The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure
Abstract
Aims
Clinical and animal studies suggest that beta1-adrenergic and M2 muscarinic receptor autoantibodies (beta1-AAbs and M2-AAbs) play important roles in the pathophysiological process of chronic heart failure (CHF). Removal of these autoantibodies improved haemodynamic parameters and left ventricular ejection fraction patients with CHF. The goal of this project is to evaluate whether beta1-AAbs and M2-AAbs predict prognosis and sudden cardiac death (SCD) in CHF.
Methods and results
A total of 2062 patients with CHF and 824 control subjects were recruited. Beta1-AAbs and M2-AAbs were detected by the enzyme-linked immunosorbent assay (ELISA) method, and the correlation between these autoantibodies and the prognosis of CHF was analysed. During a median follow-up period of 36 months (0.40 ± 65 months), 379 (21.56%) cases died—164 had dilated cardiomyopathy (DCM) and 215 had ischaemic cardiomyopathy (ICM). Of these, SCD occurred in 69 cases (40.37%) of DCM and in 84 cases (39.07%) of ICM. Positivity for beta1-AAbs in DCM and ICM was significantly higher than for the controls (8.1% and 8.25% v.s 2.2%, both P < 0.01). However, positive M2-AAbs did not show any statistical difference between the three groups. Cox regression analysis revealed that positive beta1-AAbs were associated with higher mortality in CHF and that it predicted SCD for DCM [hazard ratio (HR) 4.51, 95% confidence interval (CI) 2.405–8.471] and ICM (HR 3.749, 95% CI 2.389–5.884) patients, but not non-SCD (NSCD) patients.
Conclusions
The rates of positive beta1-AAbs were higher in CHF patients than in the controls. Positive beta1-AAbs might serve as an independent predictor for SCD in patients with CHF.
Introduction
Chronic heart failure (CHF) is a major public health issue worldwide. Recent data revealed a 1 in 5 lifetime risk for both genders in >5 million currently affected patients in the USA. CHF is a cardiovascular disease with increassing incidence, prevalence, and cost. Its estimated economic burden in the USA was US$37.2 billion in 2009.1,2 In patients who died of CHF, >50% of deaths were due to severe arrhythmias such as sustained ventricular tachycardia (VT)/ventricular fibrillation (VF) leading to sudden cardiac death (SCD).3 Therefore, the prediction and prevention of SCD is essential to the management of patients with CHF. Studies have shown a host of potential predictors of SCD in CHF patients—severe left ventricular (LV) systolic dysfunction, left ventricular ejection fraction (LVEF), electrical factors such as QRS duration and non-sustained VT,4,5 biomarkers such as N-terminal pro brain natriuretic peptide (NT-proBNP), and inflammatory factors—but they all showed relatively low sensitivity and specificity.6
In recent years, there is growing evidence suggesting an autoimmune mechanism to the pathogenesis of HF. An elevated troponin I level has been well established as a biomarker for the diagnosis and quantification of myocyte injury, and several clinical studies suggested that circulating autoantibodies against cardiac troponin I (anti-cTnI) were present in elderly patients with HF, but it was not a useful biomarker for HF diagnosis, prognosis, or monitoring.7,8. However, beta1-adrenergic receptor autoantibodies (beta1-AAbs) have been identified in the serum of patients with dilated cardiomyopathy (DCM) and they have a beta-adrenergic receptor (beta-AR) agonist effect.9 Myocardial damage caused by beta1-AAbs could be neutralized by beta1-blockers.10 Clinical studies have further shown that the removal of beta1-AAbs by immunoabsorption resulted in a significant improvement of haemodynamic parameters and LVEF in these patients.11,12.
M2 muscarinic receptor autoantibodies (M2-AAbs) as well as acetylcholine attenuate the beta-AR-mediated increase in cyclic adenosine monophosphate (cAMP) and induce negative inotropic effects on ventricular muscle in the presence of beta-AR stimulation.13 Although M2-AAbs has been associated with atrial tachyarrhythmias (since acetylcholine innervations are mainly disseminated to the atria),14 recent studies demonstrated that ∼40% of patients with idiopathic VT,15 Chagas heart disease, and circulating M2-AAbs had higher QT dispersion (QTd),16 which is a marker of repolarization heterogeneity that is associated with higher rates of ventricular arrhythmias (VAs) and SCD.17 Thus, we set out to evaluate whether beta1-AAbs and M2-AAbs might predict SCD in patients with CHF in the Chinese Han population.
Methods
Patients enrolment
From July 2005 to December 2009, 2062 patients with CHF referred from 10 hospitals in mainland China were recruited. Enrolment criteria included: CHF caused by DCM or ischaemic cardiomyopathy (ICM); in NYHA (New York Heart Association) functional class II–IV despite optimized medical therapy; and LVEF ≤45% in DCM and ≤50% in ICM. DCM was diagnosed according to the guidelines for the study of familial DCM.18 ICM was defined as ≥70% luminal stenosis of at least one major coronary artery diagnosed by coronary angiography and a history of myocardial infarction >3 months before enrolment. All cases were excluded if they had malignant tumours, severe liver and kidney dysfunctions, or other uncontrollable system diseases, pregnancy, or unwillingness to participate in the study.
Control subjects
A total of 824 cases were selected as the control group. Of these, 423 patients who underwent radiofrequency ablation for supraventricular tachycardia without structural heart disease were enrolled as ‘ward controls’ and 401 cases of community-based inhabitants who had an annual health examination and were free of structural heart disease were selected as ‘community controls’. The baseline data of these two control groups showed no significant difference. The exclusion criteria were the same as for the patients.
The investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Cardiovascular Hospital (Beijing, China). All subjects who participated in the study provided written informed consent and reported themselves as being of Chinese Han nationality.
Serum sampling and peptide synthesis
A 2 mL aliquot of blood was taken from the antecubital vein and separated by centrifugation (3000 r.p.m., Sigma Centrifuge) for 10 min. Serum samples were stored at –80°C until needed for assay. Peptides corresponding to the amino acid sequence (residues 197–222) of the second extracellular loop of the human beta1-AR (H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F) as well as the amino acid sequence (residues 169–193) of the second extracellular loop of the human M2-AAbs (V-R-T-V-E-D-G-E-C-Y-I-Q-F-F-S-N-A-A-V-T-F-G-T-A) were synthesized by a commercial source (CL BIO-SCIENTIFIC Co. Ltd). The purity of the peptides was determined by high-performance liquid chromatography (HPLC) and direct sequence analysis with an automated amino acid analyser.
Endpoint assessment
The patients were followed up during regular outpatient clinics or through telephone contact, as well as by mail. The median follow-up period was 36 months (0.40 ±65 months). The endpoints included all-cause death, SCD (ICD appropriate discharge counted as SCD), and NSCD (heart transplantation regarded as NSCD). SCD was defined as unexpected death within 1 h of the onset of acute symptoms or death during sleep without witness or unexpected death of someone last seen in a stable medical condition<24 h previously with no evidence of a non-cardiac cause.6
Autoantibody quantification
For enzyme-linked immunoabsorbent assay (ELISA), ELISA plates were coated with 100 μL per well of coating solution (pH 9.6) containing 5 μg/mL of peptide. The plates were incubated at 37°C for 1 h or overnight at 4°C followed by four washes with phosphate-buffered saline–Tween (PBST) solution. Non-specific binding sites were blocked with fat-free milk blocking solution. The plates were incubated for 1 h and washed four times before incubation with the first antibody. After incubation for 1 h at 37°C, the plates were washed four times and incubated with horseradish peroxidase–streptavidin (Go a Hu, IgG-HRP) solution (1:500) for another 1 h at 37°C, washed four times, and followed by developing for 10 min with substrate solution [4 mg of o-phenylenediamine (OPD) + 11 mL of substrate solution + 25 µL of 30% H2O2] avoiding light. The reaction was stopped by adding stop solution. The optical density (OD) was read using an ELISA plate reader (Bio-Rad model 550, USA) at 490 nm wavelength.
Statistical analysis
Continuous values were expressed as the mean ± standard deviation (SD). Student's t-test was used to compare the means between two groups, while the χ2 test was used for comparison between groups for categorical variables shown as numbers (%). P < 0.05 was considered significant. Autoantibody positive status was defined as a ratio (patient OD – blank OD/control OD – blank OD) ≥2.1. Person-months of the follow-up period started from the date of enrolment up to December 2010. Survival analysis was performed in CHF patients. The 304 (14.74%) patients who were lost during follow-up were not included in the survival analysis. Hazard ratios (HRs) for time to cardiac death and SCD from baseline were estimated using Cox proportional hazards models. In addition to considering the influence of these autoantibodies, we analysed Kaplan–Meier curves for time to probability of survival using the log rank test according to the presence or absence of these autoantibodies. The SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used in this study.
Results
Clinical characteristics
The clinical characteristics of CHF patients and controls are shown in Table 1. Briefly, age and body mass index (BMI) distribution did not differ between the groups. Other possible risk factors of CHF including hypertension, diabetes mellitus, hyperlipidaemia, LVEF, left ventricular end-diastolic diameter (LVEDD), mean heart rate (MHR), VAs [including frequent premature ventricular contractions (PVCs) and VT], or atrial fibrillation (AF) were more prevalent in the CHF group than in the controls. There was a trend towards higher NYHA classification in DCM compared with ICM (NYHA II, 21.31% vs. 50.27%; NYHA III, 42.05% vs. 31.02%; NYHA IV, 36.64% vs. 16.71%, all P < 0.05). Haemodynamic parameters were similar between patients with DCM and ICM. Cardiac arrhythmias were more frequent in DCM than in ICM. Furthermore, patients with DCM received more digitalis, diuretics, and beta-blockers, while patients with ICM took more angiotensin-converting enzyme inhibitors (ACEIs), since more ICM patients tolerated ACEIs because of hypertension.
| Clinical characteristic | Controls (n = 824) | CHF | |||
|---|---|---|---|---|---|
| DCM (n = 704) | P-value | ICM (n = 1054) | P-value | ||
| Male, n (%) | 457 (55.45%) | 532 (75.57%) | <0.001 | 847 (80.36%) | <0.001 |
| Age (years) | 56.24 ± 4.46 | 57.72 ± 14.43 | 0.181 | 66.34 ± 10.56 | 0.017 |
| NYHA class, n (%) | |||||
| I | 824 (100%) | 0 | – | 0 | – |
| II | 0 | 150 (21.31%) | – | 551 (52.27%) | – |
| III | 0 | 296 (42.05%) | – | 327 (31.02%) | – |
| IV | 0 | 258 (36.64%) | – | 176 (16.71%) | – |
| Hypertension, n (%) | 0 | 234 (33.24%) | – | 648 (61.48%) | – |
| Hyperlipidaemia, n (%) | 53 (6.44%) | 75 (10.61%) | <0.001 | 307 (29.12%) | <0.001 |
| Diabetes mellitus, n (%) | 60 (7.28%) | 120 (17.05%) | <0.001 | 311 (29.51%) | <0.001 |
| BMI | 24.40 ± 11.43 | 24.58 ± 11.92 | 0.236 | 24.88 ± 3.78 | 0.376 |
| Electrocardiography | |||||
| MHR (b.p.m.) | 69.46 ± 10.14 | 79.21 ± 14.14 | <0.001 | 71.18 ± 13.62 | <0.05 |
| AF (n) | 0 | 170 (24.15%) | – | 125 (11.86%) | – |
| PVC (n) | 0 | 189 (26.84%) | – | 209 (19.83%) | – |
| QTc (ms) | 418.60 ± 105.17 | 446.57 ± 120.81 | 0.024 | 442.11 ± 88.51 | 0.045 |
| QRS duration (ms) | 98.35 ± 84.08 | 113.25 ± 46.77 | <0.001 | 105.87 ± 51.55 | 0.035 |
| Haemodynamic parameters | |||||
| LVEF (%) | 60.34 ± 5.37 | 35.07 ± 13.18 | <0.001 | 42.89 ± 11.25 | <0.001 |
| LVEDD (mm) | 45.32 ± 6.48 | 65.29 ± 12.26 | <0.001 | 57.68 ± 9.53 | <0.001 |
| Medications, n (%) | |||||
| ACEI | 0 | 430 (61.08%) | – | 699 (66.32%) | – |
| Diuretic | 0 | 551 (78.27%) | – | 734 (69.64%) | – |
| Aldosterone antagonists | 0 | 498 (70.73%) | – | 731 (69.35%) | – |
| Digoxin | 0 | 472 (67.05%) | – | 684 (64.89%) | – |
| Beta-blocker | 0 | 545 (77.41%) | – | 743 (70.49%) | – |
| ICD, n (%) | 0 | 10 (1.42%) | – | 28 (2.66%) | – |
- a Values are the mean ± SD or number (%).
- b P-values indicate the significance compared with controls; P < 0.05 was significant.
- c Premature vascular contraction (PVC) indicated >3000 beats/24 h).
- d ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; BMI, body mass index; CHF chronic heart failure; DCM, dilated cardiomyopathy; ICD, implantable carioverter defibrillator; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MHR, mean heart rate; NYHA, New York Heart Association.
Table 2 shows the relationship between clinical characteristics and outcome of patients in regard to SCD and NSCD. Univariate analysis indicated that in ICM, the presence of beta1-AAbs was significantly higher in the SCD group than in the NSCD group. This is not the case with the DCM groups. Although positivity for beta1-AAbs was higher in the SCD group, it did not reach significance compared with that in the NSCD group. M2-AAbs and other suspected risk factors affecting the prognosis of CHF such as NYHA classification, QTc interval, BNP, and medications, e.g. ACEI and beta-blockers, were not significantly different between the SCD and NSCD groups (all P > 0.05).
| Characteristics | DCM (n = 704) | P-value | ICM (n = 1054) | P-value | ||
|---|---|---|---|---|---|---|
| NSCD (n = 95) | SCD (n = 69) | NSCD (n = 131) | SCD (n = 84) | |||
| Age (years) | 57.93 ± 15.36 | 55.35 ± 13.80 | 0.296 | 70.98 ± 9.87 | 68.16 ± 10.54 | 0.203 |
| Male gender, n (%) | 74 (77.08) | 47 (68.12) | 0.160 | 31 (23.66) | 13 (15.48) | 0.147 |
| MHR (b.p.m.) | 74.73 ± 13.74 | 76.63 ± 18.09 | 0.642 | 71.77 ± 10.54 | 65.17 ± 10.55 | 0.006 |
| Hypertension, n (%) | 30 (31.58) | 22 (31.88) | 0.967 | 76 (58.02) | 51 (60.71) | 0.695 |
| Hyperlipidaemia, n (%) | 9 (9.47) | 5 (7.25) | 0.614 | 33 (25.19) | 19 (22.62) | 0.702 |
| Diabetes mellitus, n (%) | 14 (14.74) | 8 (11.59) | 0.472 | 4 3(32.82) | 20 (23.81) | 0.208 |
| PVC, n (%) | 32 (33.68) | 26 (37.68) | 0.482 | 31 (23.66) | 21 (25.00) | 0.823 |
| AF, n (%) | 26 (27.36) | 18 (26.09) | 0.432 | 17 (12.98) | 10 (11.90) | 0.817 |
| QRS duration (ms) | 124.85 ± 43.19 | 120.08 ± 43.23 | 0.507 | 110.10 ± 69.43 | 102.88 ± 22.34 | 0.312 |
| QTc (ms) | 454.00 ± 103.60 | 458.63 ± 72.93 | 0.739 | 446.99 ± 105.31 | 445.54 ± 99.09 | 0.914 |
| NYHA | ||||||
| II | 24 (25.26) | 21 (30.43) | 0.464 | 50 (38.16) | 34 (40.47) | 0.632 |
| III | 38 (40.00) | 27 (39.13) | 0.507 | 49 (37.40) | 30 (35.71) | 0.551 |
| IV | 33 (34.74) | 21 (30.44) | 0.563 | 32 (24.44) | 20 (23.82) | 0.918 |
| LVEF (%) | 36.86 ± 11.02 | 35.44 ± 11.89 | 0.231 | 40.09 ± 12.05 | 38.41 ± 9.91 | 0.250 |
| LVEDD (mm) | 63.13 ± 9.41 | 63.80 ± 12.39 | 0.572 | 58.60 ± 9.52 | 61.18 ± 8.69 | 0.034 |
| hs-CRP (mg/L) | 6.63 ± 2.42 | 6.77 ± 2.26 | 0.766 | 8.98 ± 3.99 | 10.32 ± 3.26 | 0.249 |
| BNP (pg/mL) | 2096.6 ± 132.7 | 2093.5 ± 144.5 | 0.998 | 1856.2 ± 152.9 | 2006.8 ± 121.2 | 0.568 |
| Beta1-AAbs | 18 (18.94%) | 16 (23.18%) | 0.508 | 16 (12.21%) | 25 (29.76%) | 0.001 |
| M2-AAbs | 5 (5.26%) | 3 (4.35%) | 0.546 | 4 (3.05%) | 4 (4.76%) | 0.383 |
| ACEI, n (%) | 53 (55.79) | 36 (52.17) | 0.646 | 82 (62.59) | 56 (66.67) | 0.544 |
| Diuretic, n (%) | 59 (62.11) | 38(55.07) | 0.366 | 79 (60.31) | 52 (61.90) | 0.732 |
| Digoxin, n (%) | 63 (66.32) | 43 (62.31) | 0.597 | 80 (61.07) | 53 (63.09) | 0.381 |
| Beta-blocker, n (%) | 73 (76.84) | 52 (75.36) | 0.826 | 85 (64.89) | 58 (69.05) | 0.528 |
| ICD, n (%) | 1 (1.05) | 1 (1.45) | 0.666 | 4 (3.05) | 2 (2.38) | 0.564 |
- a Values are the mean ± SD or number (%).
- b P-values indicate the significance compared with the non-sudden cardiac death group; P < 0.05 was significant.
- c Premature vascular contraction (PVC) indicated >3000 beats/24 h).
- d AAbs, autoantibodies; ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; BNP, brain natriuretic peptide; DCM, dilated cardiomyopathy; hs-CRP, high sensitivity C-reactive protein; ICD, implantable carioverter defibrillator; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MHR, mean heart rate; NSCD, non-sudden cardiac death; NYHA, New York Heart Association; SCD, sudden cardiac death.
Beta1-adrenergic receptor autoantibodies indicated higher risk of all-cause death and sudden cardiac death but not non-sudden cardiac death
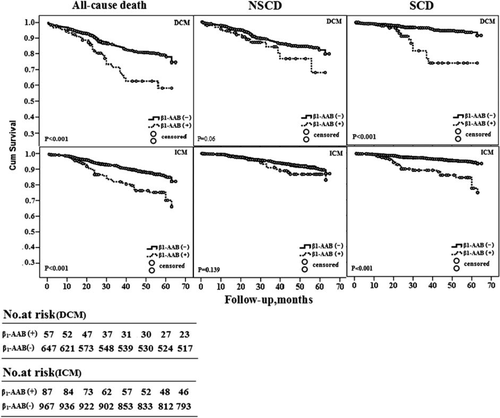
A total of 1758 cases (85.26%), made up of 704 (85.44%) cases of DCM and 1054 (85.14%) cases of ICM, were successfully followed up. During a median of 36 months (0.40–65months) of follow-up, 379 (21.56%) cases died (164 cases DCM and 215 cases ICM), and in 153 (40.37%) cases the cause was SCD (69 cases DCM and 84 cases ICM). The rest were due to NSCD. Comparing the CHF vs. the control groups, the presence of beta1-AAbs was 8.19% vs. 2.2% (P < 0.01) and of M2-AAbs was 3.01% vs. 1.84% (P > 0.05). The presence of beta1-AAbs in all-cause mortality patients was 20.73% in the DCM group and 19.07% in the ICM group; in patients with SCD it was 23.18% in the DCM group and 29.76% in the ICM group. After adjustment for risk factors such as age, gender, and other factors including NYHA classification, hypertension, diabetes mellitus, hyperlipidaemia, BMI, blood pressure, MHR, PVC, or AF, LVEF, LVEDD, pulmonary capillary wedge pressure (PCWP), and medications such as beta-blockers, ACEIs, etc., Cox regression analysis revealed that the presence of beta1-AAbs remained a positive predictor for all-cause death (HR 2.420, 95% CI 1.605–3.649 for DCM; and HR 2.339, 95% CI 1.673–3.271 for ICM) as well as SCD (HR 4.514, 95% CI 2.405–8.471 for DCM; and HR 3.749, 95% CI 2.389–5.884 for ICM) but not for NSCD, see Table 3. Kaplan–Meier curves using the log-rank test for time to probability of survival according to the presence or absence of beta1-AAbs in the patients with DCM and ICM showed that patients carrying beta1-AAbs were more susceptible to all-cause death and SCD. However, beta1-AAbs status was not correlated with NSCD (see Figure 1).
| Classification | Control (n = 824) | DCM (n = 704) | P-value | ICM (n = 1054) | P-value |
|---|---|---|---|---|---|
| Total, n (%) | |||||
| All-cause death | 0 | 164 (23.29%) | – | 215 (20.39%) | – |
| NSCD | 0 | 95 (57.93%) | – | 131 (60.93%) | – |
| SCD | 0 | 69 (42.07%) | – | 84 (39.07%) | – |
| Beta1-AAbs (+) | 18 (2.2%) | 57 (8.1%) | <0.001 | 87 (8.25%) | <0.001 |
| M2-AAbs (+) | 15 (1.82%) | 21 (2.98%) | 0.135 | 32 (3.03%) | 0.094 |
| Autoantibody strata | |||||
| Beta1-AAbs | |||||
| No death: beta1-AAbs (+) | 18 (2.2%) | 23 (3.26%) | >0.05 | 46 (4.36%) | >0.05 |
| HR (95% CI) | 1 | 1 | 1 | ||
| All-cause death: beta1-AAbs (+) | 0 | 34 (20.73%) | <0.001 | 41 (19.07%) | <0.001 |
| HR (95% CI) | 1 | 2.420 (1.605–3.649) | <0.001 | 2.339 (1.673–3.271) | <0.001 |
| NSCD: beta1-AAbs (+) | 0 | 18 (18.94%) | <0.001 | 16 (12.21%) | <0.001 |
| HR (95% CI) | 1 | 1.691 (0.969–2.951) | 0.064 | 1.475 (0.877–2.480) | 0.143 |
| SCD: beta1-AAbs (+) | 0 | 16 (23.18%) | <0.001 | 25 (29.76%) | <0.001 |
| HR (95% CI) | 1 | 4.514 (2.405–8.471) | <0.001 | 3.749 (2.389–5.884) | <0.001 |
| M2-AAbs | |||||
| No death: M2-AAbs (+) | 15 (1.82%) | 13 (2.40%) | >0.05 | 24 (2.86%) | >0.05 |
| HR (95% CI) | 1 | 1 | 1 | ||
| All-cause death: M2-AAbs (+) | 0 | 8 (4.87%) | <0.001 | 8 (3.72%) | <0.001 |
| HR (95% CI) | 1 | 1.403 (0.576–3.417) | 0.456 | 1.072 (0.530–2.169) | 0.846 |
| NSCD: M2-AAbs (+) | 0 | 5 (5.26%) | <0.001 | 4 (3.05%) | <0.001 |
| HR (95% CI) | 1 | 1.179 (0.374–3.711) | 0.779 | 0.915 (0.339–2.472) | 0.861 |
| SCD: M2-AAbs (+) | 0 | 3 (4.35%) | <0.001 | 4 (4.76%) | <0.001 |
| HR (95% CI) | 1 | 2.068 (0.500–8.555) | 0.316 | 1.294 (0.476–3.518) | 0.613 |
- a HR and 95% CI after adjustment for age, gender, and other suspected risk factors for all-cause mortality, NSCD, and SCD (including causes of chronic heart failure, body mass index, blood pressure, hypertension, diabetes mellitus, hyperlipidaemia, New York Heart Association class, left ventricular ejection fraction, maximum heart rate, premature vascular contractions or atrial fibrillation, and medications).
- b P <0.05 was considered significant.
- c AAbs, autoantibodies; CI, confidence interval; DCM, dilated cardiomyopathy; HR, hazard ratio; ICM, ischaemic cardiomyopathy; NSCD, non-sudden cardiac death; SCD, sudden cardiac death.
Discussion
The main finding of this prospective study was that the positivity for beta1-AAbs was higher in patients with CHF than in controls. Beta1-AAbs were associated with all-cause death and predicted SCD in patients with CHF. Beta1-AAb-positive patients had an approximately four- to five-fold risk increase for SCD compared with beta1-AAb-negative patients. This was consistent with the results from a previous report on 104 patients with DCM showing a higher SCD rate in autoantibody-positive patients during a mean follow-up of 2.5 years.19 However, beta1-AAb positivivity in the present study was 2.2% (controls), 8.1% (DCM), and 8.25% (ICM). This differed markedly when compared with previously published data from Jahns et al.20 which showed a beta-AAb-positive rate of 25%. Possible reasons for the difference of beta-AAbpositivitymight be as follows. (1) The definition of autoantibodypositivity was different. In our study, autoantibody positivity was defined as a ratio (patient OD – blank OD/control OD – blank OD) ≥2.1 rather than 1.5 or 2.0 in their study. (ii) Our follow-up period was relative long and since the plasma samples were stored for ∼3 years, the autoantibody activity might have decreased. Furthermore, our study showed that the presence of anti-beta1-AAbs predicted mortality in patients with both DCM and ICM, whereas Störk et al.21 showed that the presence of anti-beta1-AAbs was associated with increased all-cause and cardiovascular mortality risk in DCM but not in ICM. The possible reasons for this might be as follows. (i) We had a larger patient population, at total of 1758 patients (704 cases of DCM and 1054 cases of ICM) vs. 105 patients in their study (65 cases of DCM and 40 cases of ICM). (ii) The enrolment criteria were significantly different: they included DCM patients with LVEF <55% vs. LVEF ≤45% in our study; and they included ICM with a significant stenosis of one or more of the main coronary arteries >70% vs. luminal stenosis of at least one major coronary artery ≥70% and a history of myocardial infarction in our study. In our study all patients with ICM had a history of myocardial infarction. (iii) They did not analyse the relationship between positive beta1-AAbs and SCD in patients with ICM, instead looking at cardiovascular death, which was assumed to be due to myocardial infarction, a cerebrovascular event, decompensated heart failure, or sudden death. Our patients were actually the survivors of a myocardial infarction, and cerebrovascular death was not counted as SCD. Considering the findings in experiments showing that beta1-AAbs stimulated cardiac beta1-AR, increased the L-type calcium current, and activated the sodium/calcium exchanger, it would then play roles in the pathogenesis of cardiac arrhythmia in both patients with DCM and ICM. In contrast, positive M2-AAbs were similar between SCD and NCSD groups.
Evidence for an autoimmune mechanism in the pathogenesis of cardiovascular diseases is mounting. Limas22 reported a substance that inhibited the binding of radioactive ligands to beta1-AR in patients with DCM. Jahns20 also revealed that such antibodies against the second extracellular receptor (ECII-loop) could bind and stimulate beta1-AR in patients with DCM or ICM. Other studies also confirmed the presence of an autoantibody targeting the human beta1-AR in patients with DCM22 and also in patients with ICM at a lower frequency.21 Freedman and co-workers23 found that antibodies that stimulated cardiac beta1-AR might play an important role in development and/or progression of DCM. Further studies showed that the presence of beta1-AAbs in patients with DCM was associated with both the occurrence of severe VAs and SCD,24 and removal of beta1-AAbs by immunoabsorption resulted in a significant improvement of haemodynamic parameters, LVEF, and BNP levels,25consistent with our study. Although the sensitivity of beta1-AAbs for predicting SCD was low in our study (26.79%), the specificity was 84.69%, implying that beta1-AAb-negative patients had only an ∼10% risk for SCD. One possible major reason for such a low sensitivity might due to the limitation of the ELISA method used in this study; there are now methods with high sensitivity and specificity such as a complex three-step screening strategy (ELISA, immunofluorescence, cAMP radioimmunoassay) and fluorescence resonance energy transfer using a highly sensitive cAMP sensor (Epac1-based fluorescent cAMP sensor).26−28
Basic science research has shown that beta1-AAbs could modify the electrophysiological properties of the heart by increasing the intracellular production of cAMP and protein kinase A (PKA), and thus the phosphorylation of membrane L-type calcium channels after a calcium influx into the myocyte.29 The sustained calcium influx in the presence of autoantibody was found to activate the sodium/calcium exchanger in both human and animal cardiomyocytes and to lead to intercelluar calcium overload which resulted in slow but steady progressive myocyte destruction, fibrotic repair, subsequent heart muscle dysfunction, and electric instability of the heart.19,30–31 When the beta1-AAbs purified from DCM patients were added to neonatal rat ventricular myocytes, the repolarization phase of the action potential was prolonged and accompanied by an increase of the L-type calcium current. These effects were inhibited by bisoprolol. In a rat model, repetitive injections of beta1-antibodies caused adverse long-term effects on myocardial force development and relaxation.32 Rabbits chronically immunized with peptides corresponding to the ECII-loop of the beta1-AR had a prolonged repolarization phase of the action potential33 and increased frequency of VT, whereas the transient outward potassium current and delayed rectifier potassium current were decreased. Thus, autoantibody-mediated activation of the beta1-AR induces myocardial damage and provides the substrate for fatal VAs that might lead to SCD.
Our study found that M2-AAbs were not associated with the prognosis of CHF, consistent with previous studies, which suggests that M2-AAbs are associated with idiopathic sinus node dysfunction. It is believed that the M2 receptor-mediated regulation of the heart is more important in the atria, because of prevailing parasympathetic innervations. Although there have been reports refuting this,32,34 further studies might be of interest in this field.
In short, our study provided the evidence that beta1-AAbs are associated with poorer outcome in patients with CHF and might be useful as an independent predictor for SCD. It might also be helpful for the risk stratification of patients with CHF along with other known risk factors.
Our study was subject to four limitations. First, we were not able to find another study population in China to confirm our findings. Secondly, our study focused on clinical outcomes and we did not conduct any experiments to elucidate the function of autoantibodies in the setting of CHF. Thirdly, ∼15% patients were lost during follow-up, which might have created a bias in our results, although there were no significant differences in the baseline data between the missing group and group which was followed up. Finally, the ELISA method that was used in the study had low sensitivity and specificity compared with the other methods mentioned above.
Acknowledgements
The authors greatly appreciate Drs Ying Yang from Qingdao Fuwai Hospital, Chuanshi Xiao from the Second Hospital of Shanxi Medical University, Daowen Wang from Wuhan Tongji Hospital, JiangHong from Shanghai First People's Hospital, Jianzhong Zhou from first Affiliated Hospital of Chongqing Medical College, Xueqi Li from Fourth Affiliated Hospital of Harbin Medical College, Weibin Huang from Xiamen University Zhongshan Hospital, and He Jia from Hospital of Jilin Oilfield for assistance in collecting data and sera. The authors also thank Yaoli Pu (University of Rochester Medical Center) for reading and editing the manuscript.
Funding
The National Basic Research Program of China [973 program projects, program number: 2007CB512000, and project number: 2007CB512008 to J. Pu].
Conflict of interest: none declared.




