Circulating microRNAs as candidate markers to distinguish heart failure in breathless patients
Abstract
Aims
Since their identification in the circulation, microRNAs have received considerable interest as putative biomarkers of cardiovascular disease. We have investigated the diagnostic utility of microRNAs in differentiating between patients with heart failure (HF) and non-HF-related breathlessness, and between HF with reduced (HF-REF) and preserved (HF-PEF) EF.
Methods and results
MicroRNA profiling was performed on plasma from 32 HF and 15 COPD patients, as well as 14 healthy controls. Seventeen microRNAs were selected for validation in 44 HF, 32 COPD, 59 other breathless, and 15 controls. Cases of HF were split evenly between HF-REF and HF-PEF. Diagnostic utility was compared with NT-proBNP and high sensitivity troponin T (hs-troponin T). MiR-103 [area under the curve (AUC) = 0.642, P = 0.007], miR-142-3p (AUC = 0.668, P = 0.002), miR-199a-3p (AUC = 0.668, P = 0.002), miR-23a (AUC = 0.637, P = 0.010), miR-27b (AUC = 0.642, P = 0.008), miR-324-5p (AUC = 0.621, P = 0.023), and miR-342-3p (AUC = 0.644, P = 0.007) were associated with HF diagnosis in regression and receiver operating characteristic (ROC) analyses. Individually, NT-proBNP (AUC = 0.896, P = 9.68 × 10−14) and hs-troponin T (AUC = 0.750, P = 2.50 × 10−6) exhibited greater sensitivity and specificity. However, combining significantly associated microRNAs with NT-proBNP improved the AUC of NT-proBNP by 4.6% (P = 0.013). Four microRNAs, miR-103, miR-142-3p, miR-30b, and miR-342-3p, were differentially expressed between HF and controls, COPD, and other breathless patients (P = 0.002–0.030). Eight microRNAs that distinguished between HF-REF and HF-PEF in screening (P = 0.017–0.049) were not replicated in the validation.
Conclusions
Four microRNAs distinguished between HF and exacerbation of COPD, other causes of dyspnoea, and controls. Seven were associated with HF diagnosis in regression and ROC analysis. Although individually NT-proBNP was far superior in predicting HF, combining microRNA levels with NT-proBNP may add diagnostic value.
Introduction
Heart failure (HF) represents a major health problem worldwide.1 Despite significant advances in the treatment and management of HF, initial and recurrent hospitalization rates remain high, as do the associated economic costs. In countries with an ageing population, this burden is expected to increase in the future, further highlighting the need for an improved approach for predicting the onset and progression of this complex disease.
Recently, a novel class of non-coding RNA, microRNAs, has been proposed as a potential biomarker for many diseases, including cardiovascular disease. Small sequences 20–25 nucleotides in length, microRNAs have been identified as important post-transcriptional regulators of gene expression and are implicated in almost every cellular process.2 By associating with a complementary 2–8 nucleotide sequence termed the ‘seed’ region located in the 3'-untranslated region of the target mRNA, microRNA negatively regulates gene expression through the promotion of mRNA degradation or inhibition of mRNA translation.2 Several studies have investigated the diagnostic utility of microRNA in many cardiovascular diseases including myocardial infarction3,4, HF,4–8 and CAD.9 In 2010 in a study of 39 healthy controls, 30 HF cases, and 20 patients with dyspnoea, Tijsen et al. identified an association between circulating microRNA levels and HF diagnosis. More specifically, it was reported that miR-423-5p was enriched in the blood of patients suffering from HF, and receiver operating characteristic (ROC) curves revealed that miR-423-5p was a strong diagnostic predictor of HF, with an area under the curve (AUC) of 0.91.5 However, this association has yet to be validated in a larger patient group. Elucidating the extensive functions of microRNAs in the cardiovascular system is providing new insights into the pathophysiology of cardiovascular disease, and has resulted in these important sequences becoming intriguing pharmacological targets.
In this current study, we sought not only to replicate the reported association between miR-423-5p and HF diagnosis, but also to compare the diagnostic utility of this microRNA with the established cardiac markers NT-proBNP and high sensitivity troponin T (hs-troponin T). By conducting a microRNA screen to identify candidate microRNA diagnostic markers, and then by validating these candidates in a larger group of patients presenting to the Emergency Department (ED) with the chief complaint of shortness of breath, we also aimed to identify additional microRNAs capable of distinguishing HF from other causes of breathlessness including exacerbation of COPD. Finally, we wished to assess whether any microRNAs are differentially regulated between HF with reduced EF (HF-REF) and HF with preserved EF (HF-PEF). HF-PEF is a common form of HF, and treatments effective in HF-REF do not alter outcomes in HF-PEF, reflecting our poor understanding of the pathophysiology underlying this disease phenotype.10,11 Circulating microRNA profiles may offer insight into the underlying mechanisms distinguishing HF-PEF from HF-REF. To our knowledge, this is the first study to undertake a comparison of microRNA profiles in patients with HF-REF and HF-PEF.
Methods
Christchurch dyspnoea cohort
Inclusion criteria for the Christchurch dyspnoea cohort included age of at least 18 years, and presentation at the ED with the chief complaint of shortness of breath not resulting from trauma. At initial presentation to the ED, blood was collected into chilled EDTA tubes and stored on ice. Plasma was separated within 20 min by centrifugation and stored at –80°C. Diuretics, fluid intake and output, and other medications administered in the first 24 h were recorded, as were all test results from the hospital stay. Transthoracic echocardiography was performed on HF patients, and on patients with other non-HF causes of dyspnoea if requested as part of standard clinical care. Echocardiography was carried out using a GE Vivid 3 ultrasound system (GE Medical Systems, USA). The standardized imaging protocol included apical four- and two-chamber views according to the recommendations of the American Society of Echocardiography.12 Adjudicated diagnoses were made by two cardiologists with access to all test results available from the hospital stay including echocardiography, computed tomography (CT) scan, ventilation perfusion lung scan, pulmonary function, and inpatient laboratory tests. Each cardiologist was blinded to the other's evaluation and biomarker (including microRNA) results. At the time of this study, 450 patients were enrolled. All patients provided written informed consent. Of this cohort, 32% received a final diagnosis of HF, 24% exacerbation of COPD, and the remainder had other causes of their dyspnoea.
Canterbury healthy volunteer cohort
Volunteers aged between 20 and 108 years, randomly selected from the Canterbury electoral roll, were recruited into the Canterbury healthy volunteers study (n = 3000). Study participants were screened prior to recruitment using Hospital Patient Management Systems (PMS) databases, and had no personal diagnosed history of overt cardiovascular disease including CAD, myocardial infarction, and peripheral vascular disease. Medical history was obtained along with measurements of blood pressure, heart rate, and weight, and an ECG was performed. A blood sample was taken for neurohormone (including NT-proBNP13) and genetic analyses. Echocardiography was carried out on a subset of participants, including those that were included in the microRNA validation study. Each participant provided written, informed consent.
Sample selection
MicroRNA screening study (n = 61)
Exiqon Human Panel I and II microRNA assays, for profiling of 742 microRNAs, were run on 14 samples selected from the Canterbury healthy volunteers, and 47 selected from the Christchurch dyspnoea cohort. Within the dyspnoea subgroup, 15 had an adjudicated diagnosis of COPD, and 32 of HF. An equal number (n = 16) of HF patients with HF-REF and HF-PEF were included using an EF cut-off of 45%. Within both the <45% and >45% groups, the 16 patients with the highest NT-proBNP levels were selected for analysis. HF patients with a secondary diagnosis of pneumonia were avoided. COPD patients (n = 15) were randomly selected. COPD patients with a secondary diagnosis of acute coronary syndromes (including myocardial infarction) and/or and pneumonia were excluded. Healthy volunteers (n = 14) were age and gender matched to dyspnoea cohort samples.
MicroRNA validation (n = 150)
From the initial microRNA screen, 17 microRNAs identified as being the most significantly altered between healthy controls, COPD, and HF (including HF-REF and HF-PEF) were selected for validation in a larger group of Christchurch dyspnoea patients (n = 135) and healthy controls (n = 15). The microRNAs assayed were miR-423-5p, miR-185, miR-29a, miR-342-3p, miR-324-5p, miR-150, miR-199a-3p, miR-598, miR-142-3p, miR-1909, miR-23a, miR-27b, miR-103, let-7b, miR-30b, miR-2110, and miR-940. Dyspnoea patients with adjudicated diagnoses of HF (n = 44, 32%), COPD (n = 32, 24%), and breathlessness including all other diagnoses (n = 59, 44%) were randomly selected at the proportions at which they were present in the cohort. The HF samples were split evenly between HF-REF (n = 22, 16%) and HF-PEF (n = 22, 16%) using an EF cut-off of 45%.
RNA extraction and cDNA synthesis
RNA was extracted from 200 μL of plasma using Qiagen miRNeasy RNA extraction kits (Qiagen, Valencia, CA, USA). cDNA synthesis was carried out using 8 μL of RNA product and miRCury LNA™ Universal RT microRNA PCR, Polyadenylation, and cDNA synthesis kits (Exiqon, Copenhagen, Denmark). In order to reduce the effects of differences in reaction efficiency between batches, two separate cDNA syntheses were performed for each sample. Sample processing order for RNA extraction and cDNA synthesis was randomized to ensure that samples from each study group were distributed between experimental batches. Both RNA extraction and cDNA synthesis were carried out according to the manufacturers' instructions.
MicroRNA analysis
MicroRNA primer sets were pre-aliquoted onto 384-well plates during the Exiqon manufacturing process. Reverse transcription–PCR (RT–PCR) was performed according to product instructions. In summary, 20 μL of each cDNA reaction was diluted in nuclease-free water and added 1:1 with SYBR Green Master Mix. After mixing, 10 μL was dispensed into each well. RT–PCR was performed on a Roche LightCycler 480 (Roche, Diagnostics, Indianapolis, IN, USA). Preliminary data analysis using instrument software utilized the second derivative method, and raw Ct values were exported for further analysis in GenEx software.
Data analysis
For both the screening and validation studies, initial data processing and microRNA analysis were carried out with GenEx Software according to the product instructions. First, interplate calibration was performed using the calibration assay included on each plate during the manufacturing process. This interplate calibrator, assayed in three wells of each plate, was used to control and adjust for any run to run variation. A Ct cut-off of 37 was implemented for each microRNA. Signals exceeding this maximum were considered background and assigned a Ct of 38. Similarly, signals falling within three Ct values of the negative control (no RNA template added) were considered primer dimer and were additionally removed from analysis. In the screening study, microRNA assays that failed or had Ct values >37 in > 10% of samples were removed from subsequent analysis.
For the screening study (742 microRNAs), data normalization was performed using the global mean expression of all genes, as recommended for large-scale microRNA expression profiling.14 For microRNA validation (17 microRNAs), data were normalized to the expression of four reference genes (miR-20a, miR-106b, miR-363, and miR-140-3p) (Supplementary material, Figure S2), selected from screening data using geNorm15 and NormFinder software (Molecular Diagnostic Laboratory, Aarhus, Denmark). Ct values for each cDNA duplicate were averaged. All microRNA data were log2 transformed.
After data quality control and normalization in GenEx Software, analysis of variance (ANOVA) and t-tests were performed to identify microRNAs that were differentially expressed between patient groups. In the validation study, associations between microRNAs and EF, NT-proBNP, and hs-troponin T were assessed using Pearson correlation. Neurohormone data exhibited a skewed distribution and were log10 transformed. Binary regression and ROC analyses were used to test the diagnostic utility of microRNAs, and to compare these findings with NT-proBNP and hs-troponin T. For all statistical analyses, significance levels were set at P < 0.05. However, if highly conservative Bonferroni correction was applied to account for multiple testing, a P-value of ≤ 3.88 × 10−4 (0.05/129) for the screening study and ≤ 0.0029 (0.05/17) for the validation study would indicate statistical significance. All analyses were performed using SPSS, version 19.
Results
Cohort characteristics
Baseline characteristics of study participants are shown in Table 1. In the validation study, age (P = 0.012), NT-proBNP (P = 2.8 × 10−18), and hs-troponin T (P = 5.1 × 10−6) levels differed between study groups. Patients with HF were almost 4 years older than both healthy volunteer and COPD individuals, and ∼ 9 years older than patients with another breathless diagnosis. The NT-proBNP and hs-troponin T levels were highest in HF patients and lowest in healthy volunteers. As expected, EF was greater in the HF-PEF group compared with the HF-REF group (P = 7.9 × 10−20).
| Screening study (n = 61) | ||||||
|---|---|---|---|---|---|---|
| Healthy volunteers (n = 14) | Heart failure (n = 32) | HF-REF (n = 16) | HF-PEF (n = 16) | COPD (n = 15) | ||
| Age (years)a | 70.7 ± 8.0 | 76.6 ± 11.2 | 75.9 ± 10.7 | 77.4 ± 12.0 | 75.1 ± 10.0 | |
| Gender (male) | 57% | 75% | 75% | 75% | 73% | |
| Ejection fraction (%)a | – | 42.5 ± 17.4 | 27.3 ± 9.0 | 57.8 ± 7.0 | – | |
| NT-proBNP (pmol/L)b | 18.8 (4.0–134.9) | 460.8 (141.3–2511.9) | 778.6 (323.6–2511.9) | 272.8 (141.3–741.3) | – | |
| Validation study (n = 150) | ||||||
| Healthy volunteers (n = 15) | Heart failure (n = 44) | HF-REF (n = 22) | HF-PEF (n = 22) | COPD (n = 32) | Other breathless (n = 59) | |
| Age (years)a | 71.4 ± 10.9 | 75.0 ± 13.2 | 72.0 ± 14.2 | 78.1 ± 11.7 | 71.1 ± 10.8 | 66.7 ± 13.1 |
| Gender (male) | 60% | 70% | 77% | 64% | 52% | 59% |
| Ejection fraction (%)a | 66.0 ± 5.2 | 44.5 ± 19.0 | 27.0 ± 7.7 | 62.0 ± 6.4 | – | – |
| NT-proBNP (pmol/L)b | 13.7 (5.0–213.8) 9 | 493.2 (25.7–3801.9). | 571.5 (75.9–3801.9) | 425.5 (25.7–2089.3) | 47.4 (1.0–1023.3) | 48.4 (1.0–1000.0) |
| hs-troponin T (pmol/L)b | 8.5 (3.0–28.2) | 29.6 (3.0–512.9) | 31.1 (5.0–251.2) | 28.2 (3.0–512.9) | 13.8 (3.0–91.2) | 10.4 (3.0–1174.9) |
- a HF-PEF, heart failure with preserved ejection fraction; HF-REF, heart failure with reduced ejection fraction; hs-troponin T, high sensitivity tropononin T.
- a Arithmetic mean ± standard deviation.
- b Geometric mean and range.
Screening study
MicroRNA levels were compared between healthy volunteers, COPD, HF-REF, HF-PEF, and combined HF (Supplementary material, Tables S1–S5). For microRNAs with significantly altered expression, a combination of P-value, fold change (FC), and t-statistic guided selection into the validation study. MicroRNAs with altered expression between more than one patient group were prioritized for inclusion. Based on P-value, the final candidates selected for validation included the top four microRNAs (and seven of the top eight) when an ANOVA comparison of all study groups was undertaken. The three most differentially expressed microRNAs (and seven of the top eight) when comparing HF with controls were also selected. All microRNAs selected based on differential expression between healthy volunteers and HF achieved significance after Bonferroni correction (P < 3.88 × 10−4), as did all microRNA's, except let-7b, when comparing levels across all study groups. Finally, four of the top five microRNAs were selected when comparing HF patients with COPD, as were seven of the top eight microRNAs for distinguishing between HF-REF and HF-PEF. The candidate microRNAs for distinguishing between healthy volunteers and HF were miR-185 (P = 3.43 × 10−6), miR-29a (P = 3.47 × 10−5), miR-342-3p (P = 7.38 × 10−5), miR-1909 (P = 0.0001), let-7b (P = 0.0003), miR-324-5p (P = 0.0003), and miR-150 (P = 0.0004); HF and COPD, miR-598 (P = 0.013), miR-142-3p (P = 0.014), miR-103 (P = 0.03), miR-342-3p (P = 0.04), and miR-30b (P = 0.059); between COPD and healthy volunteers, miR-324-5p (P = 0.0004), miR-185 (P = 0.0005), miR-1909 (P = 0.0009), and miR-150 (P = 0.001); and between HF-REF and HF-PEF, miR-342-3p (P = 0.017), miR-199a-3p (P = 0.020), miR-150 (P = 0.024), miR-29a (P = 0.027), miR-2110 (P = 0.029), miR-27b (P = 0.030), miR-940 (P = 0.050), and miR-23a (P = 0.028) (Supplementary material, Figure S1).
Validation study
Identification of differentially expressed microRNAs
The 17 microRNAs identified in screening were investigated in 135 dyspnoea patients and 15 healthy volunteers (Figure 1, Table 2). For the validation study, the average Ct value for each microRNA was between 25.1 and 33.7 (Supplementary material, Table S6). MiR-103, miR-142-3p, miR-30b, and miR-342-3p were present at significantly lower levels in HF patients compared with healthy volunteers (P = 0.0019–0.019), COPD (P = 0.006–0.026), and other breathless participants (P = 0.009–0.030). Fold change difference in expression ranged between –1.41 and –2.40. The difference in miR-30b expression between HF and controls met statistical significance after Bonferroni adjustment for multiple comparisons (P < 0.0029).
| miR | Overall P-value | HF vs. HC P-value (t-statistic, FC) | HF vs. COPD P-value (t-statistic, FC) | HF vs. other breathless P-value (t-statistic, FC) | HF-PEF vs. HF-REF P-value (t-statistic, FC) | Other breathless vs. COPD P-value (t-statistic, FC) |
|---|---|---|---|---|---|---|
| let-7b | 0.17 | 0.54 (t = 0.61, FC = 1.07) | 0.078 (t = 1.78, FC = –1.20) | 0.290 (t = 1.06, FC = –1.10) | 0.65 (t = 0.46, FC = –1.06) | 0.40 (t = 0.85, FC = –1.09) |
| miR-103 | 0.023 | 0.019 (t = 2.41, FC = –1.62) | 0.011 (t = 2.61, FC = –1.51) | 0.022 (t = 2.32, FC = –1.41) | 0.68 (t = 0.41, FC = –1.09) | 0.69 (t = 0.40, FC = –1.06) |
| miR-142-3p | 0.007 | 0.012 (t = 2.58, FC = –2.40) | 0.006 (t = 2.80,FC = –2.08) | 0.009 (t = 2.66, FC = –1.91) | 0.58 (t = 0.55, FC = –1.22) | 0.73 (t = 0.34, FC = –1.09) |
| miR-150 | 0.010 | 0.009 (t = 2.68, FC = –2.63) | 0.092 (t = 1.71, FC = –1.58) | 0.051 (t = 1.98, FC = –1.54) | 0.50 (t = 0.68, FC = –1.32) | 0.89 (t = 0.14, FC = –1.03) |
| miR-185 | 0.023 | 0.014 (t = 2.53, FC = 1.43) | 0.89 (t = 0.13, FC = –1.02) | 0.34 (t = 0.95, FC = –1.11) | 0.25 (t = 1.16, FC = –1.21) | 0.48 (t = 0.71, FC = 1.09) |
| miR-1909 | 0.94 | 0.61 (t = 0.52, FC = –1.32) | 0.98 (t = 0.02, FC = 1.01) | 0.87(t = 0.16, FC = –1.06) | 0.82 (t = 0.23, FC = –1.14) | 0.84 (t = 0.20, FC = 1.07) |
| miR-199a-3p | 0.015 | 0.16 (t = 1.41, FC = –2.08) | 0.014 (t = 2.52,FC = –2.63) | 0.008 (t = 2.69, FC = –2.52) | 0.38 (t = 0.89, FC = –1.67) | 0.89 (t = 0.13, FC = –1.04) |
| miR-2110 | 0.30 | 0.25 (t = 1.15, FC = 1.27) | 0.52 (t = 0.65, FC = –1.14) | 0.25 (t = 1.16, FC = –1.22) | 0.45 (t = 0.77, FC = –1.19) | 0.74 (t = 0.33, FC = 1.07) |
| miR-23a | 0.036 | 0.26 (t = 1.13, FC = –1.62) | 0.019 (t = 2.40, FC = –2.17) | 0.018 (t = 2.39, FC = –2.04) | 0.46 (t = 0.74, FC = –1.43) | 0.83 (t = 0.21, FC = –1.06) |
| miR-27b | 0.036 | 0.10 (t = 1.67, FC = –1.87) | 0.025 (t = 2.30,FC = –1.93) | 0.020 (t = 2.37, FC = –1.82) | 0.54 (t = 0.62, FC = –1.30) | 0.83 (t = 0.22, FC = –1.06) |
| miR-29a | 0.13 | 0.06 (t = 1.92, FC = –1.71) | 0.20 (t = 1.30, FC = –1.31) | 0.11 (t = 1.62, FC = –1.34) | 0.69 (t = 0.40, FC–1.13) | 0.86 (t = 0.17, FC = 1.03) |
| miR-30b | 0.007 | 0.0019 (t = 3.26, FC = –2.32) | 0.026 (t = 2.27, FC = –1.59) | 0.030 (t = 2.21, FC = –1.48) | 0.79(t = 0.26, FC = –1.07) | 0.72 (t = 0.35, FC–1.07) |
| miR-324-5p | 0.14 | 0.61 (t = 0.51, FC = –1.12) | 0.11 (t = 1.63, FC = –1.35) | 0.032 (t = 2.18,FC = –1.44) | 0.45 (t = 0.75, FC = –1.19) | 0.74 (t = 0.33, FC = 1.07) |
| miR-342-3p | 0.009 | 0.010 (t = 2.65,FC = –2.09) | 0.013 (t = 2.55, FC = –1.70) | 0.020 (t = 2.37,FC = –1.57) | 0.80 (t = 0.25, FC–1.08) | 0.66 (t = 0.44, FC = –1.09) |
| miR-423-5p | 0.069 | 0.07 (t = 1.82, FC = 1.39) | 0.57 (t = 0.57, FC = –1.09) | 0.19 (t = 1.31, FC = –1.20) | 0.50 (t = 0.69, FC = –1.15) | 0.57 (t = 0.57, FC = 1.10) |
| miR-598 | 0.11 | 0.12 (t = 1.57, FC = –1.56) | 0.12 (t = 1.58, FC = –1.51) | 0.02 (t = 2.31, FC = –1.67) | 0.45 (t = 0.77, FC = 1.28) | 0.70 (t = 0.38, FC = 1.10) |
| miR-940 | 0.51 | 0.68 (t = 0.41, FC = –1.15) | 0.20 (t = 1.28, FC = –1.40) | 0.23 (t = 1.21, FC = –1.32) | 0.74 (t = 0.34, FC = –1.13) | 0.83 (t = 0.22, FC = –1.05) |
- a FC, fold change; HC, healthy controls; HF-PEF, heart failure with preserved ejection fraction; HF-REF, heart failure with reduced ejection fraction.
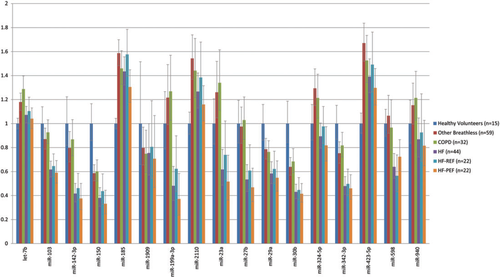
Levels of miR-150 (P = 0.009, FC = –2.63) were lower and of miR-185 (P = 0.014, FC = 1.43) higher when comparing HF patients with healthy volunteers. Compared with HF, miRs 199a-3p (P ≤ 0.014), 23a (P ≤ 0.019), and 27b (P ≤ 0.025) were up-regulated in both COPD and other breathless patients. No significant difference in microRNA levels was identified between HF-REF and HF-PEF (P ≥ 0.05) when dividing HF into these two patient subgroups. Levels of miR-423-5p, previously reported to be a diagnostic predictor of HF, did not differ between HF and healthy volunteer participants (P = 0.07, FC = 1.39), COPD (P = 0.57, FC = –1.09), and other breathless patients (P = 0.19, FC = –1.20). There was no significant difference in microRNA levels between COPD and patients with other non-HF causes of breathlessness (P ≥ 0.05).
Correlations with N-terminal pro brain natriuretic peptide, high sensitivity troponin T, and left ventricular ejection fraction
The strongest inverse correlation with NT-proBNP (r = –0.385, P = 1.0 × 10−6) and hs-troponin T (r = –0.391, P = 7.0 × 10−7) was observed for miR-30b. miR-185 exhibited the next most significant associations and was positively correlated to NT-proBNP (r = 0.320, P = 7.0 × 10−5) and hs-troponin T (r = 0.221, P = 0.007). Weaker positive correlations with both NT-proBNP (r = 0.249–0.257, P = 0.002) and hs-troponin T (r = 0.161–0.188, P = 0.021–0.048) were identified for miR-423-5p and let-7b. Weaker negative correlations with NT-proBNP (r = –0.180 to –0.247, P = 0.002–0.027) and hs-troponin T (r = –0.170 to –0.285, P = 0.0004–0.038) were observed for miR-103, miR-142-3p, miR-150, miR-199a-3p, miR-27b, and miR-342-3p. MiR-2110 was correlated with NT-proBNP (r = 0.242, P = 0.003) and miR-29a with hs-troponin T only (r = –0.198, P = 0.015). No correlation between microRNA levels and LVEF were identified; however, EF data were mostly unavailable for COPD and other breathless patients (Supplementary material, Table S7).
Associations with heart failure diagnosis
Within the dyspnoea cohort, let-7b [β = 0.49, 95% confidence interval (CI) = 0.25–0.97, P = 0.040], miR-103 (β = 0.64, 95% CI = 0.44–0.93, P = 0.019), miR-142–3p (β = 0.75, 95% CI = 0.60–0.94, P = 0.012), miR-199a-3p (β = 0.81, 95% CI = 0.69–0.96, P = 0.012), miR-23a (β = 0.80, 95% CI = 0.66–0.96, P = 0.016), miR-27b (β = 0.80, 95% CI = 0.64–0.99, P = 0.036), miR-324-5p (β = 0.70, 95% CI = 0.51–0.96, P = 0.026), and miR-342-3p (β = 0.73, 95% CI = 0.55–0.98, P = 0.036) were significantly associated with the diagnosis of HF after adjustment for age and gender. However, both NT-proBNP (β = 31.5, 95% CI = 9.16–107.98, P = 4.27 × 10−8) and hs-troponin T (β = 4.70, 95% CI = 1.74–12.70, P = 0.002) exhibited far stronger associations with HF diagnosis than any microRNA (Table 3).
| miR | β | 95% CI | P-value |
|---|---|---|---|
| let-7b | 0.49 | 0.25–0.97 | 0.040 |
| miR-103 | 0.64 | 0.44–0.93 | 0.019 |
| miR-142-3p | 0.75 | 0.60–0.94 | 0.012 |
| miR-150 | 0.82 | 0.64–1.05 | 0.116 |
| miR-185 | 0.70 | 0.41–1.18 | 0.181 |
| miR-1909 | 0.99 | 0.84–1.17 | 0.954 |
| miR-199a-3p | 0.81 | 0.69–0.96 | 0.012 |
| miR-2110 | 0.80 | 0.57–1.11 | 0.176 |
| miR-23a | 0.80 | 0.66–0.96 | 0.016 |
| miR-27b | 0.80 | 0.64–0.99 | 0.036 |
| miR-29a | 0.82 | 0.62–1.10 | 0.192 |
| miR-30b | 0.76 | 0.56–1.03 | 0.080 |
| miR-324-5p | 0.70 | 0.51–0.96 | 0.026 |
| miR-342-3p | 0.73 | 0.55–0.98 | 0.036 |
| miR-423-5p | 0.72 | 0.49–1.08 | 0.112 |
| miR-598 | 0.80 | 0.64–1.01 | 0.059 |
| miR-940 | 0.85 | 0.67–1.08 | 0.190 |
| NT-proBNP | 31.5 | 9.16–107.98 | 4.27 × 10–8 |
| Hs-troponin T | 4.70 | 1.74–12.7 | 0.002 |
- a Non-genetic variables included in the model are age and gender.
- b MicroRNA data are log2 transformed, NT-proBNP and high sensitivity troponin T (hs-troponin T) are log10 transformed.
- c CI, confidence interval
In ROC analyses, miR-103 (AUC = 0.642, P = 0.007), miR-142-3p (AUC = 0.668, P = 0.002), miR-199a-3p (AUC = 0.668, P = 0.002), miR-23a (AUC = 0.637, P = 0.010), miR-27b (AUC = 0.642, P = 0.008), miR-30b (AUC = 0.629, P = 0.016), miR-324-5p (AUC = 0.621, P = 0.023), miR-342-3p (AUC = 0.644, P = 0.007), and miR-598 (AUC = 0.650, P = 0.005) were significant predictors of HF in the dyspnoea cohort. Individually, both NT-proBNP (AUC = 0.896, P = 9.68 × 10−14) and hs-troponin T (AUC = 0.750, P = 2.50 × 10−6) exhibited greater specificity and sensitivity than the microRNAs examined. Combining all significant microRNAs in ROC analysis did not greatly improve predictive value beyond what was achieved individually (AUC = 0.678) (Figure 2A). Similarly, combining NT-proBNP and hs-troponin T did not improve above what was obtained from using NT-proBNP alone (AUC = 0.897).
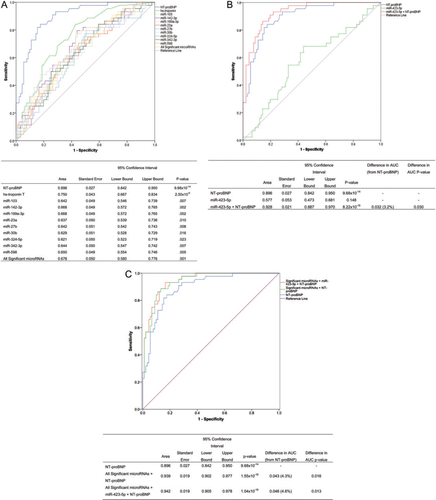
MiR-423-5p was not significantly predictive of HF diagnosis in ROC analysis (AUC = 0.577, P = 0.148). However, when miR-423-5p was added to NT-proBNP, a significant 3.2% increase in the AUC compared with NT-proBNP alone was identified (P = 0.030) (Figure 2B). Combining all those microRNAs significantly associated individually with NT-proBNP did not improve diagnostic utility (Supplementary material, Figure S3). Yet, when all significantly associated microRNAs were combined with NT-proBNP, the predictive value of NT-proBNP was increased by 4.3%, and this difference in the AUC was statistically significant (P = 0.016). The greatest improvement in predictive value was observed when all significantly associated microRNAs, miR-423-5p, and NT-proBNP were combined in the analysis (difference in AUC = 4.6%, P = 0.013) (Figure 2C).
Discussion
Circulating microRNAs have emerged as attractive putative biomarkers across a spectrum of cardiovascular disease. In addition to testing their ability to distinguish HF from other causes of breathlessness, we have assessed whether a panel of microRNAs were differentially expressed between HF-REF and HF-PEF.
Few studies have investigated the diagnostic utility of circulating microRNAs in the clinical setting of HF. In 2010, in a study of 30 HF patients, 20 non-HF dyspnoea cases, and 39 healthy controls, Tisjen et al. identified miR-423-5p as the strongest candidate marker able to discriminate between HF and other breathless individuals.5 The association between miR-423-5p and HF diagnosis was recently replicated in a study of 30 stable chronic HF patients and 30 controls.8 In our study, although miR-423-5p trended towards higher levels in HF individuals compared with healthy controls, levels did not differ from those observed in non- HF-related cases of dyspnoea. Mir-423-5p was not a predictor of HF diagnosis in either regression or ROC analyses; however, the addition of miR-423-5p to NT-proBNP significantly improved the AUC by 3.2%. Although this lack of association between miR-423-5p and HF diagnosis may be a result of insufficient statistical power due to our limited sample size, it could alternatively reflect differences in patient characteristics between studies, particularly for NT-proBNP and EF.
In our study, miR-103, miR-142-3p, miR-30b, and miR-342-3p were all significantly down-regulated in HF patients compared with non-HF dyspnoea and healthy control groups. For these microRNAs, we observed between a 1.4- and a 2.6-fold difference in expression. The microRNAs miR-103, miR-142-3p, and miR-342-3p, as well as miR-199a-3p, miR-23a, miR-27b, and miR-324-5p, were associated with HF diagnosis in ROC analysis and in regression analyses that adjusted for age and gender. However, for any individual microRNA the AUC did not exceed 0.67, which is much lower than what has previously been reported for miR-423-5p (AUC = 0.91).5
MicroRNAs have been shown to be dysregulated in multiple models of human HF. In these predominantly tissue models, microRNA expression was mostly elevated with disease, whereas in our study of plasma, many microRNAs were down-regulated in the HF group. This may be explained by differences in disease phenotype, or could alternatively reflect the processes involved in the release of microRNAs into the circulation.16 As these microRNAs are also probably expressed in other tissues, plasma levels will mirror the pathologies of not only the heart but also these other organs.
Previously, the microRNAs 30b, 103, 199a-3p, 23a, 27b, 324-5p, 342-3p, and 142-3p have been found to be dysregulated in end-stage HF,17 mitral stenosis,18 ischaemic cardiomyopathy,19 dilated cardiomyopathy,19−21 and aortic stenosis.19 Functional investigations have demonstrated a plausible role for these microRNAs in cardiovascular biology. The miR-30 family is one of the most abundant families in the murine heart,22 with potentially important predicted cardiovascular targets including fibroblast activation protein, alpha cold shock domain protein A, catalase, and interferon-γ.20 The miR-199 family play an important role in hypoxia-induced cell death through regulation of hypoxia-inducible factor-1α (HIF-1α) and the stabilization of the proapoptotic factor p53.23 MiR-103 is also induced in response to hypoxia24 and is additionally involved in pyruvate and lipid metabolism.25 Directly regulated via nuclear factor of activated T cells and cytoplasmic, calcineurin-dependent-3 (NFATc3), miR-23 is necessary for mediating the effects of the calcineurin–NFATc3 pathway during cardiac hypertrophy.26 MiR-27b is induced in response to hypoxia24 and increased in angiogenesis.17 Interestingly, miR-324-5p has been implicated in the Hedgehog signalling pathway27 which has been shown to be involved in cardiovascular development and ischaemia.28 In hepatocellular carcinoma cells, miR-142 was identified as a negative regulator of the ras-related C3 botulinum toxin substrate 1 (RAC1) gene which is associated with hypertrophy, vascular smooth muscle proliferation, and change of endothelial cell shape.29,30 The role of miR-342 in cardiovascular pathology has yet to be elucidated; however, it has been shown to induce apoptosis in colorectal cancer cells.31
Cardiac natriuretic peptides play an important role in regulating salt and water excretion and vasodilation, and constitute a pivotal part of the endogenous compensatory response to HF.32 In myocardial infarction and HF patients, BNP and NT-proBNP are independent sensitive and specific markers of both morbidity and mortality.33 International guidelines for the management of HF currently recommend the use of plasma BNP for assisting in the diagnosis of HF in the breathless patient presenting to the ED.34 Cardiac troponin is the pivotal marker in defining and diagnosing acute myocardial infarction, identifies even minor myocardial necrosis with high precision, and is related to prognosis in HF.35,36 Although circulating levels of the microRNAs examined lacked the sensitivity and specificity of hs-troponin T and NT-proBNP, adding these microRNAs to NT-proBNP produced a 4.6% improvement (P = 0.013) in the AUC of the ROC for discriminating HF from other causes of breathlessness.
Heart failure presents with preserved EF in 35–55% of cases, and outcomes in Western populations are comparable with those of HF-REF.11 Treatments effective in HF-REF provide no mortality benefit in HF-PEF, reflecting the limited understanding of the processes contributing to this phenotype.10,37 Although in microRNA profiling we identified a number of microRNAs that were differentially expressed between HF-REF and HF-PEF, we were unable to replicate these findings in the validation study. This lack of replication may be in part explained by subtle differences in the selection criteria and thus the phenotype for each arm of the study. Studies in a larger group of individuals will be required to determine if microRNAs can provide insight into the distinct biological processes involved in the development and progression of HF-PEF compared with HF-REF.
We performed microRNA profiling in patients presenting to the ED with the primary complaint of recent onset of severe shortness of breath. Although we identified seven microRNAs that distinguished between HF and other non-HF causes of dyspnoea, each lacked the sensitively and specificity of NT-proBNP and hs-troponin T. However, we provide evidence that the addition of microRNAs to NT-proBNP could be of diagnostic value. Further work is required to elucidate fully the diagnostic utility and function of these circulating microRNAs, both independently and in combination with NT-proBNP and other cardiovascular risk factors.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
Health Research Council of New Zealand, New Zealand Lotteries Grant Board, and the Heart Foundation of New Zealand. A.M.R. holds the New Zealand Heart Foundation Chair of Cardiovascular Studies.
Conflict of interest: none declared.
Acknowledgements
We thank the study participants, study coordinators, clinical research staff, and Endolab assay staff of the Christchurch Heart Institute.




