A double-blind, randomized, parallel, placebo-controlled study examining the effect of cross-linked polyelectrolyte in heart failure patients with chronic kidney disease
Abstract
Aims
This double-blind, randomized, parallel, placebo-controlled investigation evaluated the effects of cross-linked polyelectrolyte (CLP) on serum potassium and measures of congestion in patients with heart failure (HF) and chronic kidney disease (CKD).
Methods and results
The primary endpoint was change in serum potassium over time. Exploratory endpoints included: weight, physician and patient assessment of exertional dyspnoea, effect on N-terminal pro brain natriuretic peptide (NT-proBNP) levels, New York Heart Association (NYHA) classification, 6 min walk test (6MWT), and quality of life by Kansas City Cardiomyopathy Questionnaire (KCCQ). Serum potassium was similar in CLP (n =59) and placebo (n =52) groups throughout the 8-week study. Weight loss was greater in the CLP than in the placebo group at Weeks 1 (P =0.014) and 2 (P =0.004), and this trend continued until the end of the study. After 8 weeks, by physician assessment, the percentage of patients experiencing marked or disabling dyspnoea tended to be lower in the CLP than in the placebo group (7.3% vs. 23.9%, P =0.128). Fewer patients in the CLP than in the placebo group had NT-proBNP levels >1000 pg/mL at Week 4 (P =0.039) and Week 8 (P =0.065). The proportion of patients improving by at least one NYHA functional class over the study was higher in the CLP than in the placebo group (48.8% vs. 17.4%; P =0.002). Effects on 6MWT at Week 8 (p =0.072) and quality of life (overall KCCQ score) at Week 4 (p =0.005) and 8 (P =0.062) all favoured the CLP cohort. Four treatment-unrelated deaths occurred in the CLP group and none in the placebo group (P =0.056).
Conclusion
In advanced, symptomatic HF with CKD, CLP is associated with beneficial clinical effects without significant serum potassium changes.
Trial registration: NCT01265524.
See page 870 for the editorial comment on this article (doi: 10.1093/eurjhf/hfs098)
Introduction
Chronic heart failure (HF) is defined by the signs and symptoms associated with fluid overload.1 Evidence-based therapies have markedly improved survival in HF patients with reduced ejection fraction; still, management of congestion is essential to the control of symptoms. Although the mercurial diuretics were introduced early in the 20th century, it was the development of loop diuretics in the 1960s that provided rapid and effective therapy of both acute and chronic symptoms.2 However, concerns about the safety and therapeutic efficacy of diuretic-based treatment have been raised.3,4 The success of diuretic therapy made the kidney the focus of much of HF therapy, including drugs that block the renin–angiotensin–aldosterone system.
Unfortunately, the kidneys bear their own particular burden in HF. Renal function is often negatively influenced by both the same co-morbidities responsible for ventricular dysfunction and the syndrome of HF itself.5 The majority of patients admitted with HF have at least moderate kidney dysfunction and this profoundly influences prognosis.6 Physiological changes produced in the kidney by chronic diuretic use can result in diuretic resistance so that congestion becomes increasingly more difficult to control.7
Alternative therapies may influence fluid overload in HF patients. The gastrointestinal (GI) tract has been the principle path for drug ingestion for millennia. For most drugs, it provides the means for absorption into the circulation; additionally, its large surface area provides an opportunity for ion exchange. Several pharmaceuticals have taken advantage of this process including the recently introduced potassium binder RLY5016.8
Cross-linked polyelectrolyte (CLP) is a polymer that, given orally, absorbs both water and electrolytes [sodium (Na+) and potassium (K+)] in the GI tract with eventual elimination in the faeces.9 The GI tract is the portal of entry for Na+ and other electrolytes into the body and might provide an alternative means to control volume in addition to the kidney, especially when there is co-existent renal impairment. In several phase I studies with 51 healthy volunteers and 15 patients with end-stage renal disease (ESRD), treatment with 15 g of CLP was well tolerated and resulted in dose-dependent increases in stool weight, and faecal sodium and potassium excretion. Moreover, reductions in serum potassium concentrations were observed in both healthy volunteers and dialysis patients (Sorbent, unpublished data).
Fluid, Na+, and K+ retention remain a significant problem for HF patients with concomitant renal dysfunction despite diuretics and neurohormonal blockade. The current study was undertaken to explore the use of CLP as an adjunct to these therapies.
Methods
Study design
This phase II study was a double-blind, randomized, parallel, placebo-controlled investigation that examined the effect of CLP vs. placebo in preventing clinically significant hyperkalaemia and improving symptoms of fluid overload in patients with HF and renal insufficiency. The study was conducted in accordance with the principles described in the Declaration of Helsinki (1964) up to and including the Seoul revision (2008). A common clinical protocol was approved for each investigational site (located in Armenia, Georgia, and Moldova; for details of the participating countries and sites, see the Appendix) by the appropriate Ethics Committee, and all subjects provided written informed consent prior to participation.
Major inclusion criteria included age over 18 years with New York Heart Association (NYHA) class III or IV HF and chronic kidney disease (CKD) Stage 3 or 4 [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2]; not receiving the indicated spironolactone therapy based on the degree of HF; a recent hospitalization for HF (within 1–6 months); at least two signs of current fluid overload [jugular venous pressure (JVP) >8 cm; peripheral odema or ascites; pulmonary congestion on chest X-ray; pulmonary rales on auscultation]; ongoing stable treatment with a beta-blocker in addition to an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a screening serum K+ value of 4.3–5.1 mEq/L, and a serum N-terminal pro brain natriuretic peptide (NT-proBNP) level >1000 pg/mL. Exclusion criteria included concomitant use of sodium bicarbonate, antacids containing magnesium or calcium, polystyrene sulfonate; any cardiovascular, renal, hepatic, endocrine, neurological, or other disease or condition that made the patient's study participation unsafe; history of clinically significant GI pathology; severe, uncontrolled hypertension; use of aldosterone antagonist in the last 30 days or of K+-sparing medications or K+ supplements; liver transaminases >3 times the upper limit of noraml (ULN); serum creatinine value of ≥3 mg/dL; uncorrected primary severe valvular disease, known obstructive or restrictive cardiomyopathy, or uncontrolled arrhythmias; coronary artery bypass graft or percutaneous cardiac interventions within 3 months; heart transplant recipient; myocardial infarction, transient ischaemic attack, stroke, or acute coronary syndrome within 1 month; and current dialysis patient or kidney transplant recipient or need for such during the study.
The study incorporated a screening period, baseline visit, and an 8-week fixed dose treatment period during which patients were randomized at the baseline visit to 15 g/day of CLP or identical placebo capsules containing microcrystalline methyl cellulose, a white powder similar in appearance to CLP. Spironolactone was initiated at 25 mg/day and, if the serum K+ was ≤5.1 mEq/L, the dose was increased to 50 mg/day at the Week 4 visit. Doses of ACEI and ARB were not to be changed during the study. A central laboratory was utilized for laboratory assessments. Serum chemistry, clinical status, and HF assessments were monitored throughout the study a mean of every 1–2 weeks. Single 6 min walk tests (6MWTs) were performed using standard techniques at baseline, and Weeks 4 and 8.9 Evaluation of the subjective dyspnoea level using a 7-point Likert scale, and the Kansas City Cardiomyopathy Questionnaire (KCCQ) were evaluated at baseline, and at Weeks 4 and 8.10,11
Study endpoints
The primary endpoint was the change in serum K+ from baseline to the end of the study (or last observation). Several secondary endpoints focused on measures of congestion and functional capacity, including changes in 6MWT, NYHA functional class, KCCQ, physician assessment of exertional dyspnoea, and patient-reported dyspnoea level (7-point Likert scale). Additional exploratory endpoints included incidence of serum K+ elevation >5.5 mEq/L; achieving the target 50 mg spironolactone dose; HF-related hospitalization; systolic and diastolic blood pressure (BP); body weight; and NT-proBNP levels.
Statistical analysis
The analysis plan was finalized prior to study unblinding or performance of statistical analyses. Given that this was a phase II study, formal power/sample size calculations were not performed, and 100 patients were targeted for enrolment. The primary analyses were performed on the intent to treat (ITT) population, and end of study results for non-completers were excluded. Sensitivity analyses were performed for the primary and secondary endpoints using a last observation carried forward approach which included Week 8/end of study results for non-completers. Categorical data were evaluated using the χ2 test for homogeneity of proportions. Continuous or ordinal data were analysed with repeated measures analysis of covariance (RM-ANCOVA) using change from baseline values to compare the CLP and placebo treatments. Using this model, a restricted maximum likelihood approach was used to estimate and compare mean profiles between the two treatment groups, assuming an unstructured covariance matrix. Given that this was a phase II study, corrections for multiplicity were not performed and a P-value ≤0.05 was considered statistically significant. As this was a phase II study, statistical testing was not performed for any of the non-efficacy variables. No clinically important differences in baseline characteristics were observed between treatment groups, with the exception of the gender imbalance (Table 1).
| Characteristic | CLP (n =59) | Placebo (n =52) |
|---|---|---|
| Age (years), mean ± SD (range) | 68 ± 8 (51–84) | 70 ± 10 (49–86) |
| Male gender, n (%) | 42 (71.2) | 29 (55.8) |
| Co-morbidities, (%) | ||
| Hypertension | 78.0 | 92.3 |
| Coronary artery disease | 67.8 | 75.0 |
| Chronic obstructive pulmonary disease | 11.9 | 7.7 |
| Diabetes mellitus | 23.7 | 30.8 |
| Atrial fibrillation | 20.3 | 25.0 |
| NYHA functional class, n (%) | ||
| II | 0 (0.0) | 2 (3.8) |
| III | 52 (88.1) | 45 (86.5) |
| IV | 7 (11.9) | 5 (9.6) |
| KCCQ Overall Summary Score, mean ± SD (range) | 37.3 ± 16.1 (6.3–84.9) | 38.1 ± 17.1 (3.9–78.6) |
| Vital signs, mean ± SD (range) | ||
| Weight (kg) | 75.6 ± 13.6 (45.4–109.6) | 75.0 ± 16.6 (40.9–119.1) |
| Systolic blood pressure (mmHg) | 129.1 ± 14.1 (100–160) | 132.6 ± 15.7 (100–165) |
| Diastolic blood pressure (mmHg) | 79.4 ± 8.5 (60–100) | 80.7 ± 8.5 (70–100) |
| Heart rate (b.p.m.) | 74.9 ± 10.1 (53–102) | 77.0 ± 14.0 (58–139) |
| Laboratory measurements, mean ± SD (range) | ||
| Blood urea nitrogen (mg/dL) | 25.2 ± 12.8 (13–72) | 25.5 ± 10.4 (12–56) |
| Serum creatinine (mg/dL) | 1.7 ± 0.6 (0.7–3.2) | 1.6 ± 0.6 (0.9–3.3) |
| eGFR (mL/min/1.73 m2) | 45.0 ± 14.2 (19.2–75.3) | 44.9 ± 17.3 (11.3–95.9) |
| Serum sodium (mg/dL) | 140.2 ± 2.8 (132–144) | 141.4 ± 2.5 (137–149) |
| Serum potassium (mg/dL) | 4.73 ± 0.4 (3.3–5.7) | 4.9 ± 0.3 (4.3–5.6) |
| Serum bicarbonate (mmol/L) | 25.7 ± 4.4 (11.7–38.5) | 26.5 ± 2.9 (17.5–34.6) |
| NT-proBNP (mg/dL) | 5169.8 ± 6181.3 (1085–29 753) | 4868.3 ± 6603.7 (924–35 382) |
| Haematocrit (mg/dL) | 39.4 ± 4.7 (28.9–50.2) | 40.3 ± 5.6 (29.4–53.7) |
| Medication, n (%) | ||
| ACE inhibitor/ARB | 56 (95) | 49 (94) |
| Beta-blocker | 56 (95) | 46 (89) |
| Aldosterone antagonists | 59 (100) | 52 (100) |
| Diuretics | 49 (83) | 48 (92) |
- a ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association.
Results
Baseline characteristics
Of 223 subjects screened at 24 sites, 113 were randomized to either CLP (n = 59) or placebo (n = 54). The most frequent reasons for screen failures were eGFR above the 60 mL/min/1.73 m2 inclusion threshold, serum creatinine ≥3 mg/dL, serum K+ outside the 4.3–5.1 mEq/L range, or low NT-proBNP <1000 pg/mL. Demographic characteristics were similar between groups except that a larger percentage of males were randomized to CLP than to placebo (71.2% vs. 55.8%; P =0.091) (Table 1). Clinical and laboratory variables were similar between groups. By design, all patients had advanced HF (≥1 HF hospitalizations in the 6 months preceding randomization, baseline NYHA functional class 3.1 ± 0.4, presence of signs and symptoms of congestion, and baseline mean NT-proBNP values of 5028 ± 6355 pg/mL) and CKD (average eGFR 44.9 ± 15.7 mL/min/1.73 m2). At study onset, ∼90% of patients were receiving guideline-recommended medical therapy. From baseline to the end of Week 8, compliance with the study drug was excellent, as indicated by the fact that the CLP and placebo patients took 96.3% and 97.2%, respectively, of the prescribed capsules.
Efficacy evaluation
Primary endpoint
Serum K+ was similar in the CLP and placebo groups at baseline and throughout the 8 week study period. The two groups were similar in terms of incidence of hyperkalaemia (K+ >5.5 mEq/L), 13 (22.4%) vs. 11 (21.2%), and hyperkalaemia prompting discontinuation of study drug, 6 (10.2%) vs. 5 (9.3%). At the end of Week 4, the percentages of patients eligible to increase their daily spironolactone dose to 50 mg because their serum K+ level was ≤5.1 mEq/L were similar in the CLP and placebo groups (64.4% vs. 73.1%; P =0.327).
Secondary endpoints
Measures of fluid overload
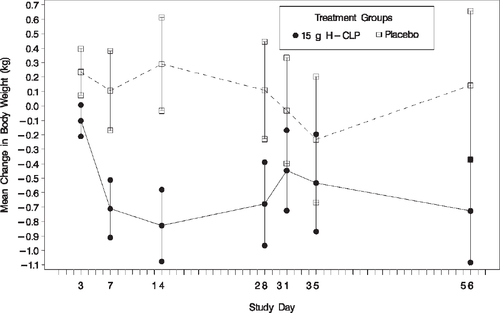
Weight loss was significantly greater in the CLP than in the placebo group at Week 1 (–0.71 ± 1.5 kg vs. 0.11 ± 2.0 kg; P =0.014) and at Week 2 (–0.83 ± 1.8 kg vs. 0.29 ± 2.3 kg; P =0.004) and a trend toward greater weight loss in the CLP group continued at Weeks 4 (P =0.066) and 8 (P =0.212) (Figure 1).
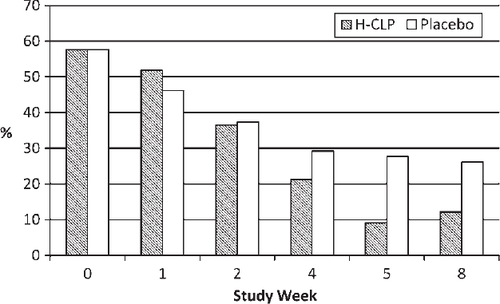
The frequency of marked or disabling exertional dyspnoea by physician assessment decreased over time for the entire study population. However, fewer patients in the CLP group than in the placebo group had this degree of dyspnoea at Week 4 [10 (21.3%) vs. 14 (29.2%) (P =0.376)] and Week 8 [5 (12.2%) vs. 12 (26.1%) (P =0.103)] (Figure 2). The percentage of patients reporting ‘moderately or markedly better’ breathing (by the 7-point Likert scale) was 21.3% in the CLP group and 16.7% in the placebo group (P =0.567) at Week 4 and 36.6% and 21.7%, respectively (P =0.127) at Week 8.
The two groups were similar throughout the study in terms of NT-proBNP levels. However, at Weeks 4 and 8, fewer patients in the CLP group than the placebo group had NT-proBNP levels >1000 pg/mL [Week 4, 43 (91.5%) vs. 48 (100 %); P =0.039; and Week 8, 36 (87.8%) vs. 45 (97.8%); P =0.066, respectively].
Changes in diuretic doses during the study period were modest and similar in the two groups. During the study, two CLP patients and one placebo subject had an HF-related hospitalization.
Measures of functional capacity and quality of life
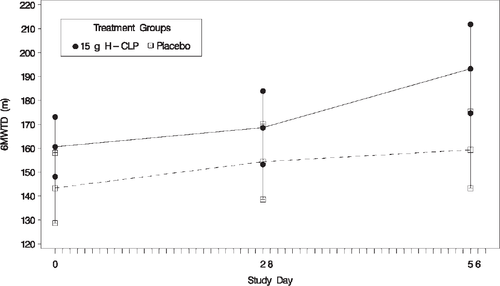
The proportion of patients improving by at least one NYHA functional class from baseline to Week 8 was substantially higher in the CLP than in the placebo group [20 (48.8%) vs. 8 (17.4 %); P < 0.001]. At Week 4, the 6MWT distance increased from baseline by ∼20 m in both groups. However, the 6MWT distance from baseline to Week 8 tended to increase more in the CLP than in the placebo group (39.3 ± 53.39 m vs. 19.7 ± 39.17 m; P =0.072) (Figure 3).
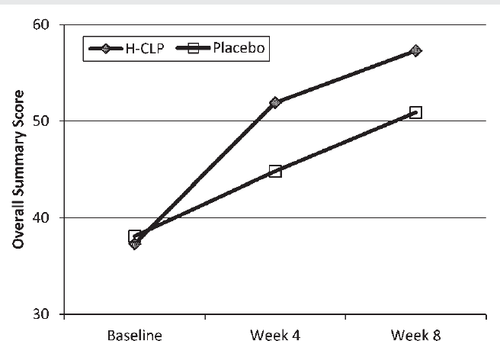
Quality of life improvement by the KCCQ Overall Score was greater in the CLP than in the placebo group at the end of Weeks 4 (13.53 ± 12.23 vs. 6.40 ± 12.3; P =0.005) and 8 (18.3 ± 17.4 vs. 12.5 ± 13.6; P =0.062) (Figure 4). Similar directional differences occurred in all of the individual components of the KCCQ, including social limitations, physical limitations, symptom frequency, and clinical summary scores.
Systemic blood pressure
For the entire cohort, there were no differences in BP between groups throughout the course of the study.
Safety evaluation
Adverse events
A summary of total, cardiac, and GI adverse events reported for the CLP and placebo groups is shown in Table 2. No statistically significant differences were identified.
| MeDRA SOC preferred term | 15 g CLP (n =59) | Placebo (n =52) |
|---|---|---|
| Total no. of patients with adverse events | 21 (35.6%) | 16 (30.8%) |
| Cardiac disorders | ||
| Total | 3 (5.1%) | 3 (5.8%) |
| Atrial fibrillation | 1 (1.7%) | 1 (1.9%) |
| Cardiac failure | 1 (1.7%) | 0 (0/0%) |
| Cardiac failure acute | 1 (1.7%) | 1 (1.9%) |
| Cardiac failure congestive | 0 (0.0%) | 1 (1.9%) |
| Gastrointestinal disorders | ||
| Total | 14 (23.7%) | 7 (13.5%) |
| Abdominal discomfort | 7 (11.9%) | 2 (3.8%) |
| Abdominal distention | 3 (5.1%) | 1 (1.9%) |
| Abdominal pain | 1 (1.7%) | 0 (0.0%) |
| Abdominal pain (upper) | 0 (0.0%) | 1 (1.9%) |
| Constipation | 1 (1.7%) | 0 (0.0%) |
| Diarrhoea | 0 (0.0%) | 1 (1.9%) |
| Nausea | 3 (5.1%) | 0 (0.0%) |
| Vomiting | 1 (1.7%) | 3 (5.8%) |
Most GI disturbances were transient and mild to moderate. Only four patients, all in the CLP group, experienced adverse events leading to study discontinuation (constipation; nausea and vomiting; nausea, bloating, and loss of appetite; and generalized weakness, bloating, and loss of appetite). There were no clinically relevant changes in serum Na+, calcium, magnesium, or phosphorus. In the CLP group, mean serum bicarbonate level decreased ∼2–3 mmol/L from baseline to the end of Week 2, but mean values remained in the normal range and returned to pre-treatment levels by the end of Week 4. Measures of renal function [blood urea nitrogen (BUN), serum creatinine, and eGFR] did not change significantly from baseline to the end of Week 8 in either the CLP or placebo group.
Deaths
Four deaths, all in the CLP group, occurred during the study. Each death was judged by the investigator to be definitely unrelated to the study drug. The first death occurred suddenly at Week 3 in a 70-year-old NYHA class IV male with ischaemic HF, left ventricular ejection fraction (LVEF) =20%, atrial fibrillation, and eGFR =46 mL/min/1.73 m2. He was seen by the investigator 2 weeks before the event and was clinically stable with no electrocardiogram (ECG) or laboratory changes.
The second death occurred at Week 4 in a 60-year-old NYHA class III diabetic male with ischaemic HF, LVEF =20%, previous pulmonary embolism, and eGFR =48 mL/min/1.73 m2. The patient was seen by the investigator 2 days before he developed an acute HF decompensation complicated by impaired consciousness and hypoxia.
The third death occurred at Week 6 in a 63-year-old NYHA class III male with ischaemic HF. Two weeks before death the patient had refused hospitalization recommended on the basis of worsening fluid overload and progressive renal impairment, as indicated by a drop in eGFR from 48 to 20 mL/min/1.73 m2.
The fourth death occurred at Week 6 in a 67-year-old NYHA class IV male with non-ischaemic HF, atrial fibrillation, and eGFR =36 mL/min/1.73 m2 who complained of abdominal distension and loss of appetite associated with a metabolic acidosis in the absence of renal function changes 3 d before the patient stopped taking study drug. The patient was hospitalized 3 days after study drug discontinuation due to worsening dyspnoea, hydrothorax, ascites, and marked acidosis, and died 3 days later.
Discussion
The trial presented here is a phase II, double-blind, randomized, parallel group, placebo-controlled study designed to explore the effects of CLP vs. placebo on serum K+ concentration and fluid overload in HF patients with renal impairment who are on the guideline-recommended combination of an ACE inhibitor or ARB, a beta-blocker, and diuretics, and have a clinical indication for the addition of spironolactone.1213–14
The principal aim of this early study was to explore the effects of CLP on multiple clinical and laboratory variables to identify the most appropriate endpoints for future pivotal clinical trials. Based on the results of pilot studies in normal volunteers and patients with end-stage renal disease (ESRD), it was speculated that a daily 15 g CLP dose might lower serum K+ and/or reduce fluid overload in patients with advanced HF and CKD, defined as an eGFR <60 mL/min/1.73 m2.
Throughout the study, no differences were observed between CLP and placebo in serum K+ concentrations. In contrast, in phase I studies in healthy volunteers and ESRD patients, CLP produced significant faecal removal of K+ and changes in serum K+ (unpublished data on file). Based on these findings, the lack of effect of CLP on serum K+ concentrations in the present study is both surprising and difficult to explain. Neither dietary Na+ and K+ intake nor faecal K+ content were measured in this study and therefore it cannot be determined if co-existing HF and CKD influenced colonic handling of K+ in the study subjects.15 Experimental evidence shows that aldosterone increases colonic K+ secretion, whereas limiting dietary Na+ activates colonic K+ absorption.16 The use of an aldosterone antagonist in Na+-restricted HF patients may have attenuated the ability of CLP to increase faecal K+ elimination. An alternative explanation is that the addition of spironolactone was associated with a rise in serum K+, leading to comparable rates of hyperkalaemia in both treatment groups. It is well established that the use of an aldosterone antagonist in patients with reduced renal function can lead to increased rates of hyperkalaemia. For example, in a study of 105 HF patients with either a history of hyperkalaemia or CKD, an incidence of hyperkalaemia as high as 38.5% was seen in patients with an eGFR <60 mL/min/1.73 m2.8 In that study the polymeric K+ binder RLY5016 significantly lowered serum K+ levels and allowed up-titration of spironolactone to a 50 mg dose in a higher proportion of subjects.8
Several differences between the CLP and RLY5016 studies are noteworthy. Severity of HF was much greater in the current study than in the patients enrolled in the RLY5016 study, as indicated by a higher NYHA class and NT-proBNP levels and a lower eGFR in the CLP population. 8 Hypokalaemia and hypomagnesaemia did not occur in the CLP study. In contrast, compared with placebo, RLY5016 was associated with a higher frequency of hypokalaemia (0% vs. 6%) and greater reduction in Mg levels (P < 0.001). This is of considerable concern in HF patients in whom hypokalaemia and hypomagnesaemia are associated with an increased risk of arrhythmias and poorer outcomes.3,16,17
Another important difference between the RLY5016 and the CLP compounds is that the former binds very little sodium, whereas the latter can simultaneously bind K+ and Na+.18 Due to this unique feature of CLP, the present study was designed also to evaluate the effects of this polymer on HF-related congestion and functional impairment. Favourable and consistent actions of 15 g/day CLP on parameters associated with fluid overload were observed. These effects resulted in a pattern of improvement over placebo in both physician-assessed and patient-reported outcomes. During the study period, compared with the placebo group, the patients treated with CLP lost more weight and were less likely to have severe exertional dyspnoea. Compared with placebo, 15% more CLP patients described their current dyspnoea as being moderately or markedly better than before initiation of study drug. This differential improvement in dyspnoea between study drug and placebo is similar to the 12–15% differences between either relaxin or the adenosine A1 receptor antagonist rolofylline and placebo observed in controlled clinical trials of acute HF where one would reasonably expect to see larger early changes.19,20 In addition, by the end of the study, the treatment group included more patients who improved by at least one NYHA class and fewer patients with NT-proBNP levels >1000 pg/mL,21 the inclusion criteria cut-off. The decongestion occurring with CLP may be related to reduction of total body Na+ by the polymer as well as the polymer's ability to trap free water. Fluid overload in HF patients is inevitably related to an increase and abnormal distribution of total body Na+.22 Indeed, withdrawal of isotonic fluid by ultrafiltration improved the outcomes of hospitalized HF patients more than removal of hypotonic urine with diuretics.23,24 In the CLP group there is continuous trapping of Na+ and fluid both from the diet and the GI secretions. This is likely to modulate total body Na+ downward in a more physiological manner than might be expected from the ‘peak and valley’ hypotonic reductions induced by intermittent diuretic therapy.24 From baseline to the end of the study, the CLP group achieved a trend toward longer 6MWT distances and higher KCCQ scores. The totality of these findings, whether or not statistically significant, suggests that CLP produced a potentially clinically meaningful reduction in HF-related congestion. This is important, because hypervolaemia itself has been shown to worsen the outcomes of HF patients.25 The prognostic value of the 6MWT was clearly demonstrated in patients from the Studies of Left Ventricular Dysfunction (SOLVD) and from the SOLVD Registry.9
The KCCQ is a self-administered, 23-item questionnaire that quantifies physical limitations, symptoms, self-efficacy, social interference, and quality of life. In some studies of HF patients, the KCCQ has been shown to have greater responsiveness to clinical change than other quality of life assessment tools.11
The observed overall mortality rate of 4% was as expected in a population of patients with co-existing advanced HF and CKD.27 All four deaths occurred in the CLP group. None of the deaths was attributed by the attending investigator to the use of blinded study drug. An independent analysis of all available patient data by a group of HF experts did not identify a unifying aetiology that could be attributed to CLP. Regardless, this imbalance in mortality mandates cautious safety monitoring in the future development of CLP including the involvement of an independent safety monitoring board.
Overall, CLP was well tolerated. The majority of GI adverse events were mild or moderate and transient. In only four instances were adverse events sufficiently severe to require study discontinuation. Interestingly, GI adverse events rates are very similar for CLP and RLY5016 (23.7% vs. 21%).8 Patients with adverse events leading to study drug discontinuation appear to be similar for CLP and RLY5016 (7% vs. 7%).8 The brief decline (within the normal range) in serum bicarbonate levels observed in the CLP group was probably a result of physiological buffering to counter a minor transient increase in ingested hydrogen ion. This finding warrants further monitoring in future trials.
Conclusions
Administration of CLP is associated with favourable effects on signs and symptoms of HF without serum K+ changes in patients with advanced, symptomatic HF and CKD. In these patients, CLP therapy may represent an effective method of fluid removal, clinical improvement, and an alternative approach in diuretic-resistant states.
Future directions
Future investigation may elucidate the reason for the lack of change in serum K+ concentration by CLP and help identify which changes in the structure of the polymer may lead to increased faecal K+ elimination. The consistent reduction in both physician-assessed and patient-reported variables should encourage further clinical investigation of CLP to treat congestion-related HF morbidity.27 CLP treatment may be useful in patients with significant renal impairment because its effects are independent of renal function. Additionally, in fluid-overloaded HF patients with CKD, CLP may permit the lowering of loop diuretic doses and therefore attenuate the potential harmful effects associated with high doses of these drugs.28,29 CLP may also be useful in optimizing decongestion in hospitalized HF patients nearing discharge.30
Acknowledgements
The authors would like to thank Howard Dittrich, MD, for critical evaluation of the manuscript, Thomas Oliphant (Innovative Analytics, Kalamazoo, MI) and Ed Vonesh for statistical support, Alena Shevchenko for clinical monitoring, and Ric Newman for support in design and conduct of the study. Last, but not least, the authors would like to thank all study sites and the patients who participated in this study.
Funding
Sorbent Therapeutics, Inc.
Conflict of interest: M.R.C., J.T.H., B.M.M., P.S., and D.L.v.V. have received consultancy fees from Sorbent Therapeutics, Inc. L.H., D.A., and J.L are employees of sorbent Therapeutics, Inc. M.M., H.S., and H.H have received research funding from Sorbent Therapeutics, Inc.
Appendix: Participating countries and sites
Armenia (‘Armenia’ Republic Medical Centre Closed Joint-Stock Company, Yerevan, M. Drambyan, A. Ter-Margaryan, A. Baghdasaryan; Department of Urgent Cardiology of ‘Erebouni’ Medical Center Closed Joint-Stocks Company, Yerevan, H. Hayrapetyan, Z. Mirzoyan, S. Ghulijanyan; ‘St. Grigor Lusavorich’ Medical Centre Closed Joint-Stocks Company, Yerevan, L. Kocharyan, A. Sargsyan, S. Ghumashyan, N. Aghabekyan; Yerevan State Medical University Muratsan Hospital, Yerevan, A. Piruzyan, A. Sargsyan, A. Mshetsyan, L. Antonyan; Department of General and Invasive Cardiology of Yerevan State Medical University, Yerevan, H. Sisakyan, T. Sargsyan, L. Sahakyan; Center of Preventive Cardiology ‘Barekam’ LLC, Yerevan, P. Zelveian, Z. Hakobyan, L. Tumasyan, T. Hamazaspyan); Georgia (Emergency Cardiology Center named by acad. G. Chapidze, Tbilisi, G. Chapidze, L. Rigvava, K. Gabunia, T. Kavtaradze; Emergency Cardiology Center named by acad. G. Chapidze, Tbilisi, V. Chumburidze, T. Kikalishvili, M. Agladze, K. Lekishvili, N. Kharchilava; LTD Medical Diagnostic Centre ‘Samgori Medi’, Tbilisi, N. Egiashvili, M. Bolkvadze, G. Abjandadze; Tbilisi State Medical University A. Aladashvili University Clinic, Tbilisi, N. Emukhvari, I. Khintibidze, R. Napetvaridze, K. Khijakadze; MediClubGeorgia Medical Centre, Tbilisi, D. Gochitashvili, D. Mindiashvili, P. Pitiurishvili; Cardio-Reanimation Centre LTD, Tbilisi, B. Kobulia, I. Jashi, A. Kadzhaya, I. Maisuradze; Ltd ‘LJ Clinic’, Kutaisi, Z. Lominadze, G. Sharvadze, L. Ushkhvani, T. Charkhalashvili; Adapti Angio-Cardiology Clinic LTD, Tbilisi, M. Mamatsashvili, T. Kajaia, N. Gvelesiani; Cardiology Clinic LTD, Tbilisi, I. Megreladze, N. Gagua, K. Berdzenishvili; LTD ‘Cito’, Tbilisi, Z. Pagava, R. Agladze; Multiprofile Clinical Hospital of Tbilisi #2, Tbilisi, K. Paposhvili, N. Chkhaidze, A. Kvitsiani, I. Gvenetadze, M. Shushania, E. Porchkhidze; Tbilisi Heart and Vascular Clinic LTD, Tbilisi, T. Shaburishvili, G. Khabeishvili, I. Verulava, E. Narchemashvili; Health Center ‘Medina’, Sharabidzeebi, Khelvachauri, Ajarian Autonomic Republic, I. Sikharulidze, M. Mishvelidze, G. Nikolaishvili; Samgori Medi, Tbilisi, N. Egiashvili); Moldova (IMSP Clinical Hospital of Health Ministry, Chisinau, V. Calancea, D. Barba, A. Zlatoycena; Research Department in Emergency Cardiology and Rhythm Disturbances Institute of Cardiology, Chisinau, A. Grosu, L. David, A. Raducan, V. Racila; Municipal Clinic Hospital ‘Sfinta Treime’, Chisinau, S. Matcovschi, T. Dumitras, N. Capros; Cardiology Policlinics, Chisinau, L. Morcov, A. Marina, V. Rudi; Municipal Clinic Hospital ‘Arhanghelul Mihail’, Chisinau, I. Tibirna, A. Scripnic, G. Bezu).




