Expression of Angiogenic Growth Factors in Paragangliomas†
Financial support for this research was provided by grants from the American Cancer Society and the National Organization for Hearing Research to Dr. Jyung and the National Institutes of Health to Dr. Tuan. Dr. LeClair is the recipient of an institutional National Institutes of Health training grant (AR07583) and an individual National Research Service Award (HD08368).
Abstract
Objective/Hypothesis: To determine if angiogenic growth factors including vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PD-ECGF) are expressed in human paragangliomas.
Study Design: A histopathologic and molecular examination of paraganglioma specimens obtained from surgical cases or retrieved from the Pathology Department of the Massachusetts Eye and Ear Infirmary.
Methods: Fresh tumor or archival, paraffin-embedded paraganglioma specimens were analyzed by immunohistochemistry, Western blotting, and ELISA.
Results: Positive immunohistochemical staining for VEGF was observed in five of nine surgical specimens and in six of eight archival specimens (11/17, or 65%). PD-ECGF immunoreactivity was detected in four of five surgical specimens and six of eight archival specimens (10/13, or 77%). The presence of PD-ECGF was confirmed by Western blot assay and ELISA confirmed the presence of VEGF in tumor extract.
Conclusions: Both VEGF and PD-ECGF are expressed in paragangliomas and may contribute to the extreme vascularity of these tumors.
INTRODUCTION
Paragangliomas, commonly known as glomus tumors, are highly vascular neoplasms arising from paraganglia, which are derived from the embryonic neural crest. The distribution of paraganglia indicates that these tumors can develop at the carotid bifurcation (carotid body tumor), in the adventitia of the jugular bulb (glomus jugulare), in the no-dose ganglion of the vagus nerve (glomus vagale), in the middle ear (glomus tympanicum), as well as in other regions, including the nasal cavity, orbit, and larynx. A distinguishing feature of these tumors is their extreme vascularity, which produces a characteristic “blush” on angiography and multiple serpiginous flow voids on magnetic resonance imaging.1 During surgical resection of these tumors, significant blood loss usually occurs unless preoperative embolization is performed. Histologically, clusters of tumor cells (zellballen) are invested in a highly vascular stroma containing capillary-sized blood vessels. The mechanism by which paragangliomas become so highly vascularized, however, has not yet been determined.
Angiogenesis, defined as the sprouting and development of new blood vessels from established vasculature, was recognized long ago as a critical step in the progression of solid tumors.2 To sustain the neovascularization process, many types of tumors synthesize angiogenic growth factors. Vascular endothelial growth factor (VEGF) appears to be one of the most important signals in tumor angiogenesis, having been identified in glioblastoma,3 renal,4 colon,5 and breast carcinoma,6 as well as Kaposi's sarcoma.7 The fact that VEGF mediates tumor angiogenesis is not surprising, given its central role in physiological angiogenesis and vascular development in the embryo.8 VEGF, which was first identified as vascular permeability factor (VPF),9 is also important in the angiogenesis of non-neoplastic lesions such as rheumatoid arthritis,10 as well as the retinal neovascularization of diabetes.11 VEGF exerts its biological effect by interacting with its cell surface receptors, flt-1 and flk-1/KDR.12-14 VEGF induces proliferation and migration of endothelial cells through the flk-1/KDR receptor, while it stimulates organization of endothelial cells into tubules through the flt-1 receptor.15
Platelet-derived endothelial cell growth factor is another potent angiogenic factor that has been implicated in the neovascularization of lung,16 breast,17 colon,18 gastric,19 and bladder carcinomas.20 It is distinguished from other angiogenic factors by its identity with the enzyme thymidine phosphorylase (dThdPase) and the lack of a hydrophobic leader sequence, suggesting that it is not secreted through the classic endoplasmic reticulum pathway.21, 22 The mechanism by which dThdPase confers angiogenic activity to PD-ECGF is not fully understood but may be partly explained by its chemotactic activity for endothelial cells.21
In this study, we hypothesized that growth factor-mediated mechanisms were involved in the neovascularization of paragangliomas and that specific growth factors should be identifiable in tumor specimens. Recently, it has been demonstrated that VEGF and one of its receptors (flk-1, or KDR) are upregulated in association with the marked angiogenesis and hyperplasia of the rat carotid body after chronic hypoxia.23 We hypothesized that a similar mechanism was responsible for the extreme vascularity of paragangliomas. We first examined the tumor specimens by immunohistochemistry and then verified the expression of growth factors at the protein level with Western immunoblotting and ELISA. Immunohistochemical staining was initially carried out on formalin-fixed, paraffin-embedded paraganglioma specimens using antibodies directed against VEGF and its receptors, PD-ECGF, as well as other angiogenic factors including basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1), and angiogenin. Because our initial immunohistochemical results showed positive staining for VEGF and PD-ECGF, we focused our further studies on these two factors.
MATERIALS AND METHODS
Immunohistochemistry
Nine tumor specimens were obtained at the time of surgical resection, fixed for 24 hours in 10% buffered formalin, and then embedded in paraffin. An additional eight archival formalinfixed, paraffin-embedded specimens were obtained from the Massachusetts Eye and Ear Infirmary/Massachusetts General Hospital. Sections were cut at 6-μm thickness and mounted on Superfrost Plus slides (Baxter Healthcare, Glendale, CA). Table I shows the distribution of specimens according to site.
To a varying degree, specimens displayed the characteristic organoid clustering of type I (chief) and type II (sustentacular) tumor cells into zellballen. The majority of specimens were confirmed to be paragangliomas based on immunostaining for chromogranin (a marker of type I/chief cells) and S-100 (a marker for type II/sustentacular cells), based on protocols used in the Department of Pathology at Massachusetts General Hospital.
Immunohistochemical staining was performed according to a biotinylated tyramine amplification protocol published previously.24 Briefly, sections were deparaffinized and rehydrated, treated with 3% hydrogen peroxide to quench endogenous peroxidases, incubated in 5% normal horse serum to block nonspecific binding sites, and then incubated with the primary antibodies, using relatively dilute titers as allowed by the amplification protocol.
The following antibodies and concentrations were used: anti-VEGF, 1:5 K (polyclonal rabbit antihuman, Santa Cruz Biotechnology, Santa Cruz, CA), anti-VEGF, 1:5 K (polyclonal goat antihuman, R&D Systems, Minneapolis, MN), anti-flt-1, 1:8 K (polyclonal rabbit antihuman, Santa Cruz Biotechnology), anti-KDR/flk-1, 1:8 K (polyclonal rabbit antihuman, Santa Cruz Biotechnology), anti-PD-ECGF, 1:8 K, (polyclonal goat antihuman, R&D Systems), anti-bFGF, 1:5 K (monoclonal mouse antibovine, Upstate Biotechnology, Lake Placid, NY), anti-PDGF-AB, 1:32 K (polyclonal rabbit antihuman, Upstate Biotechnology), anti-von Willebrand factor, 1:32 K (polyclonal rabbit antihuman, Dako, Carpinteria, CA), anti-TGF-β1, 1:4 K (polyclonal rabbit antihuman, a generous gift of Dr. Kathleen Flanders, Laboratory of Chemoprevention, National Institutes of Health), and anti-β1-LAP, 1:10 K (polyclonal goat antihuman, R&D Systems). All dilutions were made in phosphate-buffered saline (PBS), pH 7.4.
Biotinylated secondary antibodies directed against the species of the primary antibodies were applied to the sections, followed by incubation with avidin-biotin-horseradish peroxidase (Standard ABC kit, Vector, Burlingame, CA). For the amplification step, sections were exposed to a solution of approximately 7 μmol/L biotinylated tyramine/0.01% hydrogen peroxide for 10 minutes and then re-incubated with ABC for 1 hour. Finally, the immunostaining reaction was visualized with 0.05% 3,3-diaminobenzidine in 0.1 mol/L PBS containing 0.01% hydrogen peroxide.
Positive controls for VEGF included normal ovarian tissue as well as diseased thyroid tissue (Grave's disease).25, 26 Negative controls consisted of tumor sections incubated with PBS only. For VEGF immunostaining, preadsorption experiments using a 10-fold molar excess of recombinant human VEGF165 peptide (R&D Systems) were performed on selected specimens to determine if the positive stain could be abolished by exposure of the primary antibody to purified antigen. As an additional control, sections from a reported “avascular” paraganglioma were immunostained to determine if there was a gross difference in angiogenic growth factor expression between typical tumor specimens and a relatively avascular tumor.27
Protein Extraction
Paragangliomas and adjacent normal tissues were pulverized under liquid nitrogen and extracted by overnight agitation in cold (4°C) lysis buffer (50 mmol/L Tris-buffered saline, pH 7.6, 1% Triton-X 100, 1% NP-40, and protease inhibitors including phenylmethylsulfonyl fluoride, aminocaproic acid, and benzamidine-HCl). After centrifugation to remove debris, protein concentration in the supernatant was estimated by BCA assay (Pierce Co., Rockford, IL) and aliquots were stored at −80°C.
Western Blotting
To detect VEGF and PD-ECGF, 100 μg of total protein extract (from paraganglioma or normal tissue) was fractionated by SDS polyacrylamide gel electrophoresis (4% stacking gel and 10% resolving gel), followed by electroblotting onto nitrocellulose. As a positive control, 100 ng of recombinant human protein (VEGF or PD-ECGF) was loaded in a separate lane. To detect VEGF, blots were probed with polyclonal rabbit antihuman VEGF antibodies (1:2500, Santa Cruz Biotechnology) followed by alkaline phosphatase-conjugated goat antirabbit immunoglobulin G. To detect PD-ECGF, separate blots were probed with goat antihuman PD-ECGF antibodies (1:5000, R&D Systems) followed by alkaline phosphatase-conjugated rabbit antigoat immunoglobulin G. Alkaline phosphatase enzyme detection was carried out by incubation in NBT/BCIP substrate solution (Zymed, South San Francisco, CA).
ELISA
Protein extracts were further analyzed for VEGF levels using a commercial ELISA kit designed to detect picogram quantities of this protein (Quantikine VEGF kit, R&D Systems). Supernatants from both paragangliomas and normal tissue were diluted to an equivalent total protein concentration and assayed according to the manufacturer's instructions. A standard dilution of recombinant human VEGF was included each time, as was a no-enzyme-conjugate negative control. Absorbance measurements at 450 nm were converted into picograms of VEGF. The entire assay was replicated on three separate occasions using different aliquots of the same protein extracts.
RESULTS
Immunohistochemistry
Antibodies directed against VEGF and PD-ECGF produced immunostaining patterns that were reproducible between specimens and appeared to localize to specific cell types. Immunostaining for other angiogenic growth factors, including bFGF, PDGF, TGF-β1, and angiogenin, did not yield positive results. Although the tumor site did not clearly influence the degree of immunoreactivity, staining patterns could be more readily discerned in larger specimens (carotid body and glomus vagale tumors) in which the zellballen cytoarchitecture was more apparent.
Vascular endothelial growth factor immunoreactivity was detected in five of nine surgical specimens and six of eight archival specimens (11/17, or 65%). VEGF immunoreactivity was largely localized to the cytoplasm of type I or chief cells, highlighting the zellballen architecture of immunostained specimens (Fig. 1A). In many type I cells, the immunoreactivity was quite intense, highlighting the vacuolated cytoplasm of these cells. This finding was readily apparent in specimens that had not been embolized previously, because the cytoarchitecture was preserved. Within a given specimen, the level of VEGF immunoreactivity was variable, with some regions of the tumor being highly stained and other regions being only faintly reactive. Regardless of the level of staining, VEGF immunoreactivity was localized to the type I cells. No obvious gradient of VEGF immunoreactivity was seen from the periphery of the tumor to its core; however, the regions highly reactive for VEGF were usually in the periphery of the specimen, near the tumor capsule. In some specimens, VEGF immunoreactivity was observed within vessels of the tumor capsule, particularly in the smooth muscle cells of the vessel media (Fig. 1C). This finding is consistent with the known capacity of smooth muscle cells to synthesize VEGF.28 In embolized specimens, cytoarchitecture was significantly distorted, with diffuse areas of necrosis manifested by pyknotic type I cell nuclei; embolization material was observed extending throughout the tumor microvasculature. Even in these specimens, however, faint VEGF immunoreactivity was discerned throughout the remnants of the type I cells (not shown). When primary antibodies for VEGF were preadsorbed with purified VEGF antigen, clear reduction in immunoreactivity was observed, although it was not completely abolished (not shown).
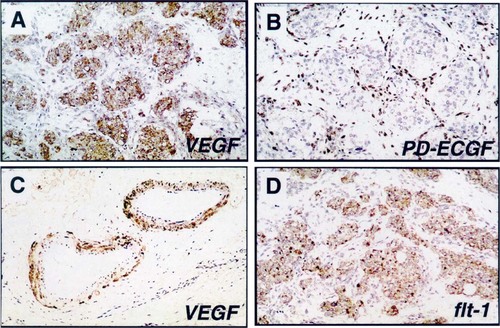
Immunostaining of paraganglioma specimens. A. Vascular endothelial growth factor (VEGF) staining highlights type I cells, which show the characteristic zellballen architecture. B. Platelet-derived endothelial cell growth factor (PD-ECGF) was localized to the fibrovascular stroma surrounding the zellballen clusters. C. VEGF was also detected in the smooth muscle layer lining tumor vessels. D. Flt-1 immunoreactivity was also observed in some type I cells.
Immunoreactivity for PD-ECGF was observed in four of five surgical specimens and six of eight archival specimens (10/13, or 77%). In contrast to VEGF, PD-ECGF immunoreactivity was localized to cells within the fibrovascular stroma (Fig. 1B). PD-ECGF-immunoreactive cells were easily distinguished from the VEGF-immunoreactive type I cells, and no cell types appeared to be positive for both growth factors. Likewise, the cell population immunoreactive for PD-ECGF appeared to be distinct from the S-100+, type II cells, because PD-ECGF-reactive cells were largely outside of the zellballen clusters.
Immunoreactivity for the VEGF receptor flt-1 was observed in five of six surgical specimens, primarily localized to the type I cells, as seen with VEGF immunostaining, particularly in nonembolized specimens (Fig. 1D). Flt-1 immunoreactivity was also observed within some peripheral tumor vessels but was not observed within most of the tumor microvasculature. In contrast, flk-1/KDR immunoreactivity was not observed in any of the specimens, even within the tumor vessels. This did not appear to be related to a general lack of antigen preservation, because factor VIII (von Willebrand factor) antigenicity, a useful marker of endothelial cells, was apparent in almost all specimens.
Interestingly, the single avascular paraganglioma case demonstrated similar immunoreactivity patterns that were observed in typical tumor cases, with VEGF immunoreactivity localized to the type I cells and PD-ECGF immunoreactivity localized to stromal cells in the fibrovascular tissue. Type I cells also demonstrated flt-1 receptor immunoreactivity in this specimen.
Western Blotting
Having noted significant immunostaining for VEGF and PD-ECGF in our tissue sections, we next sought to confirm these results by Western blotting of paraganglioma protein extracts. Using antihuman PD-ECGF antibodies, we were able to colorimetrically detect a strong band running at approximately 65 kD, the approximate molecular weight of the PD-ECGF standard (Fig. 3). In a similar assay, antihuman VEGF antibodies showed no visible band migrating at 23 kD, although the recombinant protein was easily observed.
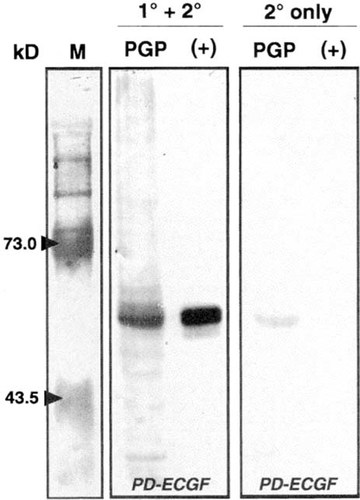
Western blotting of paraganglioma extracts. The two right panels show tumor protein extracts (PGP) next to recombinant human PD-ECGF (+). The blots were probed with antihuman PD-ECGF primary antibodies followed by secondary antibody detection as described in Materials and Methods (1° and 2°) or with omission of secondary antibodies (2° only) as a control. PD-ECGF was clearly detected in the tumor protein extract, with only background staining seen in the control. The far left panel contains molecular weight markers with relevant sizes indicated in kilodaltons.
ELISA
We were subsequently able to detect a strong VEGF enrichment in paragangliomas using a more sensitive immunodetection method. When equal amounts of protein from a thymic paraganglioma and adjacent normal thymus tissue from the same patient were compared, the tumor tissue showed a strong positive color reaction, whereas the normal tissue showed only background levels (Fig. 2A). By comparing these colorimetric levels with a known standard, we were able to quantify a nearly 80-fold enrichment of VEGF protein in the tumor tissue compared with the adjacent normal cells (Fig. 2B).
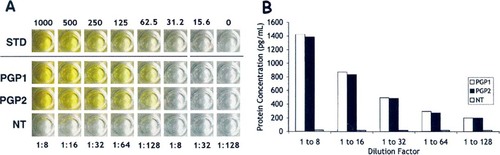
ELISA for VEGF in paragangliomas. A. Colorimetric detection of VEGF protein (yellow product). STD = recombinant VEGF protein standards; PGP1 and PGP2 = paraganglioma protein extract; NT = adjacent normal tissue extract. B. Quantitative comparison of VEGF levels in duplicate lanes of the tumor sample (PGP1 and PGP2) and in adjacent normal tissue (NT).
DISCUSSION
The angiogenic nature of paragangliomas is a central issue in the pathophysiology of these lesions, and ultimately it dictates many aspects of their management. It is well known that surgical treatment of these tumors can be associated with significant blood loss, usually mandating preoperative angiography and embolization, which imposes additional risks to the patient. Radiation therapy results in a variable arrest of tumor growth that may reflect a temporary blunting of the angiogenesis within these lesions. It has been shown that radiation therapy does not eradicate the tumor cells and probably exerts its major effect by inducing a vasculitis within tumor vessels.29 Pharmacologic inhibition of angiogenesis is a potential treatment modality for these tumors that could provide a valuable alternative or adjunct to surgery or radiation.
Our findings indicate that VEGF and PD-ECGF may be critical angiogenic factors responsible for the extreme vascularity of paragangliomas. Immunohistochemical colocalization of these factors has been shown in other tumors such as breast carcinoma30, 31 and has also been described in the physiological angiogenesis of the placenta.32 While one growth factor may exert the predominant angiogenic effect, the simultaneous synthesis of multiple growth factors may be a strategy utilized by tumors to ensure an optimal angiogenic response from the host tissue.33 An additive or synergistic effect between VEGF and PD-ECGF might occur, because these factors operate through distinct mechanisms. VEGF, an endothelial mitogen, is secreted and interacts with its cell-surface receptors; in contrast, PD-ECGF is not secreted by the classic endoplasmic reticulum pathway and possesses dThdPase activity, which is essential for its angiogenic effects.22 Exactly how dThdPase elicits an angiogenic response is not known, because it is not an endothelial mitogen. However, the mechanism may involve endothelial cell chemotaxis or angiogenic by-products of enzymatic activity.21, 22 Recently, co-expression of VEGF and PD-ECGF has been demonstrated in squamous cell carcinoma of the head and neck by investigators who questioned the angiogenic role of PD-ECGF in squamous cell carcinoma, because PD-ECGF immunoreactivity did not correlate with microvessel density, whereas VEGF staining did.34
Vascular endothelial growth factor has been implicated in the neovascularization of many solid tumor types, and its presence in paragangliomas is therefore not surprising. The cytosolic pattern of VEGF immunoreactivity we observed in paragangliomas is consistent with VEGF staining patterns seen in other tumors.34 Interestingly, VEGF immunoreactivity was not accompanied by clear flk-1/KDR immunoreactivity, while flt-1 staining was observed in type I cells and occasional tumor vessels. The finding of flt-1 immunoreactivity in type I tumor cells is intriguing, because very few tumor types have been shown to express this receptor.28 However, in a transgenic mouse model of glioblastoma multiforme, neoplastic astrocytes express both VEGF and the flt-1 receptor, suggesting the presence of an autocrine loop.35 If it were established that type I paraganglioma cells truly express the flt-1 receptor, a potential autocrine growth mechanism using VEGF would exist, with pathological angiogenesis possibly occurring as a by-product. Immunoreactivity for flt-1 without any detectable flk-1/KDR in tumor specimens was not unexpected, given the fundamentally different signal transduction properties of these two receptors.36, 37 The upregulation of flk-1/KDR observed in the carotid bodies of hypoxic rats23 might reflect VEGF-mediated proliferation and migration of endothelial cells, whereas flt-1 immunoreactivity seen in human paragangliomas might reflect maintenance of more established vasculature by VEGF signaling from tumor cells.
The immunoreactivity for PD-ECGF in paragangliomas is consistent with patterns of expression in normal tissues as well as in other tumor types; specifically, stromal cells and macrophages are strongly immunoreactive in organs such as the thyroid and prostate glands, the kidneys, and the colon.38 The proportion of PD-ECGF-immunoreactive stromal cells in paragangliomas that were macrophages was not demonstrated in this study but could be determined in the future by double-label immunohistochemical studies.
Regarding our immunohistochemical findings of VEGF, PD-ECGF, and flt-1 in the “avascular” paraganglioma specimen, it is important to recognize that this specimen was deemed avascular by its angiographic appearance, but its appearance on magnetic resonance imaging revealed strong contrast enhancement.27 Strong enhancement on magnetic resonance imaging suggests some degree of neovascularization, and therefore our findings in this specimen do not necessarily contradict our hypothesis.
We did attempt to verify expression of VEGF and PD-ECGF at the mRNA level using in situ hybridization. However, many specimens were archival and therefore were not processed optimally for RNA preservation, likely accounting for the absence of hybridization signal in many of the specimens. Using a radiolabeled probe for VEGF mRNA, we found that two specimens did demonstrate a clear concentration of silver grains over the zellballen. Our inability to detect VEGF by Western blotting while observing significant reactivity by ELISA most likely resulted from cross-linking of VEGF to high molecular weight extracellular matrix molecules.
Both genetic and environmental factors contribute to the development of paragangliomas. In sporadic cases, the development of these tumors may involve the combination of environmental influences in a genetic background that is permissive for tumor growth. However, in familial cases in which individuals develop multiple paragangliomas, the genetic defect by itself may be sufficient for tumor growth. Although the causative gene for familial paragangliomas has been localized to the long arm of chromosome 11, the identity of this gene and the molecular mechanism for the genesis of these tumors are still unknown.39 It is tempting to speculate whether the genetic mechanism underlying familial paragangliomas might be similar to von Hippel-Lindau disease, in which a protein that negatively regulates hypoxia-inducible genes is inactivated.40 Tumors that arise in von Hippel-Lindau disease are hypervascular and overexpress a hypoxia-inducible angiogenic growth factor, VEGF.41
Hypoxia is an important environmental stimulus that has been implicated in the development of paragangliomas of the carotid and aortic bodies in animals.42, 43 In humans, hyperplasia of the carotid body occurs in patients with hypoxia secondary to high altitude, emphysema, cyanotic congenital heart disease, or cystic fibrosis.44-46 In addition, carotid body tumors occur 10 times more frequently in populations living at high altitudes compared with populations at sea level.47 Hypoxia may also be a predisposing factor in patients with chronic obstructive pulmonary disease.48 Because hypoxia is known to stimulate synthesis of VEGF and PD-ECGF, expression of these hypoxia-inducible, angiogenic growth factors by tumor cells could explain the influence of hypoxia on paraganglioma development.49, 50
The influence of estrogens is another environmental factor that may accelerate the development of paragangliomas. Carotid body tumors in high-altitude populations show a marked female preponderance, as high as 12 to 1,47 and even at sea level, the predominance of carotid body tumors in women in still apparent, at a ratio of 2 to 1.51 This suggests an interaction between hypoxic and estrogenic factors in the development of paragangliomas. This interaction might occur at the level of VEGF or PD-ECGF synthesis, both of which are regulated by hypoxia as well as estrogen levels.50, 52-54 Finally, oncogene expression in paragangliomas may also stimulate “downstream” signals such as VEGF or PD-ECGF.55
CONCLUSION
We have demonstrated the presence of VEGF and PD-ECGF in paraganglioma specimens. Expression of these angiogenic growth factors may explain the highly vascular nature of these tumors and may provide a mechanism to facilitate understanding of the environmental and genetic influences on these interesting neoplasms. An animal model for paraganglioma growth is still unavailable but would be very useful for determining the biological importance of VEGF and PD-ECGF expression in these tumors. Ultimately, VEGF and PD-ECGF expression by paragangliomas might be exploited as a novel method of treatment, using anti-angiogenic strategies. Clinicopathologic correlation will be examined to determine if angiogenic growth factor expression is associated with adverse outcome. Further studies will determine if VEGF and PD-ECGF expression occurs in the setting of a generalized upregulation of hypoxia-inducible genes in paragangliomas.
ACKNOWLEDGMENT
We thank Dr. Larry Brown (Department of Pathology, Beth Israel Hospital, Harvard Medical School) for his generous gift of human VPF/VEGF cDNA and Dr. Edward Schwartz (Department of Oncology, Albert Einstein College of Medicine) for the human PD-ECGF cDNA. We also acknowledge the Cooperative Human Tissue Network for assistance in obtaining fresh paraganglioma specimens, Dr. Ruggero Balli (Istituto di Clinica Otorinolaringoiatrica, Modena, Italy) for providing sections of an avascular paraganglioma specimen, and Dr. Ben Pilch (Department of Pathology, Massachusetts Eye and Ear Infirmary) for assistance in obtaining archival paraganglioma specimens.





