVar; https://www.ncbi.nlm.nih.gov/clinvar/40497456/), has been hypothesized to be causative for Li-Fraumeni syndrome.11 Additional somatic variants are reported in Supplementary data, http://links.lww.com/HS/A92.
Interestingly, 6 years before, a 15 year-old first degree cousin of patient #1 had been diagnosed with t(8;21) positive acute myeloid leukemia (AML). The boy, henceforward referred to as patient #2, had been treated with standard chemotherapy, without bone marrow transplantation, and maintains complete remission. Following acquisition of an informed consent, the same germline variant TP53 rs730881996 was identified by mean of Sanger sequencing on patient #2 (Fig. 2B and C). Notably, the same heterozygous variant was detected on patient #2 father (henceforth patient #3), who subsequently developed both a follicular lymphoma and an intra-pancreatic mucinous neoplasm and on patient #1 father (the 2 men are brothers), henceforth indicated as patient #4, with silent medical history (Fig. 2B). Unfortunately, also due to geographical reasons, it was not possible to obtain neither a detailed family history nor additional DNA samples. Family history has been summarized in Figure 2C. At this time, no tests for TP53 mutations have been performed on sister of patient #2 and brother of patient #1, who served as a HSCT donor, who are currently healthy.
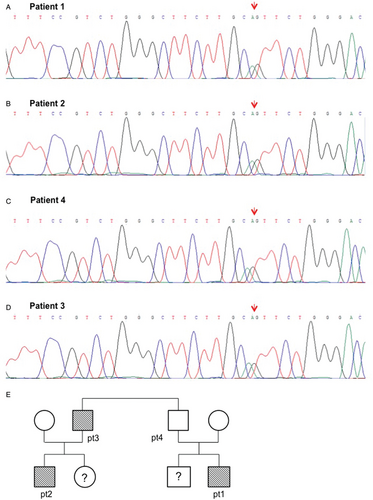
Sanger sequencing validation of TP53 mutation detected in patient #1 (A) and #2 (B), in father of patient 1- namely patient 4 (C) and patient #3 (D). (E) Available family tree of our patients.
All together, these findings suggest that the germline TP53 p.His115Arg mutation increased the risk of hematological malignancies in this family, and may represent the phenotypic expression of a hereditary cancer-predisposing syndrome.
This TP53 mutation is classified as of uncertain clinical significance. However, the association of this mutation with malignancies in this family is highly suggestive for a Li-Fraumeni-like phenotype. Although Li-Fraumeni syndrome is not typically associated to clonal blood disorders, there are consistent reports indicating that it may cause a genetic predisposition to hematologic malignancies (notably myelodysplasia or hypodiploid acute lymphoblastic leukemia).12, 13
Besides being important in leukemogenesis, the role of the identified mutation on DNA damage response could also explain the reduced sensitivity to HU therapy in patient #1. HU specifically acts on the S-phase of the cell cycle by inhibiting the enzyme ribonucleoside diphosphate reductase, resulting in impaired reductive conversion of ribonucleotides to deoxyribonucleotides and thus limiting de novo DNA synthesis.14, 15 Consequently, HU can induce DNA double-strand breaks by causing replication fork arrest upon nucleotide pool depletion. Effective DNA repair is crucial in order to kill tumor cells with HU. Consistently, cell lines that harbor Fanconi Anemia mutations (ie, DNA repair mutations) are often resistant to HU treatment.16
In conclusion, we described here a peculiar case of unclassifiable MDS/MPN which is however suggestive of CMML/atypical CML. We hypothesize that the occurrence of his leukemia was related to a germline TP53 mutation, which was also found in his first grade cousin, who developed AML, in his uncle, who developed follicular lymphoma and intra-pancreatic mucinous neoplasm, and his father, who is at present clinically silent, thus indicating an incomplete penetrance.
To our knowledge, this case is unique since it is in between CMML and atypical CML in the context of a novel mutation likely associated with a Li-Fraumeni syndrome and hematological cancer predisposition.
Present findings underline the role of an extensive investigation of the family history and highlight the contribution of NGS in unusual hematological diseases, as well as the difficulties of interpretation of mutations classified as of uncertain significance in a clinical setting. Concerning cancer predisposition, it also poses the question whether healthy relatives, if HSCT donor, should be tested for TP53 mutation and eventually included in Li Fraumeni programs.
Supplementary Figure 1, http://links.lww.com/HS/A93
Sources of Funding
This research was supported by Comitato Maria Letizia Verga (program ‘Passaporto genetico’), by the Associazione Italiana per la Ricerca sul Cancro (AIRC, grant IG2018-ID 21999 to GC), by the Associazione “La Vale c’è”, SR is the recipient of a fellowship by Associazione “Beat Leukemia” (www.beat-leukemia.org).
Author Contributions
Silvia Nucera and Grazia Fazio have equal contribution as first author. Gianni Cazzaniga and Adriana Balduzzi have equal contribution as senior author.