Continuous ambulatory peritoneal dialysis as a therapeutic alternative in patients with advanced congestive heart failure
Abstract
Aims
Continuous ambulatory peritoneal dialysis (CAPD) has been proposed as an additional therapeutic resource for patients with advanced congestive heart failure (CHF). The objective of this study was to determine the therapeutic role of CAPD, in terms of surrogate endpoints, in the management of patients with advanced CHF and renal dysfunction.
Methods and results
A total of 57 candidates with New York Heart Association (NYHA) class III/IV CHF, renal dysfunction (glomerular filtration rate < 60 mL/min/1.73 m2), persistent fluid congestion despite loop diuretic treatment, and at least two previous hospitalizations for acute heart failure (AHF) were invited to be included in the CAPD programme; however, 25 patients were finally included. The primary outcome was evaluated by the change at 6 and 24 weeks for the Minnesota Living With Heart Failure Questionnaire (MLWHFQ), the 6 min walk test (6MWT), NYHA class, serum natriuretic peptides [brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP)], serum carbohydrate antigen 125 (CA125), and hospitalization rates for AHF. CAPD was associated with a substantial improvement in the MLWHFQ (−21.3, P < 0.001; and −20.4, P < 0.001), the 6MWT (54.0, P < 0.001; and 45.6, P = 0.023), and NYHA class (−1.0, P < 0.001; and −1.4, P < 0.001) at 6 and 24 weeks, respectively. The Ln(CA125) decreased markedly (−0.8, P = 0.003; and −0.98, P = 0.003), with no effect on BNP and NT-proBNP. There was a marked reduction in the number of days hospitalized for AHF (6 month post-CAPD vs. 6 months pre-CAPD: −84%; P < 0.001).
Conclusions
In advanced CHF and renal dysfunction, CAPD was associated with short/mid-term improvement in severity parameters, with an acceptable rate of side effects.
Introduction
In patients with advanced heart failure (HF), the pathophysiological mechanisms resulting in systemic congestion play an important role in the progression of the disease, and systemic congestion usually is the main indication for hospitalization.1 Moreover, recent evidence has highlighted the role of fluid retention in the pathogenesis of renal dysfunction and subsequent diuretic resistance.2 The co-existence of previous renal functional impairment, diuretic resistance, and worsening renal function is often found to occur in patients with HF and persistent volume overload. This clinical presentation represents the most extreme clinical feature of the cardiorenal syndrome,3 a condition with an ominous prognosis, and where the available therapeutic options are scarce and limited.4,5 Indeed, in this scenario, there are no data from randomized studies supporting the beneficial effect on survival for most of the pharmacological tools used (such as diuretics).6,7 In this particular setting, intermittent ultrafiltration has emerged as an alternative therapeutic option for reducing volume overload, with potential advantages over standard treatment in acute situations.6,7 Likewise, some authors have proposed the use of peritoneal dialysis (PD) as an additional resource for the treatment of advanced congestive heart failure (CHF), by advocating a more physiological (and continuous) ultrafiltration.8,9 In fact, some studies have described improvement in the clinical functional class and echocardiographic/haemodynamic parameters, and reduction in hospitalization rates associated with the use of PD in these patients.10–16 In addition, lower cost compared with standard care has been recently reported.12 Nevertheless, most of these studies were retrospective analyses of small series, and have included patients with co-existence of end-stage renal failure.
The primary objective of this study was two-fold. (i) To determine the therapeutic role of continuous ambulatory peritoneal dialysis (CAPD) in improving patient clinical status at 6 and 24 weeks. The primary outcome will be measured by favourable changes in parameters of disease severity such as the 6 min walk test (6MWT), the Minnesota Living With Heart Failure Questionnaire (MLWHFQ), New York Heart Association (NYHA) functional class, serum natriuretic peptides [brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP)], and serum carbohydrate antigen 125 (CA125). (ii) To evaluate whether CAPD confers benefit in terms of hospitalization rates at 6 months.
The following endpoints were also tested (secondary outcomes): (i) evolution of renal function at 6 and 24 weeks; and (ii) rates of adverse effects related to the technique.
Methods
Study group and protocol
We prospectively studied a cohort of 57 patients, followed in the heart failure unit of the Hospital Clínico Universitario de Valencia, from 1 August 2008 to 1 February 2011, who met the following inclusion criteria: (i) at least two non-planned admissions for acute heart failure (AHF), the last episode being in the past 6 months; (ii) NYHA functional class III/IV; (iii) persistent congestion despite optimal loop diuretic therapy; and(iv) presence of renal dysfunction [estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2] documented at least once in the last 12 months.
Acute heart failure was defined as the rapid onset of symptoms and signs secondary to abnormal cardiac function and the presence of objective evidence of structural or functional abnormality of the heart at rest (such as cardiomegaly, third heart sound, cardiac murmur, abnormality of the echocardiogram, or raised natriuretic peptides).17 Persistent congestion was assessed following current recommendations.1
Following their last hospitalization, patients were invited to participate in a CAPD programme as a complementary therapeutic option. Peritoneal dialysis is a renal replacement therapy used in patients with end-stage renal disease that consists of the introduction of an osmotic solution into the abdomen through a catheter. The process uses the patient's peritoneum as a membrane across which fluid excess and waste products are removed from the blood. Among the 57 patients that fulfilled the inclusion criteria, 18 refused to participate, 7 were excluded because of major wall abdominal defects, and 1 patient, who was initially selected, was subsequently withdrawn due to undergoing a cardiac transplantation (Figure 1). Thirty-one patients agreed to participate and an abdominal catheter was surgically implanted at a median of 26 days [interquartile range (IQR) = 20–31 days) after the last hospitalization. Because of complications inherent to the technique, poor patient collaboration, and one death, six patients did not start the CAPD programme (Figure 1). Thus, CAPD was initiated in 25 patients at a median of 54 days (IQR = 38–59 days) after the catheter implantation. Our protocol followed the current international guidelines regarding PD-related infections18,19 and peritoneal access.20 The double-bag Fresenius AG. Stay-safe system was used in all patients. The CAPD programme consisted of 2–3 times/day exchange with dialysate solution (1.36–2.27% glucose) according to the evolution of the grade of congestion.
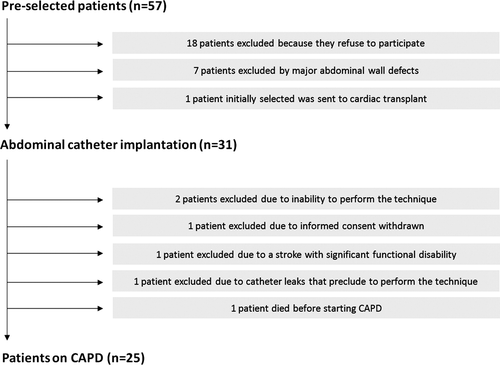
Demographic information, medical history, vital signs, 12-lead electrocardiogram (ECG), echocardiography, laboratory data, and pharmacological treatments were routinely assessed at each pre-specified visits and collected using pre-established questionnaires. The use of medical treatment was individualized and in line with established guidelines;17 as stated in the protocol, every patient was to have the same scheduled number of visits pre- and post-CAPD. Baseline measurements were performed 24–48h before PD onset and all patients were in stable clinical condition. By protocol, loop diuretic dosage was not initially modified until a clinical reduction of systemic congestion was verified; then a reduction was suggested, but the final decision was left to the discretion of the nephrologist/cardiologist in charge of the clinical follow-up of the patient.
Outcomes
Primary
To measure the success of the CAPD, the primary endpoint was defined by changes that occurred pre- and post-CAPD onset (at 6 and 24 weeks) in the self-reported MLWHFQ, the 6MWT, the NYHA functional class, serum natriuretic peptides (BNP and NT-proBNP), and serum CA125 (a biomarker related to the presence of fluid overload/redistribution and the risk of death and readmission).21–23 The number of days spent in the hospital for AHF 24 weeks before and 24 weeks after CAPD was also evaluated.
Secondary
The secondary family of endpoints was comprised of the following: (i) assessing the effect of the intervention on renal function evaluated by changes pre-/post-CAPD in serum urea, serum creatinine, and in the eGFR; (ii) determining the rate of peritonitis and other adverse effects related to the technique; and (iii) assessing the effect of CAPD on other variables related to CHF severity [heart rate, systolic blood pressure, serum haemoglobin, serum sodium, and echocardiographic parameters: left ventricular ejection fraction (LVEF), pulmonary artery systolic pressure (PASP), tricuspid annular plane systolic excursion (TAPSE), and E/e‘ values].
The protocol was approved by the ethics committee of our centre in accordance with the principles of the Declaration of Helsinki and national regulations. All patients gave written informed consent to participate in this study.
Statistical analysis
Data analysis was performed by Cuore International, Inc. (Scottsdale, AZ, USA). Continuous variables were expressed as the mean ±1 standard deviation (SD) or median (IQR) when appropriate. Discrete variables were presented as percentages.
Pre- and post-continuous ambulatory peritoneal dialysis comparisons
Mixed linear models were used for the analyses of the primary objective, which involved the comparison of baseline values with the repeated measures over time (at the 6 and 24 week visits) for the following variables: the self-reported MLWHFQ, the 6MWT, the NYHA functional class, serum BNP, serum NT-proBNP, serum CA125, number of days spent in the hospital for AHF, serum creatinine, serum urea, and eGFR. Because of the lack of normality or severe skewing of variable distributions, serum BNP, NT-proBNP, and CA125, number of days spent in the hospital for AHF, serum creatinine, serum urea, and eGFR were logarithmically transformed. Contrasts between post-CAPD visits vs. baseline (or pre-CAPD) were adjusted for multiple comparisons using the Sidak method. For each variable, random intercept and random slopes models were compared using likelihood ratio tests, in order to select the best model to fit the data. Normality of residuals was checked for each variable. All data shown in the figures are expressed as the mean with their 95% confidence interval (CI). In order to provide a clinically and meaningful unit of change, the percentage Δ change (%Δ change) from baseline was estimated for each variable using the following formula: %Δ change = (post – pre ×100)/pre.
A two-sided P-value of < 0.05 was considered to be statistically significant for all analyses. All analyses were performed using STATA 12.0 (STATA Statistical Software: Release 12, College Station, TX, USA).
Results
The clinical characteristics of the patients included in the CAPD programme are presented in Table 1. Twenty-four patients were still alive and undergoing CAPD at 6 months.
| Demographic and medical history | |
|---|---|
| Age, years | 75.1 (8.2) |
| Male, n (%) | 18 (72) |
| Weight, kg | 77 (65–86) |
| Hypertension, n (%) | 24 (96) |
| Dyslipidaemia, n (%) | 20 (80) |
| Diabetes mellitus, n (%) | 9 (36) |
| Current smoker, n (%) | 2 (8) |
| Previous smoker, n (%) | 9 (36) |
| Ischaemic heart disease, n (%) | 17 (68) |
| Valvular heart disease, n (%) | 10 (40) |
| COPD, n (%) | 8 (32) |
| Peripheral oedema, n (%) | 23 (92) |
| NYHA class III–IV, n (%) | 25 (100) |
| Charlson co-morbidity index | 5 (2) |
| Vital signs | |
| Heart rate, b.p.m. | 74 (69–88) |
| Systolic blood pressure, mmHg | 119 (107–127) |
| Diastolic blood pressure, mmHg | 68 (12) |
| ECG | |
| Atrial fibrillation, n (%) | 10 (40) |
| QRS >120 ms, n (%) | 11 (44) |
| Left bundle branch block, n (%) | 6 (24) |
| Laboratory | |
| Haemoglobin, g/dL | 12 (11–13) |
| Serum creatinine, mg/dL | 1.88 (1.51–2.7) |
| Urea, mg/dL | 91 (61–140) |
| eGFRa, mL/min/1.73 m2 | 33 (21–42) |
| Sodium, mEq/L | 138 ± 6 |
| BNP, pg/mL | 616 (391–1380) |
| NT-proBNP, pg/mL | 6420 (3749–12 873) |
| CA125, U/mL | 71 (42–207) |
| Echocardiography | |
| LVEF, % | 40 ± 14 |
| LVDD, mm | 60 ± 11 |
| PASPb, mmHg | 53 ± 16 |
| Medical treatment and devices | |
| Beta-blockers, n (%) | 14 (56) |
| Furosemide dosage, mg | 120 (120–160) |
| Tiazide, n (%) | 5 (20) |
| Spironolactone, n (%) | 8 (32) |
| ACEI, n (%) | 7 (28) |
| ARB, n (%) | 5 (20) |
| Statins, n (%) | 17 (68) |
| Oral anticoagulants, n (%) | 14 (56) |
| Nitrates, n (%) | 7 (28) |
| Digoxin, n (%) | 5 (20) |
| Pacemaker, n (%) | 3 (12) |
| ICD, n (%) | 4 (16) |
| CRT, n (%) | 2 (8) |
- a Values are expressed as median (interquartile range) or mean ± standard deviation; categorical variables are presented as percentages.
- b ACEI, angiotensin-converting enzyme inhibitor; AHF, acute heart failure; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; CA125, carbohydrate antigen 125; CAPD, chronic ambulatory peritoneal dialysis; COPD, chronic pulmonary obstructive disease; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
- a Estimated GFR (eGFR) using the Modification of Diet in Renal Disease (MDRD) formula.
- b Data available in 21 patients.
A significant, but modest, decrease in diuresis was also registered following CAPD (1765 ± 488 vs. 1485 ± 661 mL, P= 0.023). The mean daily peritoneal ultrafiltration was 679 ± 379 mL.
Primary outcomes
The Minnesota Living With Heart Failure Questionnaire
Continuous ambulatory peritoneal dialysis was associated with a substantial improvement at 6 and 24 weeks (−21.3, P < 0.001; and −20.4, P < 0.001, respectively) (Figure 2A). Expressing these changes as %Δ change from baseline, there was a 35.4% and 30.5% reduction in the MLWHFQ at 6 and 24 weeks, respectively.
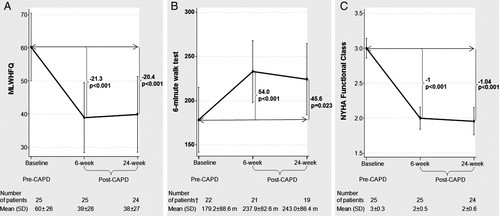
The 6 min walk test
The distance walked in 6 min (m) increased at 6 and 24 weeks after CAPD (54.0, P < 0.001; and 45.6, P < 0.023, respectively) (Figure 2B). Expressing these changes as %Δ change from baseline, there was a 56.6% and 89.1% increase in the 6MWT at 6 and 24 weeks, respectively.
New York Heart Association class
After CAPD, there was a significant improvement in the NYHA functional classification at 6 and 24 weeks (−1.0, P < 0.001; and −1.4, P < 0.001, respectively) (Figure 2C). This improvement translated into a 32.7% and 34.0% reduction in the NYHA class at 6 and 24 weeks, respectively.
Biomarkers
No favourable changes were seen with either BNP or NT-proBNP (Figure 3A and B). However, the Ln(CA125) decreased markedly post-CAPD at 6 weeks (−0.8, P = 0.003) and 24 weeks (−0.98, P = 0.003) (Figure 3C). Expressing these changes as %Δ change from baseline, there was a 17.9% and 35.1% reduction at 6 and 24 weeks, respectively, for Ln(CA125).
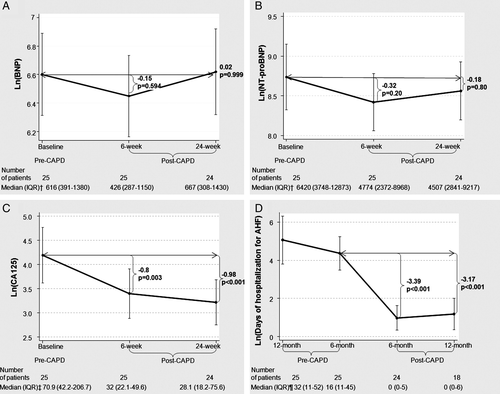
Hospitalization days for acute heart failure
The number of days hospitalized for AHF was measured at 6 months pre-CAPD and compared with 6 months post-CAPD. Patients who underwent CAPD had a marked reduction in the number of days hospitalized for AHF (Figure 3D). Expressing these changes in absolute terms, they went from a median of 16 days (IQR = 11–45) to 0 days (IQR = 0–5) at 6 months; in relative units, it meant a %Δ change reduction of 84%.
Secondary outcomes
During a median follow-up of 14 months (IQR = 6–18), 21 episodes of peritonitis (in 11 patients) were registered, accounting for a study rate of 0.75 episodes per patient and year at risk (one episode every 16–18 months). Most cases (n = 15) responded to ambulatory antimicrobial therapy. One death resulted from a complicated peritonitis. In two patients, CAPD was interrupted indefinitely due to further relapses. Furthermore, in one patient, an exit site leak precluded starting the technique, and in two additional patients CAPD was stopped due to hydrothorax and severe scrotal swelling.
Looking at the effect on renal function, there was a significant decrease in serum urea at the 6 and 24 week visits (Figure 4A), whereas no significant changes were noted with respect to serum creatinine and eGFR (Figure 4B and C, respectively).
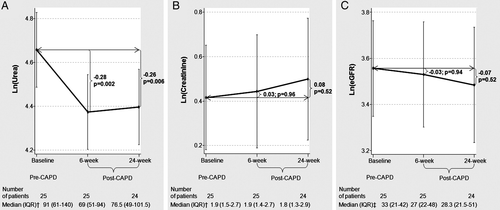
Table 2 depicts the changes that occurred in other clinical and laboratory parameters. From these data, a significant improvement at 6 months in serum haemoglobin was observed; however, no significant changes were seen in systolic blood pressure, heart rate, or serum sodium, and echocardiographic parameters such as LVEF, PASP, E/e', and right ventricular systolic function at 6 weeks. Likewise, non-significant weight changes were registered. Six months after PD onset, all patients (except one) safely tolerated a reduction in oral furosemide of up to 80 mg per day.
| Variables | Pre-CAPD | Post-CAPD | Overall P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | 24 weeks | |||||||||
| n | Absolute value at baseline | n | Absolute value at 6 weeks | %Δ change from baseline | P-value | n | Absolute value at 24 weeks | %Δ change from baseline | P-value | ||
| Weight, kg | 25 | 78.6 ± 15.7 | 25 | 75.7 ± 13.2 | −2.0 ± 4.0 | 0.257 | 24 | 77.8 ± 13 | 0.7 ± 7.0 | 0.754 | 0.0357 |
| Systolic blood pressure, mmHg | 25 | 119 (107–127) | 25 | 123 (115–135) | 6.6 ± 22.4 | 0.618 | 24 | 120 (108–130) | 2.2 ± 16.6 | 0.999 | 0.4935 |
| Heart rate, b.p.m. | 25 | 74 (69–88) | 25 | 75 (64–83) | −0.1 ± 20.7 | 0.891 | 24 | 69 (60–80) | −3.7 ± 24.9 | 0.327 | 0.2713 |
| Haemoglobin, g/dL | 25 | 12 (11–13) | 25 | 12 (12–14) | 13.1 ± 16.7 | <0.001 | 24 | 13 (12–14) | 8.9 ± 14.8 | 0.015 | 0.0001 |
| Serum sodium, mEq/L | 25 | 138 ± 6 | 25 | 137 ± 4 | −0.5 ± 3.2 | 0.697 | 24 | 138 ± 2 | −0.1 ± 3.8 | 0.992 | 0.5997 |
| LVEF, % | 25 | 40 ± 14 | 24 | 39 ± 14 | −2.5 ± 19.3 | 0.348 | |||||
| E/e’ | 18 | 22.6 ± 11.6 | 18 | 23.2 ± 12.7 | 24.4 ± 77.2 | 0.526 | |||||
| LV diastolic diameter, mm | 25 | 60 ± 11 | 24 | 61 ± 11 | 3.3 ± 10.9 | 0.156 | |||||
| LV systolic diameter, mm | 25 | 47 ± 12 | 24 | 47 ± 12 | 1.3 ± 12.2 | 0.819 | |||||
| PASP, mmHg | 21 | 53 ± 16 | 17 | 51 ± 14 | −4.1 ± 14.6 | 0.171 | |||||
| TAPSE, mm | 15 | 15.6 ± 4.9 | 18 | 14.9 ± 5.1 | −9.9 ± 27.4 | 0.128 | |||||
- a CAPD, chronic ambulatory peritoneal dialysis; LV, left ventricular; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
- b Values are expressed as median (25–75 percentile) or mean ± standard deviation; categorical variables are presented as percentages.
Discussion
This preliminary study indicates that CAPD is a feasible alternative for the treatment of symptomatic patients with advanced CHF, persistent fluid overload (despite loop diuretic therapy), and the co-existence of any grade of renal failure. It was initiated in up to 64% of patients that met the inclusion criteria and agreed to be included, and in up to 80% of those in whom a peritoneal catheter was implanted. CAPD was associated with a substantial improvement in NYHA functional class, physical performance (6MWT), quality of life (MLWHFQ), and biochemical profile. Moreover, the implementation of this procedure had an acceptable safety profile.
The clinical relevance of our findings stems from the fact that this is a non-traditional approach used for the treatment of a population with a prohibitively high morbidity/mortality, and where most of the pharmacological treatments are often contraindicated, not tolerated, or have failed to improve symptoms or prognosis.
Recent evidence has highlighted the role of congestion, not only as a marker of HF severity, but also as influencing the progression of the disease through neurohormonal (favouring sodium retention), renal (reducing renal perfusion), cardiac (predisposing to subendocardial ischaemia), intestinal (endotoxin translocation), and possibly complex endothelial interactions.1,24,25 Associated with this procedure, in addition to clinical and physical improvement, we found an important reduction in surrogate markers indicative of systemic and renal venous congestion [serum CA125 and blood urea nitrogen (BUN)].2,21–23 The fact that these findings were also present 6 months post-CAPD allows us to speculate that, by ameliorating the severe fluid overload, the effect of this intervention would not only translate into a temporary clinical relief but might slow down the progression of the disease. The results are also in agreement with the theoretically advantageous characteristics attributable to this technique, such as slow and daily UF, preservation of the residual renal function, haemodynamic stability, and sodium sieving with maintenance of normonatraemia. Furthermore, PD does not require special and complex technologies to be carried out, which is the case with other UF techniques.
In agreement with other authors who have failed to correlate serum natriuretic peptides values with the volume status,26−28 we did not observe significant changes in natriuretic peptides following CAPD. However, we cannot exclude that moderate to severe renal function impairment present in our sample could act as an important masking factor in natriuretic peptide reduction.
Previous studies
Our results are consistent with various case reports and some observational cohorts that have shown that PD may play a role in the treatment of patients with advanced CHF, by showing clinical, haemodynamic, biochemical, or echocardiographic improvement.7–15 However, as limitations in these studies, the inclusion criteria were not clearly defined, or the target population included mostly exhibited severe left ventricular systolic dysfunction and/or end-stage renal disease. Indeed, in the present study, 17 patients (68%) displayed LVEF >35% and most of the patients exhibited renal dysfunction between stages 2 and 4 (median eGFR = 33 mL/min/1.73 m2). In a recent cohort (similar to our cohort concerning GFR composition), there was a marked clinical and haemodynamic improvement associated with CAPD.12 Concerning the safety of this procedure, we noticed that the rate of peritonitis in our group was higher than recommended in international guidelines,18,19 an observation that we attribute to the elevated age and high prevalence of co-morbidity in our patients. Most cases were treated in an ambulatory setting without major clinical consequences, except one patient, who died due to a CAPD-related abdominal infection, and two additional cases, where CAPD was interrupted due to peritonitis relapses (after 6 months). Despite the fact that PD might be a technique associated with potential and serious adverse effects, we would like to stress that, in this population of patients with advanced refractory CHF, this risk appears acceptable given the elevated baseline risk of these patients when treated with the usual-care approach.
Further studies are needed to unravel the uncertainties about the safety, efficacy, and plausibility of this procedure. For instance, the following questions need to be clarified. What are the optimal characteristics of patients who benefit from this technique? Does CAPD provide additional pleiotropic effect (e.g. clearance of proinflammatory cytokines) rather than resolution of fluid overload?29 What is the optimal ultrafiltration regimen? Do other osmotic agents (e.g. icodextrin) offer additional benefits compared with glucose? Are substantial benefits sustained in the long term? Do these intermediate results translate into an improved survival? Is mid- to long-term CAPD cost-effective compared with standard care?
The main limitation of this study stems from the fact that this is a small, single-centre observational study with no control group. To us, a randomized clinical trial in this setting was not a priori feasible due to the difficulty in blinding the patients and the investigator regarding the treatment intervention.
Conclusions
In this preliminary study, we found that patients with advanced refractory CHF and treated with CAPD had a significant improvement in symptoms, physical performance, quality of life, and biochemical profile, with an acceptable rate of side effects. Further studies, especially in more controlled scenarios, are warranted to confirm our results and to define the role and clinical utility of this technique for patients with advanced refractory CHF.
Funding
The Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III [RED HERACLES RD06/0009/1001 (Madrid, Spain)]; ayuda para proyectos de grupos emergentes año 2010 de la Conselleria de Sanitat de Valencia (DOCV 6.175, 30/12/2009–Annex III); Sociedad Española de Cardiología (Beca Esteve 2009); Fresenius Medical Care.
Conflict of interest: none declared




