A contemporary view on endothelial function in heart failure
Abstract
The assessment of different aspects of endothelial dysfunction in cardiovascular medicine in general and in heart failure (HF) has been the focus of intense research, and includes vasomotor, haemostatic, antioxidant, and inflammatory activities. Differences also exist in the pattern of endothelial dysfunction depending on aetiology, severity, and stability of HF in individual patients. In the majority of patients with ischaemic aetiology of HF, endothelial dysfunction is systemic in its nature and involves both arteries and veins, conductance vessels and microvascular beds, coronary, pulmonary, and peripheral vessels. The pattern of endothelial dysfunction is more heterogeneous in non-ischaemic HF, with fewer features of systemic abnormalities. Indeed, many subjects with non-ischaemic HF have a functionally preserved endothelium in peripheral arteries, with endothelial dysfunction seen only in coronary vessels. Endothelial dysfunction has significant prognostic value in HF, but its clinical application is hampered by methodological limitations in its assessment. Various medications (including angiotensin-converting enzyme inhibitors and statins) and regular physical activity have been shown to improve endothelial function in HF. However, there are still no pharmaceutical agents specifically targeting the vascular endothelium. Despite the large number of studies, the pathophysiological role of the vascular endothelium and its clinical potential as a therapeutic target has not yet been sufficiently developed and undoubtedly awaits further exploration.
Introduction
The prominent regulatory activity of the vascular endothelium in heart failure (HF) was discovered about two decades ago, and its assessment in different cardiovascular disorders, including heart failure (HF), has been the focus of intense research. Indeed, various aspects of endothelial function are affected in HF, including vasomotor, haemostatic, antioxidant, and inflammatory activities (Figures 1 and 2). Differences also exist in the pattern of endothelial dysfunction depending on aetiology, severity, and stability of HF in individual patients. Accumulating evidence suggests a prognostic value of different measures of endothelial dysfunction in HF. Furthermore, a number of therapeutic interventions and regular exercise can improve endothelial function in HF.1,2
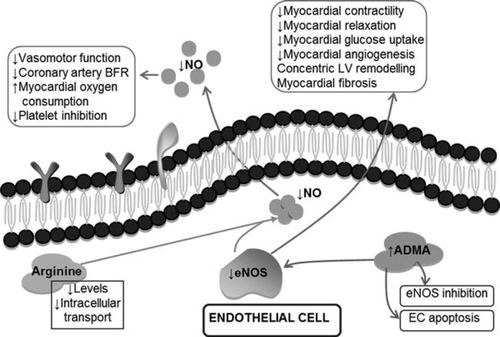
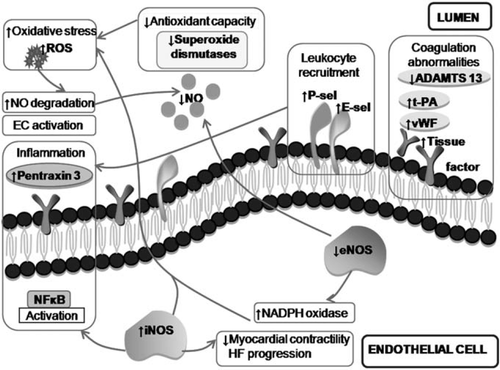
In this review article, we aim to provide a critical and contemporary analysis of the available evidence on pathological and prognostic implications of endothelial abnormalities in systolic HF. Only scarce data are available on endothelial dysfunction in HF with preserved ejection fraction. In order to provide a holistic approach to the topic, such studies will be discussed with the type of HF clearly specified. Given the vast amount of data published on endothelial function in HF, the review is more focused on clinical data with in-depth discussion of pathophysiological insights; prothrombotic transformation of the endothelium in HF and the role of endothelial progenitors and angiogenic factors in HF having recently been reviewed elsewhere.3–5 Despite the diverse activities that the endothelium is involved in, its vasomotor function has attracted most attention of researchers and it is considered under the term ‘endothelial dysfunction’. Accordingly, vasomotor abnormalities are discussed under endothelial dysfunction in this review, unless other particular endothelial functions are specified.
Clinical evidence of endothelial dysfunction in heart failure
Numerous studies have demonstrated peripheral endothelium-dependent vasomotor abnormalities in HF assessed by brachial artery flow-mediated dilation (FMD) or forearm blood flow changes in response to acetylcholine.6 However, many such studies show weak, if any, correlations between these measures of endothelial dysfunction and clinical parameters of HF severity, cardiac contractility, or wedge pressure.
Although the presence of endothelial dysfunction has been uniformly shown in ischaemic HF, the evidence is less robust in the case of non-ischaemic HF (NIHF). Both endothelial-dependent and endothelial-independent vasodilation are progressively depressed with increasing clinical severity in HF related to valvular heart disease, making it difficult to be precise on the cause of the vascular dysfunction. In one small study, HF of non-ischaemic aetiology was not associated with abnormalities in FMD, which is in contrast to ischaemic HF.7 In other studies, patients with ischaemic systolic HF have more prominent endothelial dysfunction compared with those with non-ischaemic HF of the same severity.8
Although endothelial dysfunction is a feature of HF of any aetiology, multiple cofactors typical of ischaemic HF (e.g. atherosclerosis, diabetes, etc.) seem likely to contribute towards systemic endothelial impairment per se. In contrast, patients with NIHF could be expected to have more localized endothelial dysfunction of the cardiac vasculature. The evidence supporting this concept will be discussed further below. However, the degree of vasomotor endothelial dysfunction typically established in brachial or radial arteries may show little, if any, correlation with parameters of endothelial dysfunction in other vascular beds. Data on endothelial dysfunction in HF specific to particular vascular beds are discussed in the following sections.
Endothelial dysfunction of the coronary circulation in heart failure
Significant impairment of coronary endothelial function has been observed in patients with left ventricular (LV) dysfunction. Significant impairment of coronary endothelial function is also present in most patients with stable idiopathic dilated cardiomyopathy (IDCM) despite normal epicardial coronary arteries, and profound coronary endothelial dysfunction was observed in acute-onset IDCM.9 Of note, the abnormalities of coronary blood flow reserve in IDCM correlated with cardiac geometry and N-terminal pro brain natriuretic peptide (NT-proBNP) levels, but not with FMD of peripheral arteries.10 These findings indicate that in contrast to patients with an atherosclerosis-related HF aetiology, many patients with NIHF tend to have localized (i.e. coronary/endocardial) rather than systemic endothelial dysfunction. Coronary endothelial impairment in HF involves both resistance (microvascular) and conductance (large) vessels, and it parallels deterioration of cardiac contractility.11 In addition to the regulation of cardiac haemodynamics, it is increasingly appreciated that there is a close communication of endothelial cells with cardiomyocytes and fibroblasts, which orchestrate cardiac remodelling, fibrosis, and angiogenesis, ultimately reflecting the complex nature of heart failure.12,13
In one study, the coronary response to acetylcholine correlated with the subsequent improvement in LV ejection fraction during a 7-month follow-up period.14 Furthermore, coronary endothelial dysfunction was independently associated with impaired cardiac relaxation in 160 patients with normal ejection fraction in the absence of occlusive coronary artery disease (CAD).15 Also, coronary endothelial dysfunction in CAD has been associated with future progression of myocardial diastolic dysfunction.16 These data are supported by findings showing that altered myocardial nitric oxide (NO) balance contributes to hypertrophy-mediated myocardial ischaemia and a transition to HF.17 Both endothelial nitric oxide synthase (eNOS) activity and expression in cardiac tissue were reduced in ischaemic HF, whilst inducible NOS (iNOS) was increased in human failing hearts.17 Thus, coronary endothelial perturbation may be central to the pathogenesis of HF.
Systemic nature of endothelial dysfunction in heart failure
Accumulating evidence indicates a systemic nature of endothelial dysfunction in systolic HF. It involves arterial (mentioned above), venous, and microvascular endothelial cells and affects both systemic and pulmonary circulatory beds. For example, venous endothelial cells in dogs with decompensated HF showed evidence of inflammatory activation secondary to high vascular stress and peripheral blood flow congestion.18 On a background of CAD, impaired venous endothelial function in vitro in ischaemic HF has been associated with elevated C-reactive protein levels and > 2-fold higher NADPH-dependent superoxide generation.19 Endothelium-dependent venodilation by dorsal hand veins is also significantly reduced in chronic HF, but particularly so in acute decompensated HF, and its improvement correlates with the 6 min walk test distance, thus suggesting the presence of venous vasomotor dysfunction in HF.20 In contrast, Nightingale et al. reported preserved forearm venous endothelial function in patients with chronic NIHF despite arterial endothelial dysfunction.21
Vasomotor endothelial dysfunction has also been demonstrated in relation to the pulmonary circulation. Resting exhaled NO, a marker of pulmonary endothelial NO release, is increased in HF, indicating that NO output plays a counter-regulatory role in the impaired blood flow seen in chronic HF. However, HF patients have significantly reduced ability to increase NO release during exercise, which parallels any oxygen deficit and is consistent with the presence of endothelial dysfunction.
The systemic nature of endothelial dysfunction in HF is also supported by reports of a defective endothelium-dependent dilatory response (i.e. vasomotor dysfunction) of the microvascular segment.22 Also, patients with HF have reduced capillary density that is inversely related to maximal oxygen consumption. Indeed, systemic levels of the natural eNOS inhibitor, asymmetric dimethylarginine (ADMA), are increased in HF patients and independently predict a reduced effective renal plasma flow.23
Systemic vascular dysfunction in HF also involves impaired arterial elastic properties. These changes in HF seem to be secondary to arterial remodelling, with hypertrophy of the vascular wall layer seen in severe chronic HF. Indeed, the vascular wall hypertrophy correlates with both impaired FMD and the arterial response to glyceryl trinitrate (GTN), thus suggesting that increased arterial stiffness may affect the vascular dilatory response irrespective of endothelial dysfunction. However, an analysis of the local arterial elastic characteristics (e.g. distensibility and compliance) in HF shows their correlation with forearm FMD, but not with the response to GTN, indicating that the increased arterial rigidity may be a feature or consequence of vasomotor endothelial dysfunction per se.24
This systemic nature of endothelial dysfunction seen in HF corresponds to the concept of the endothelium as one organ. However, this approach towards the endothelium also acknowledges substantial diversity in phenotype and function of endothelial cells located within different segments of the vascular tree, although more research is needed on the differential implication of these segments in HF pathophysiology.
Endothelial dysfunction and clinical outcome in heart failure
The clinical relevance of vasomotor endothelial dysfunction in HF comes from prospective outcome studies. Although these studies are relatively small (Table 1), all invariably show an independent significant association of endothelial dysfunction with a negative outcome in HF, ranging from mild HF [New York Heart Association (NYHA) class I] with relatively preserved myocardial contractility to advanced HF (NYHA class IV) with severely depressed LV function.25
| Study | Study population | NYHA/ejection fraction | Measure of endothelial function | Follow-up duration | Outcome | Resultsa |
|---|---|---|---|---|---|---|
| Shechter et al.25 | 82 (100% IHF) | IV/22 ± 3 | FMD | 14 months | Death | HR (median FMD) 2.04; 95% CI 1.09–5.1, P = 0.03 |
| de Berrazueta et al.46 | 242 (38% IHF) | I–IV/36 ± 13 | FBF in response to ACH (VOP) | 5 years | Composite of death, heart attack, angina, stroke, NYHA class IV, or hospitalization due to HF | HR [Exp(B)] 0.67; SE 0.18, P = 0.01 |
| Heitzer et al.47 | 289 (56% IHF) | I/41 ± 7 | FBF in response to ACH (VOP) | 4.8 years | Composite of death from cardiac causes, hospitalization due to HF, heart transplantation. | HR 0.96; 95% CI 0.94–0.98, P = 0.007 |
| Katz et al.48 | 149 (33% IHF) | II–III/25 ± 1 | FMD | 28 months | Death or urgent transplantation | HR (1% decrease in FMD) 1.20; 95% CI 1.03–1.45, P = 0.027 |
| Katz et al.48 | 110 (56% IHF) | II–III/25 ± 1 | Exhaled NO production | 13 months | Death or urgent transplantation | HR 1.31; 95% CI 1.01–1.69, P = 0.04 |
| Fischer et al.49 | 67 (64% IHF) | II–III/47 ± 10 | FMD | 46 months | Composite of cardiac death, hospitalization due to HF, or heart transplantation | HR [Exp(B)] 0.665; SE 0.18, P = 0.01 |
| Kübrich et al.26 | 185 heart transplant recipients (32% IHF) | 75 ± 10 | Coronary vasomotor function | 60 months | Composite of death, progressive HF, myocardial infarction, percutaneous or surgical coronary revascularization | RR 1.97; CI 1.1–3.6, P = 0.028 |
- a ACH, acetylcholine; CI, confidence interval; FBF, forearm blood flow; FMD, flow-mediated dilation; HF, heart failure; HR, hazard ratio; IHF, ischaemic heart failure; NO, nitric oxide; NYHA, New York Heart Association; RR, relative risk; SE, standard error; VOP, venous occlusion plethismography.
- a For all, endothelial function was an independent predictor of outcome.
Both FMD and the arterial response to acetylcholine are significant predictors of a high risk of unfavourable events, and their predictive value does not seem to be affected by HF aetiology. Epicardial endothelial dysfunction is also an independent predictor of cardiovascular events and death in patients after cardiac transplantation.26 Also, FMD significantly predicts the likelihood of response to cardiac resynchronization therapy (CRT), independently of QRS duration, LV ejection fraction, or LV dyssynchrony, and the improvement in FMD 3 months following CRT positively correlates with an increase in 6 min walk distance. Further evidence of the significant prognostic role of the coronary endothelium in ischaemic systolic HF comes from recent studies employing positron emission tomography for assessment of myocardial perfusion reserve.27
Blood markers of endothelial dysfunction in heart failure
Most studies showed significant up-regulation of plasma markers of endothelial activation (e.g. E-selectin) and damage [e.g. von Willebrand factor (vWF)] in chronic HF (Table 2). However, most of these studies included patients of mixed aetiology (predominantly ischaemic), with healthy individuals serving as controls. Thus it is difficult to differentiate precisely the role of HF per se and the influence of co-morbidities (e.g. atherosclerosis and diabetes) as a driving force for the up-regulation of these markers. Indeed, Kistorp et al.28 observed increased levels of E-selectin and vWF in HF patients with diabetes but not in those without diabetes. The impact of co-morbidities may also explain a lack of correlation between vWF/E-selectin and parameters of HF severity (e.g. BNP levels, LV ejection fraction, 6 min walk test, or NYHA class) and no difference between patients with stable and decompensated HF.29 Although vWF (but not E-selectin) levels were predictive of future adverse cardiovascular outcomes in stable HF, the role of co-morbidities is in question, especially given that BNP was of no predictive value in the study cohort.30 Another index of endothelial damage, circulating endothelial cells (CECs), have been reported to be increased in HF, with no differences between acute and chronic HF.31 Nonetheless, CEC levels correlate with other markers of endothelial damage/dysfunction (FMD, plasma vWF, and E-selectin) and BNP levels, but not with ejection fraction or NYHA class.31
| Study | Study population | EF, % (inclusion criteria/actual) | Aetiology | Controls | Marker | Results |
|---|---|---|---|---|---|---|
| Kistorp et al.28 | 195 CHF with diabetes, 147 CHF without diabetes | ≤45/30 ± 8 in diabetic group, 30 ± 8 in non-diabetic group | 74% IHF in diabetic group, 51% IHF in non-diabetic group | 116 healthy | E-selectin | ↑ diabetes group |
| vWF | ↔ in non-diabetic | |||||
| ↑ diabetes group | ||||||
| ↔ in non-diabetic | ||||||
| Chong et al.30 | 35 with AHF, 40 with CHF | ≤40/30 (21–33) in AHF, 30 (29–33) in CHF | 63% IHF in AHF group, 83% IHF in CHF group | 32 healthy | E-selectin | ↑ in AHF and CHF |
| vWF | ↔ AHF vs. CHF | |||||
| sTM | ↑ in AHF and CHF | |||||
| ↔ AHF vs. CHF | ||||||
| ↑ in AHF and CHF | ||||||
| ↑ AHF vs. CHF | ||||||
| Vila et al.50 | 59 CHF | Not specified | Not specified | 59 healthy | vWF | ↑ |
| Thrombospondin-1 | ↓ | |||||
| Chong et al.29 | 137 CHF | ≤45/30 (25–35) | 61% IHF | 106 healthy | E-selectin | ↔ |
| vWF | ↔ | |||||
| Chong et al.31 | 30 with AHF, 30 with CHF | ≤40/30 (22–32) in AHF group, 30 (29–34) in CHF | 70% IHF in AHF group, 80% IHF in CHF group | 20 healthy | E-selectin | ↑ in AHF and CHF |
| vWF | ↔ AHF vs. CHF | |||||
| CECs | ↑ in AHF and CHF | |||||
| ↔ AHF vs. CHF | ||||||
| ↑ in AHF and CHF | ||||||
| ↔ AHF vs. CHF | ||||||
| Chong et al.6 | 30 CHF | <40/31 (29–35) | 77% IHF | 20 healthy | vWF | ↑ |
| sTM | ↔ | |||||
| CECs | ↑ |
- a AHF, acute heart failure; CECs, circulating endothelial cells; CHF, chronic heart failure; DCM, dilated cardiomyopathy; EF, ejection fraction; IHF, ischaemic heart failure; sTM, soluble thrombomodulin; vWF, von Willebrand factor; ↑, increased; ↓, decreased; ↔, no changes.
Endothelin-1 is produced by the endothelium and vascular smooth muscle cells, and is the most potent known vasoconstrictor. Endothelin-1 overexpression has been shown to parallel other features of endothelial dysfunction and be implicated in the pathogenesis of various cardiovascular disorders. Unsurprisingly, plasma endothelin-1 levels are increased in HF patients. Endothelin-1 promotes ADMA production in experimental HF, which explains the correlation of endothelin-1 levels with parameters of endothelial dysfunction in patients with dilated cardiomyopathy. Inhibition of the endothelin-1 pathway significantly improves vasomotor endothelial function and survival in rats with HF. Endothelium-mediated dilation in HF patients also significantly improves after 3 weeks of treatment with low (but not high) doses of endothelin A receptor blocker LU 135252. However, clinical trials of endothelin receptor(s) blockers in HF were disappointing, but beta-blockers and statins have been shown to reduce endothelin-1 production, thus possibly contributing their beneficial effects in HF.
Pharmaceutical agents, nutritional supplements, and endothelial dysfunction in heart failure
Excessive activation of the renin–angiotensin system disrupts NO downstream signalling. Accordingly, all available studies indicate that pharmacological blockade of the renin–angiotensin–aldosterone axis [e.g. angiotensin-converting enzyme (ACE) inhibitors or spironolactone] improves endothelial function and reduces vWF in HF (Figure 3). However, the ability to restore endothelial function varies between individual ACE inhibitors or depends on their dose (e.g. higher doses are needed for enalapril). In one study, responder status determined by improvement in physical capacity was linked to the improvement in endothelial function with ACE inhibitors.32 For example, in the African American HF Trial (A-HeFT), improvement in morbidity, mortality, and functional status in HF was linked to the amelioration of endothelial dysfunction.
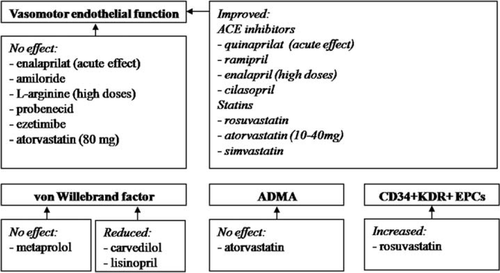
These clinical findings are supported by experimental data showing that ACE inhibition normalizes NO-dependent dilatation in HF models. Beneficial effects of ACE inhibitors on the endothelium in HF are mediated via different mechanisms including eNOS up-regulation, inhibition of endothelial apoptosis, and reduced production of vasoconstrictor prostanoids.
Pleiotropic (i.e. lipid-independent) properties of statins have attracted substantial attention of researchers, and their endothelial effects have been extensively studied. Statins have helped to maintain coronary NO production and activity in pacing-induced cardiomyopathy, markedly improved endothelium-dependent vasorelaxation, and enhanced myocardial neovascularization. Treatment with statins parallels improvement of LV function and survival via eNOS-mediated mechanisms. In the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) trial, therapy with 10 mg of rosuvastatin had no effect on mortality.33 This controversy may stem from a number of factors which include the choice of a specific statin and its dose, the population studied, or, alternatively, longer (almost 3 years) follow-up duration in the CORONA trial.
In all but one clinical studys, statins resulted in a marked improvement of endothelial function, irrespective of HF aetiology. However, the treatment of NIHF with the highest doses of atorvastatin (80 mg daily) failed to improve endothelial function.34 However, effects of statins on endothelial dysfunction can be drug specific and, recently, treatment with high-dose rosuvastatin not only improved FMD, but was also associated with up-regulation of circulating angiogenic progenitors and enhanced angiogenesis.35 Endothelial effects of the statins do not seem to depend on their lipid-lowering properties and they can improve endothelial function even in HF individuals with normal lipid levels.2 Lipid-independent effects of statins on the endothelium are also supported by failure of ezetimibe to affect endothelial dysfunction despite similar reduction in LDL.36
Statins seem to have no effect on ADMA levels, but they significantly reduce oxidative stress in HF patients.35,37 Simvastatin increases extracellular superoxide dismutase (SOD) activity, a potential contributor to the statin-induced improvement of endothelial function, but this is not seen from ezetimibe therapy.38 However, a direct causal relationship between antioxidant properties of statins and their positive endothelial effects in human HF is more difficult to prove, and recovery of endothelial function with atorvastatin alone was more potent than in combination with an antioxidant, vitamin E.
Given that endothelial NO is produced from l-arginine, supplementation with this amino acid has been used as a therapeutic option to improve endothelial function in HF. Also l-arginine might reduce the endogenous NO inhibitor ADMA, known to be increased in HF. For example, rats with HF have diminished plasma l-arginine levels, and chronic supplementation with low (but not high) l-arginine doses improves aortic endothelium-dependent relaxation ex vivo, but did not affect haemodynamics in vivo. Similarly, in human studies, a lower (e.g. 8 g daily)—but not higher (20 g daily)—dose of l-arginine has been shown to improve endothelial function. In a placebo-controlled study, 6 weeks treatment with oral l-arginine hydrochloride (5.6–12.6 g daily) significantly increased distances during a 6 min walk test, improved arterial compliance, and reduced circulating levels of endothelin-1. Thus, whilst the restoration of reduced l-arginine levels may be beneficial in some patients, excessive l-arginine consumption is unlikely to provide any additional benefits to endothelium. Moreover a significant increase in urea and aspartate transaminase levels was observed in patients who received high l-arginine doses.
Vitamin C inhibits endothelial cell apoptosis in congestive HF, and studies in this area have shown an improvement in endothelial function. Nevertheless, given the rather unsuccessful results of antioxidant vitamin supplementation in clinical trials, they should not be routinely recommended in HF patients. Although the number of other medications (e.g. allopurinol, sildenafil, etanercept, growth hormone, etc.) have been shown to have positive effects on endothelial dysfunction, it is currently difficult to speculate on the clinical relevance of these observations.
It has to be pointed out that improvement in endothelial function does not always translate into outcome benefits. This could have several explanations. First, beneficial effects of many interventions on the endothelium were mostly shown in short-term trials, while robust data on their long-term effects are less established. Secondly, such therapies (e.g. antioxidants) are usually not endothelium specific and have multiple biological targets in different tissues, as such possibly ameliorating net benefits related to the vascular effects. Thirdly, given the complex nature of HF, moderate improvement of endothelial function may not be sufficient to result in mortality reduction, being overwhelmed by other factors (e.g. reduced LV contractility per se). Development of novel potent endothelium-specific therapies may offer additional opportunities.
The important role of the circulation progenitors in endothelial/vascular repair has been recently acknowledged and reviewed.39 Development of novel approaches of selective intramyocardial delivery of angiogenic and pro-reparative growth factors [e.g. vascular enothelial growth factor (VEGF)] designed to provide their prolonged release to the damaged tissues has been recently reported and it led to better post- myocardial infarction recovery, enhanced angiogenesis, and eNOS up-regulation.40 Clinical testing of such approaches is awaited with interest.
Exercise and endothelial dysfunction in heart failure
Physical exercise has gained acceptance as a beneficial intervention for patients with HF. According to a systematic review of randomized controlled trials, exercise might slow the pathophysiological progression of HF. In chronic HF, regular exercise inhibits neurohormonal stimulation and the production of proinflammatory cytokines, reduces levels of natriuretic peptides and systemic vascular resistance, and attenuates the oxidative burden.41 These favourable effects parallel improvement in symptoms, exercise capacity, and quality of life, while patients who do not improve their exercise capacity significantly after an exercise training programme have a poorer prognosis.
The shear stress associated with exercise is a critical endothelial stimulus, commonly employed to assess endothelial dysfunction (i.e. FMD). Accordingly, it is natural to expect an effect of physical training on the endothelial functional performance. Indeed, regular aerobic training of patients with ischaemic and non-ischaemic systolic HF is associated with restoration of the vasodilatory endothelial-dependent function. In contrast, training of isolated muscle groups (e.g. handgrip training) does not seem to have any substantial effect on systemic endothelial function. Physical training in HF has been showed to up-regulate eNOS gene expression, accelerate l-arginine transport, and [0]increase basal endothelial NO formation. Additionally, in patients with HF, exercise training increases VEGF and mobilizes endothelial progenitors, presumably an indicator of the desired endothelial regeneration.42 A growing body of evidence suggests that beneficial effects of physical exercise in HF are at least partly mediated by augmentation of the regenerative capacity of bone marrow-derived angiogenic cells.43,44
The intensity and modality of exercise training are also of importance. Whereas acute bouts of exercise have been reported to enhance production in proinflammatory cytokines and markers of endothelial damage in HF, these effects are not seen when moderate exercise is performed regularly.45 Also, aerobic interval training in HF was more effective that moderate continuous training.1 Even in healthy individuals undergoing moderate intensity exercise, but not mild or high intensity exercise, there is a significant augmentation of acetylcholine-induced vasodilation. Indeed, improvement of FMD after the course of training paralleled reverse LV remodelling, prominent improvement in ejection fraction, and reduction in NT-proBNP levels, although a causative relationship between these processes is difficult to establish.1 Also it is still not clear whether exercise-related improvement of endothelial function has any independent effects on clinical outcomes.
Conclusions and future directions
Endothelial abnormalities are a common feature of HF. Various aspects of the endothelial function can be affected, including vasomotor, haemostatic, antioxidant, and inflammatory activities. Substantial differences exist in the pattern of endothelial dysfunction depending on aetiology, severity, and stability of HF in individual patients. There is considerable evidence that in the majority of patients with ischaemic aetiology, endothelial dysfunction is systemic in its nature and involves arteries, conductance vessels and microvascular beds, coronary, pulmonary, and peripheral vessels. There is also minor evidence of venous endothelial dysfunction in HF. The pattern of endothelial dysfunction is more heterogeneous in NIHF, with fewer features of systemic abnormalities. In fact, many subjects with NIHF have functionally preserved endothelium in peripheral arteries, with endothelial dysfunction seen only in coronary vessels. It is possible the coronary endothelial dysfunction in these patients mirrors local pathological processes in the heart and may reflect their activity.
Endothelial dysfunction undoubtedly has significant prognostic value in HF but its clinical application is hampered by methodological limitations. Some of these methods are invasive and some (e.g. FMD) are very operator dependent. A number of medications (including ACE inhibitors and statins) and regular physical activity have been shown to improve endothelial function in HF, but there are still no pharmaceutical agents specifically targeting the vascular endothelium. In an animal model of HF, endothelial dysfunction was successfully corrected by tyrosine phosphatase 1B inhibitors, but their effects have not yet been tested in human clinical trials. Overall, the pathophysiological role of the vascular endothelium and its clinical potential as a therapeutic target has not been adequately developed and undoubtedly we await more exciting discoveries. Things can only get better.
Funding
The Heart Failure Association of the European Society of Cardiology [a Research Fellowship to E.S.].
Conflict of interest: none declared.




