A randomized study of haemodynamic effects and left ventricular dyssynchrony in right ventricular apical vs. high posterior septal pacing in cardiac resynchronization therapy
Abstract
Aims
The effect on left ventricular (LV) systolic function and LV dyssynchrony by alternative right ventricular (RV) lead position in cardiac resynchronization therapy (CRT) is unclear. In the present study, RV apical (RV-A) was compared with RV high posterior septal (RV-HS) lead position in CRT.
Methods and results
In 85 consecutive CRT patients (mean age 66 ±11 years) the RV lead placement was randomized to RV-A (n = 43) or RV-HS (n = 42). The LV lead was targeted to the latest activated LV segment (concordant LV lead), identified by two-dimensional speckle tracking radial strain (ST-RS) echocardiography. Concordant LV leads were obtained in 72%, similar in RV-A and RV-HS (79% vs. 64%; P = 0.13). Six months after CRT, no difference was found in LV reverse remodelling (reduction of LV end-systolic volume ≥15%) according to RV-A and RV-HS leads [26 (65%) vs. 25 (64%); P = 0.93]. Superior LV reverse remodelling was observed in concordant LV leads compared with discordant LV leads [41 (73%) vs. 10 (43%); P = 0.01]. At 6-month follow-up, LV reverse dyssynchrony (reduction of anteroseptal to posterior delay ≥50%) using ST-RS imaging was similar in RV-A and RV-HS [25 (63%) vs. 24 (62%); P = 0.93]. More LV reverse dyssynchrony was found in concordant LV leads vs. discordant LV leads [39 (70%) vs. 10 (43%); P = 0.03]. A concordant LV lead was an independent predictor of LV reverse remodelling (odds ratio, 3.65; P = 0.01) and LV reverse dyssynchrony (odds ratio, 4.22; P = 0.02) 6 months after CRT.
Conclusion
RV-A and RV-HS in CRT demonstrated similar LV reverse remodelling and LV reverse dyssynchrony at 6-month follow-up. Concordant LV leads provided superior LV reverse remodelling and LV reverse dyssynchrony.
Trial registration: NCT01035489
Introduction
Cardiac resynchronization therapy (CRT) can reduce morbidity and mortality in symptomatic heart failure (HF) patients on optimal pharmacological therapy with depressed left ventricular (LV) function and bundle branch block.1 CRT may improve LV systolic function, and provide reduction of LV volumes and LV dyssynchrony.1,2 Echocardiographic analysis by using two-dimensional (2D) speckle tracking radial strain (ST-RS) imaging can assess the segmental LV contraction pattern and the degree of LV dyssynchrony in patients receiving CRT.2 LV lead placement at the segment with the latest mechanical activation (concordant LV lead) without extensive myocardial scarring, evaluated by ST-RS echocardiography, has shown additional benefits in LV function, LV volume reduction, and prolongation of survival in retrospective analysis of CRT.3–5
Alternative RV lead positions used in CRT compared with the conventional right ventricular apex (RV-A) have been reported in previous retrospective studies of non-randomly placed RV leads.6–9 However, to our knowledge, there are as yet no prospective studies on the clinical impact of a single RV lead position in CRT. We hypothesized that by placing the RV lead in the high posterior septum (RV-HS), closer to the Purkinje system, we could better utilize the intrinsic ventricular conduction system. Subsequently, the RV-HS lead position in CRT would provide a more synchronous contraction pattern than the conventional RV-A lead placement. Thus, the aim of this study was to investigate the clinical status, the haemodynamic effects, and LV dyssynchrony effected by the RV-A vs. RV-HS lead position in CRT, in a prospective randomized trial. The LV lead was prospectively targeted to the latest activated segment identified by ST-RS echocardiography.
Methods
Patient population and study design
Eighty-five consecutive HF patients scheduled for CRT were prospectively included in this study between November 2008 and August 2010. Inclusion criteria were HF patients on optimal pharmacological treatment in New York Heart Association (NYHA) functional class III or IV, LV ejection fraction (LVEF) ≤35%, and QRS duration ≥120 ms. The QRS morphologies included were left bundle branch block (LBBB) and intraventricular conduction delay, whereas right bundle branch block was not included. All patients underwent echocardiographic examination and were assessed clinically according to NYHA functional class and distance walked in 6 min at baseline and repeated 6 months after CRT. The RV lead was randomized to either RV-A or RV-HS. The LV lead was prospectively targeted to the latest activated segment identified by baseline ST-RS echocardiography. The regional ethics committee approved this study, and the patients were included after informed consent.
Cardiac resynchronization therapy device implantation
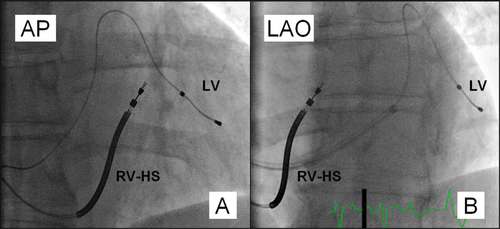
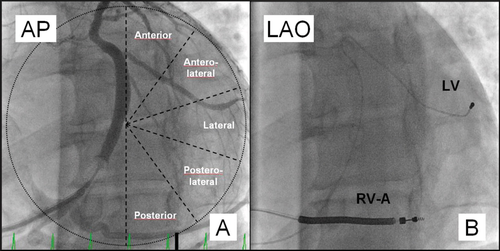
A CRT device was implanted under local anaesthesia with transvenous introduction of the pacing leads. During the CRT implant procedure, fluoroscopic imaging in anteroposterior (AP), left anterior oblique 30° (LAO) and right anterior oblique 30° (RAO) views was used to position the pacing leads. The RV lead was randomly assigned to receive either the RV-A or RV-HS lead position in a 1:1 ratio. To obtain the RV-A lead position, we used the conventional implantation technique, and RV-A lead location was demonstrated by a straight or slightly curved anterior lead direction in LAO fluoroscopic view.10 The RV-HS lead position was achieved by using a stylet-delivered technique.11 First, the RV lead was advanced into the pulmonary artery facilitated by a curved stylet. Next, a second pre-shaped stylet, resembling a swan's neck with distal posterior angulation, was introduced into the lead tip. The RV lead was pulled back during counterclockwise rotation to the posterior interventricular septum before the screw was deployed into the septal wall. Finally, the stylet was removed and the appropriate RV-HS lead position was verified with biplane fluoroscopy. The RV-HS lead position was confirmed in the RV outflow tract (RVOT) by the AP fluoroscopic view and a posterior lead tip direction to reach the posterior septum, verified in LAO fluoroscopic view (Figure 1). Guided by fluoroscopy, the coronary sinus was cannulated, and by using a balloon catheter (Attain 6215, Medtronic, Minneapolis, MN, USA), a venogram recording in LAO view of the coronary vein tributaries was obtained. The LV was divided into five equal segments from the venogram in LAO view: the anterior, anterolateral, lateral, posterolateral, and posterior segment (Figure 2).12 Subsequently, the LV lead was targeted to the latest activated segment identified by ST-RS echocardiography. The LV lead was positioned in a basal or midventricular segment, and the apical segment was avoided, visualized in RAO fluoroscopic view.12 The right atrial (RA) lead was positioned in the RA appendage. A dual-chamber biventricular CRT device was connected to the pacing leads produced by the same manufacturer (Medtronic).
Echocardiographic imaging
Transthoracic 2D echocardiography was performed using a commercially available system (Vivid 7, General Electric Vingmed, Milwaukee, WI, USA) equipped with a 3.5 MHz transducer by placing the patient in left lateral decubitus position. The images were stored digitally as cine-loops for offline post-processing (EchoPac 108.1.5, GE Medical Systems, Horten, Norway). LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were obtained from apical four- and two-chamber views and the LVEF was calculated using the biplane Simpson's method.13 LV end-diastolic diameter (LVEDd) was measured from M-mode in the parasternal long axis view.14 For each of these echocardiographic variables, three representative consecutive cardiac cycles were analysed, and the mean value was used for statistical calculations. Patients demonstrating ≥15% LVESV reduction from baseline to 6-month follow-up were considered as LV reverse remodelling responders.15 The same observer blinded to the RV and LV lead positions performed all the echocardiographic analyses.
Assessment of left ventricular dyssynchrony
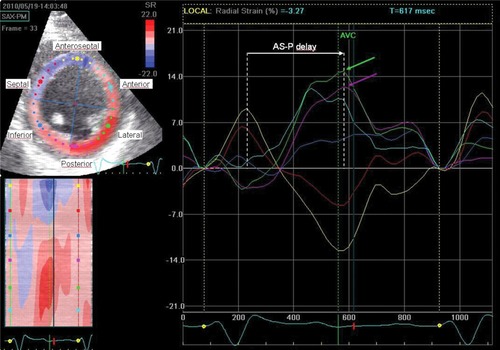
Speckle tracking analysis was feasible in all patients and performed from the 2D greyscale mid LV parasternal short axis view in end-expiration (Figure 3). All images were recorded with a frame rate of ≥30 Hz (mean, 50 ±18 Hz; range 30–89 Hz). By using a single end-systolic frame, the LV endocardial border was traced and the automatically generated second circle was adjusted to include the epicardial border. Speckle tracking software automatically generated time–strain curves, and reliable strain curves were achieved in 94% of the segments. Strain curves from five representative cardiac cycles were averaged. The difference in time between peak radial strain of the anteroseptal and posterior LV segments (AS–P delay) and the standard deviation (SD) of time from QRS onset to peak radial strain from six LV segments (SDt6) was determined from speckle tracking analysis.2 We hypothesized that CRT in some patients could reverse LV dyssynchrony as much as 50% at 6-month follow-up compared with baseline, evaluated by ST-RS echocardiography. Thus, patients demonstrating a ≥50% AS–P delay reduction 6 months after CRT were considered LV reverse dyssynchrony responders. By using the baseline echocardiograpic examination, peak radial strain from the anterior, lateral, and posterior segments was used to identify the LV segment with the latest contraction. If ≤10 ms separated two adjacent LV segments at the end of LV mechanical activation, the segment between them was assigned as the latest one. In this way, we identified five targeted LV lead positions: the anterior, anterolateral, lateral, posterolateral, and posterior segments. Segments with radial strain <10%, indicating high transmural scar burden, were excluded.4 If all LV radial strains were <10%, the latest activated segment was chosen as the targeted LV segment.
Tissue Doppler imaging (TDI) of the LV walls was obtained from apical four-chamber, two-chamber, and long axis views. The images were acquired at high frame rates (>100 frames/s) that resulted in aliasing velocities of 16–28 cm/s. The LV ejection period was determined from the apical long axis view using pulsed-wave Doppler with the sample volume placed in the LV outflow tract (LVOT). Time from QRS onset to peak LV wall velocity during the ejection period in the basal and mid LV segment from the apical views was used to assess LV dyssynchrony. The maximal time difference between peak systolic LV wall velocities among basal segments in apical four-chamber and two-chamber views (TDI-Ts 4 segments) was obtained.16 By using TDI images from apical four-chamber, two-chamber, and long axis views, the SD of time from QRS onset to peak LV wall velocity during the ejection period from the basal and mid LV segments (TDI-SDTs 12 segments) was calculated.17
Interventricular mechanical delay (IVMD) was obtained by using pulse-wave Doppler of aortic flow velocity in the apical long axis and pulmonic flow velocity in the parasternal short axis view. The pre-ejection interval from QRS onset to opening of aortic and pulmonic valves was measured, and the IVMD was calculated from the difference in time.18
Atrioventricular (AV) dyssynchrony was assessed by using transmitral pulsed-wave Doppler in apical four-chamber view. The LV filling time (LVFT) was measured and divided by the cardiac length (RR), and is presented as a percentage (LVFT/RR × 100%).18
For each of the variables indicating LV dyssynchrony, five representative cardiac cycles were analysed, and the average was used for statistical calculations.
Speckle tracking radial strain analysis and left ventricular lead concordance
Digitally stored fluoroscopic images obtained during the CRT implant procedure were used to assess the LV lead position in a double-blind set-up. LV lead concordance was accredited if the LV lead was located in the segment with the latest mechanical activation, or the adjacent one, evaluated by pre-operative ST-RS echocardiography.
Follow-up
Atrioventricular and interventricular (VV) optimization was done prior to hospital discharge and repeated at 3-month follow-up. The AV delay was obtained by the iterative method using transmitral pulsed-wave Doppler in apical four-chamber view by measuring the longest filling time from a programmed AV delay of 50–170 ms in 20 ms decrements.19 Pulsed-wave Doppler from the LVOT in the apical long axis view was used to measure velocity–time integrals of randomly programmed VV intervals using 0–80 ms RV and LV pre-excitation delays in 20 ms decrements, and the largest velocity time integral was obtained.19 At 6-month follow-up the patients were evaluated with echocardiography and clinical reassessment, with NYHA classification and distance walked in 6 min.
Sample size and statistical analysis
The study was powered to detect a difference of 30% in the number of patients demonstrating ≥50% AS–P delay reduction between RV-A and RV-HS at 6-month follow-up, with 80% power at a significance level of 5% using a two-tailed t-test. This would require 77 patients with an addition of 10% assumed to be lost 6 months after CRT implantation. Statistical analysis was conducted using SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ±SD. After normality testing, the variables were compared using paired or unpaired Student's t-test as appropriate. Categorical data are listed as frequencies and percentages, and were compared by Pearson χ2 or Fisher's exact test. Univariable and multivariable logistic regression analyses were used to assess the relationship of eight independent variables on LV reverse remodelling and LV reverse dyssynchrony responders. To calculate intra- and interobserver variability, 10 samples were randomly selected and assessed. To assess the reproducibility of the LVEF, LVESV, and AS–P delay measurements, Bland–Altman analysis and linear regression analysis were performed. A P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the 85 study patients [66 ±11 years; 74 men (87%)] are presented in Table 1. All patients had severe heart failure symptoms [65 in NYHA functional class III (77%)] and a wide QRS duration (169 ±25 ms). Almost all patients (99%) were treated with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker and a beta-blocker (95%). The patients had severely depressed LV function, with mean LVEF 24 ±4%. Baseline LV volumes were increased, mean LVEDV was 236 ±63 mL and mean LVESV was 179 ±50 mL. LV dyssynchrony at baseline evaluated by ST-RS echocardiography was severe; the mean AS–P delay was 227 ±95 ms. The two RV lead position groups were similar at baseline except for more frequent use of spironolactone in the RV-A group.
| Variables | All (n = 85) | RV-A (n = 43) | RV-HS (n = 42) | P-value |
|---|---|---|---|---|
| Age, years | 66 ± 11 | 67 ± 12 | 66 ± 11 | 0.87 |
| Gender, male (%) | 74 (87) | 38 (88) | 36 (86) | 0.72 |
| NYHA functional class III, n (%) | 65 (77) | 31 (72) | 34 (81) | 0.34 |
| Ischaemic cardiomyopathy, n (%) | 51 (60) | 26 (60) | 25 (60) | 0.93 |
| Diabetes mellitus, n (%) | 22 (26) | 11 (26) | 11 (26) | 0.95 |
| Creatinine, μmol/L | 95 ± 27 | 95 ± 30 | 96 ± 25 | 0.76 |
| NT-proBNP (pmol/L) | 301 ± 276 | 298 ± 302 | 305 ± 251 | 0.90 |
| Sinus rhythm, n (%) | 67 (79) | 33 (77) | 34 (81) | 0.64 |
| QRS duration, ms | 169 ± 25 | 173 ± 25 | 165 ± 25 | 0.14 |
| LBBB, n (%) | 73 (86) | 36 (84) | 37 (88) | 0.56 |
| LVEF (%) | 24 ± 4 | 25 ± 4 | 24 ± 4 | 0.23 |
| LVEDV (mL) | 236 ± 63 | 231 ± 54 | 241 ± 71 | 0.48 |
| LVESV (mL) | 179 ± 50 | 174 ± 41 | 184 ± 57 | 0.35 |
| AS–P delay (ms) | 227 ± 95 | 232 ± 105 | 222 ± 85 | 0.63 |
| ACEI or ARB, n (%) | 84 (99) | 43 (100) | 41 (98) | 0.31 |
| Beta-blocker, n (%) | 81 (95) | 42 (98) | 39 (93) | 0.29 |
| Loop diuretics, n (%) | 64 (75) | 35 (81) | 29 (69) | 0.19 |
| Spironolactone, n (%) | 27 (32) | 19 (44) | 8 (19) | 0.01 |
| Digitoxin, n (%) | 21 (25) | 12 (28) | 9 (21) | 0.49 |
- a ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AS–P delay, left ventricular (LV) anteroseptal to posterior time difference in peak radial strain measured by speckle tracking imaging; LBBB, left bundle branch block; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; NYHA, New York Heart Association; NT-proBNP; N-terminal pro brain natriuretic peptide; RV-A, right ventricular (RV) apical lead position; RV-HS, RV high posterior septal lead position.
Right ventricular lead position
All RV leads were successfully positioned in RV-A or RV-HS according to their randomization. In all RV-HS patients, the RVOT was verified in AP fluoroscopic view. A slight variation in the posterior lead tip angulation in RV-HS was observed; however, all RV-HS leads demonstrated a posterior lead tip direction in LAO fluoroscopic view. The CRT implant procedure duration observed in RV-A was 116 ±38 min and in RV-HS 132 ±63 min (P = 0.17). The peak radial strain in the segment containing the LV lead was similar in RV-A and RV-HS (17.4 ±9.9% vs. 19.8 ±8.2%; P = 0.23). The number of patients with LV strain <10% was not different in RV-A and RV-HS [6 (14%) vs. 3 (7%); P = 0.48]. One RV-A lead dislogmement was observed during the 6-month follow-up and successfully repositioned at the original site. The RV sensing function and pacing thresholds remained acceptable during the follow-up period. The average AV interval was 119 ±33 ms, similar in RV-A and RV-HS (114 ±34 ms vs. 123 ±32 ms; P = 0.27), and the average VV intervals were LV pre-excitation 27 ±28 ms, comparable in RV-A and RV-HS (23 ±28 ms vs. 30 ±28 ms; P = 0.24).
Left ventricular lead position
The baseline echocardiographic ST-RS analysis identified the LV segment with the latest mechanical activation, and the implanting physician was instructed to position the LV lead at the targeted LV segment. The locations of the latest activated LV segments were the anterior [9 (11%)], anterolateral [5 (6%)], lateral [27 (32%)], posterolateral [26 (31%)], and posterior [18 (21%)] position. From the double-blind set-up control using the implant procedure fluoroscopy images, the LV lead distribution was anterior [2 (2%)], anterolateral [25 (29%)], lateral [37 (44%)], posterolateral [18 (21%)], and posterior [3 (4%)]. Subsequently, concordant LV leads were achieved in 61 patients (72%) of the study population and were similar according to RV-A and RV-HS [34 (79%) vs. 27 (64%); P = 0.13]. The reasons why a concordant LV lead at the targeted LV segment was not obtained were lack of appropriate coronary vein [17 (71%)], LV lead instability [4 (17%)], diaphragmatic stimulation [2 (8%)], and no LV pacing capture [1 (4%)]. The CRT implant procedure durations in concordant and discordant LV leads were 118 ±46 min vs. 139 ±65 min (P = 0.11). The peak radial strain in the segment containing the LV lead was similar in concordant and discordant LV leads (17.8 ±8.2% vs. 20.5 ±11.0%; P = 0.22). The numbers of patients with LV strain <10% in concordant and discordant LV leads were comparable [7 (11%) vs. 2 (8%); P = 1.00]. During the 6-month follow-up period, 6 (7%) LV lead dislodgements were observed, and all leads were successfully repositioned at the original site. The LV sensing function and pacing thresholds remained acceptable to obtain long-term biventricular pacing in the studied patients.
Clinical and echocardiographic response to cardiac resynchronization therapy
During the 6-month follow-up period, one patient underwent heart transplantation and five patients died from worsening of heart failure or intercurrent disease, equally distributed in RV-A (n = 3) and RV-HS (n = 2; P = 1.0). After 6 months of CRT, the patients improved in NYHA functional class from 3.2 ±0.4 to 2.3 ±0.5 (P < 0.001), but there was no difference according to RV-A and RV-HS (3.3 ±0.5 to 2.4 ±0.5 vs. 3.2 ±0.4 to 2.3 ±0.5; P = 0.98). The distance walked in 6 min increased from 385 ±110 to 414 ±116 m (P < 0.02), but was similar with respect to RV-A and RV-HS (372 ±118 to 410 ±111 m vs. 398 ±101 to 417 ±123 m; P = 0.46).
The echocardiographic variables on LV function and LV dyssynchrony at baseline and 6 months after CRT in RV-A and RV-HS patient groups are presented in Table 2. At 6-month follow-up, the number of patients demonstrating ≥15% reduction of LVESV was 26 (65%) in the RV-A and 25 (64%) in RV-HS group (P = 0.93). Both RV lead positions demonstrated statistically significant improvement in LVEF and LV volumes after 6 months of CRT, but the haemodynamic response was not different between RV-A and RV-HS. The relative improvements effected by CRT in the RV-A and RV-HS groups, respectively, were LVEF 29.5 ±23.3% vs. 32.3 ±28.9% (P = 0.64); LVEDV −14.6 ±19.4% vs. −14.9 ±20.2% (P = 0.94); LVESV −21.4 ±20.8% vs. −22.0 ±23.3% (P = 0.90); and LVEDd −8.2 ±8.5% vs. −12.1 ±9.8% (P = 0.06).
| Variable | All (n = 85) | RV-A (n = 43) | RV-HS (n = 42) | P-value |
|---|---|---|---|---|
| LVEF (%) | ||||
| Baseline | 24 ± 4 | 25 ± 4 | 24 ± 4 | 0.23 |
| Follow-up | 31 ± 7* | 31 ± 6* | 31 ± 8* | 0.78 |
| LVEDV (mL) | ||||
| Baseline | 236 ± 63 | 231 ± 54 | 241 ± 71 | 0.48 |
| Follow-up | 200 ± 68* | 198 ± 67* | 203 ± 70* | 0.77 |
| LVESV (mL) | ||||
| Baseline | 179 ± 50 | 174 ± 41 | 184 ± 57 | 0.35 |
| Follow-up | 141 ± 56* | 138 ± 52* | 144 ± 60* | 0.68 |
| LVEDd (cm) | ||||
| Baseline | 7.1 ± 0.7 | 7.0 ± 0.7 | 7.1 ± 0.8 | 0.33 |
| Follow-up | 6.3 ± 0.9* | 6.4 ± 0.8* | 6.3 ± 0.9* | 0.54 |
| AS–P delay (ms) | ||||
| Baseline | 227 ± 95 | 232 ± 105 | 222 ± 85 | 0.63 |
| Follow-up | 121 ± 108* | 133 ± 119* | 109 ± 95* | 0.34 |
| SDt6 (ms) | ||||
| Baseline | 106.8 ± 51.1 | 110.3 ± 54.6 | 103.2 ± 47.5 | 0.52 |
| Follow-up | 59.6 ± 49.3* | 57.9 ± 50.7* | 61.4 ± 48.4* | 0.75 |
| TDI-Ts 4 segments (ms) | ||||
| Baseline | 69.5 ± 36.1 | 65.0 ± 37.1 | 74.1 ± 34.8 | 0.25 |
| Follow-up | 60.6 ± 32.0** | 58.0 ± 30.6 | 63.3 ± 33.7 | 0.46 |
| TDI-SDTs 12 segments (ms) | ||||
| Baseline | 34.8 ± 11.9 | 32.8 ± 11.4 | 36.9 ± 12.3 | 0.12 |
| Follow-up | 33.5 ± 12.7 | 32.7 ± 11.2 | 34.3 ± 14.2 | 0.58 |
| IVMD (ms) | ||||
| Baseline | 38 ± 23 | 36 ± 21 | 40 ± 25 | 0.45 |
| Follow-up | 15 ± 12* | 16 ± 14* | 14 ± 9* | 0.37 |
| LVFT/RR (%) | ||||
| Baseline | 45 ± 8 | 45 ± 9 | 45 ± 8 | 0.86 |
| Follow-up | 48 ± 5** | 49 ± 5** | 47 ± 5 | 0.18 |
- a AS–P delay, left ventricular (LV) anteroseptal to posterior time difference in peak radial strain measured by speckle tracking (ST-RS) imaging; IVMD, interventricular mechanical delay; LBBB, left bundle branch block; LVEDd, LV end-diastolic diameter; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; LVFT/RR, LV filling time/cardiac cycle length ×100%; RV-A, right ventricular (RV) apical lead position; RV-HS, RV high posterior septal lead position; SDt6, standard deviation (SD) of time from QRS onset to peak radial strain measured by ST-RS in six LV segments; TDI-Ts 4 segments, maximal difference between peak systolic velocity delay from tissue Doppler imaging (TDI) in basal apical four-chamber or two-chamber; TDI-SDTs 12 segments, SD of time from QRS onset to peak systolic velocity from TDI in 12 LV segments.
- * P < 0.001 baseline vs. follow-up;
- ** P < 0.05 baseline versus follow-up.
The number of patients demonstrating ≥50% AS–P delay reduction 6 months after CRT, assessed with ST-RS echocardiography, were in 25 (63%) in the RV-A and 24 (62%) in RV-HS group (P = 0.93). LV dyssynchrony using the AS–P delay and SDt6 from ST-RS imaging demonstrated a statistically significant reduction at 6-month follow-up, but the reduction was similar between RV-A and RV-HS patient groups. For LV dyssynchrony measured by TDI, TDI-Ts of 4 segments improved from baseline to 6-month follow-up, but TDI-SDTs 12 segments did not, and without any difference with respect to the RV lead location. IVMD was reduced at 6-month follow-up, but not the LVFT/RR, and there were similar findings in the RV-A and RV-HS groups.
By using logistic regression analysis, the reduction of AS–P delay ≥50% could predict ≥15% reduction of LVESV at 6-month follow-up [odds ratio, 2.77(1.06–7.21); P = 0.04].
Left ventricular lead concordant to the latest activated segment from speckle tracking radial strain imaging
A subgroup analysis on LV lead position according to the segment with the latest mechanical activation, assessed by ST-RS echocardiography, was performed. Clinical and echocardiographic variables at baseline in concordant and discordant LV leads are presented in Table 3. Baseline characteristics were similar, except that there were more patients with ischaemic cardiomyopathy in the discordant LV lead group. At 6-month follow-up, the numbers of patients with ≥15% reduction of LVESV were 41 (73%) in the concordant LV lead group and 10 (43%) in the discordant LV lead group (P = 0.01). The numbers of patients demonstrating ≥50% AS–P delay reduction 6 months after CRT were 39 (70%) in the concordant LV lead group and 10 (43%) in the discordant LV lead group (P = 0.03). LVEF, LV volumes, and LV dimensions at baseline and at 6-month follow-up, in concordant and discordant LV lead positions, are presented in Table 4. Patients with LV leads at the latest activated LV segment assessed by ST-RS echocardiography demonstrated greater improvement in LVEF and had more LV volume reduction compared with those with discordant LV lead position. Concordant LV leads demonstrated similar LV reverse remodelling responders in RV-A and RV-HS patient groups [22 (71%) vs. 19 (76%); P = 0.67). Discordant LV leads provided comparable LV reverse remodelling responders in the RV-A and RV-HS groups [4 (44%) vs. 6 (43%); P = 0.94].
| Variable | Concordant (n = 61) | Discordant (n = 24) | P-value |
|---|---|---|---|
| Age, years | 66 ± 12 | 67 ± 11 | 0.73 |
| Gender, male (%) | 52 (85) | 22 (92) | 0.43 |
| NYHA functional class III, n (%) | 47 (77) | 18 (75) | 0.84 |
| Ischaemic cardiomyopathy, n (%) | 32 (52) | 19 (79) | 0.02 |
| Sinus rhythm, n (%) | 49 (80) | 18 (75) | 0.59 |
| QRS duration, ms | 169 ± 25 | 168 ± 25 | 0.87 |
| LBBB, n (%) | 53 (87) | 20 (83) | 0.67 |
| LVEF (%) | 24 ± 4 | 23 ± 4 | 0.31 |
| LVEDV (mL) | 234 ± 64 | 241 ± 60 | 0.65 |
| LVESV (mL) | 177 ± 49 | 185 ± 52 | 0.49 |
| LVEDd (cm) | 7.0 ± 0.7 | 7.1 ± 0.8 | 0.60 |
| AS–P delay (ms) | 228 ± 99 | 223 ± 86 | 0.84 |
| ACEI or ARB, n (%) | 60 (98) | 24 (100) | 0.53 |
| Beta-blocker, n (%) | 58 (95) | 23 (96) | 0.88 |
| Loop diuretics, n (%) | 45 (74) | 19 (79) | 0.60 |
| Spironolactone, n (%) | 19 (31) | 8 (33) | 0.85 |
- a ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AS–P delay, left ventricular (LV) anteroseptal to posterior time difference in peak radial strain measured by speckle tracking imaging; LBBB, left bundle branch block; LVEDd, LV end-diastolic diameter; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; NYHA, New York Heart Association; RV-A, right ventricular (RV) apical lead position; RV-HS, RV high posterior septal lead position.
| Variable | Concordant (n = 61) | Discordant (n = 24) | P-value |
|---|---|---|---|
| LVEF (%) | |||
| Baseline | 24 ± 4 | 23 ± 4 | 0.31 |
| Follow-up | 32 ± 7* | 28 ± 5* | 0.003 |
| Change (%) | 35 ± 25 | 21 ± 26 | 0.04 |
| LVEDV (mL) | |||
| Baseline | 234 ± 64 | 241 ± 60 | 0.65 |
| Follow-up | 189 ± 68* | 227 ± 60*** | 0.02 |
| Change (%) | −18 ± 21 | −6 ± 15 | 0.01 |
| LVESV (mL) | |||
| Baseline | 177 ± 49 | 185 ± 52 | 0.49 |
| Follow-up | 131 ± 55* | 165 ± 52** | 0.01 |
| Change (%) | −26 ± 22 | −11 ± 17 | 0.005 |
| LVEDd (cm) | |||
| Baseline | 7.0 ± 0.7 | 7.1 ± 0.8 | 0.60 |
| Follow-up | 6.2 ± 0.9* | 6.7 ± 0.8* | 0.02 |
| Change (%) | −12 ± 10 | −7 ± 6 | 0.01 |
- a LVEDd, left ventricular (LV) end-diastolic diameter; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume.
- * P < 0.001 baseline vs. follow-up;
- ** P < 0.01 baseline vs. follow-up;
- *** P < 0.05 baseline vs. follow-up.
Ischaemic vs. non-ischaemic cardiomyopathy
At 6-month follow-up, the number of patients with ≥15% reduction of LVESV was 29 (62%) in ischaemic cardiomyopathy and 22 (69%) in non-ischaemic cardiomyopathy (P = 0.52). The number of patients demonstrating ≥50% AS–P delay reduction at 6 months after CRT was 27 (57%) in the ischaemic cardiomyopathy group and 22 (69%) in the non-ischaemic cardiomyopathy group (P = 0.31).
Regression analyses
Univariable and multivariable logistic regression analyses were performed to assess different parameters to predict ≥15% reduction of LVESV and ≥50% AS–P delay reduction 6 months after CRT. The parameters assessed to predict LV reverse remodelling and LV reverse dyssynchrony were age, male gender, QRS duration, LBBB, ischaemic cardiomyopathy, baseline AS–P delay, and concordant LV lead and RV-HS lead position (Table 5). Concordant LV lead was the only predictor positively associated with LV reverse remodelling by univariable analysis, and was also found in multivariable analysis 6 months after CRT. By univariable analysis, QRS duration, LBBB, baseline AS–P delay, and concordant LV lead were associated with LV reverse dyssynchrony. However, in multivariable analysis, only baseline AS–P delay and concordant LV lead were predictive of ≥50% AS–P delay reduction at 6-month follow-up.
| LV reverse remodelling | LV reverse dyssynchrony | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Univariable | ||||
| Age (per 1 year) | 1.02 (0.97–1.06) | 0.49 | 0.99 (0.95–1.03) | 0.59 |
| Male gender | 0.65 (0.16–2.66) | 0.54 | 0.92 (0.25–3.46) | 0.91 |
| QRS (per 1 ms) | 1.01 (0.99–1.03) | 0.17 | 1.02 (1.00–1.04) | 0.04 |
| LBBB | 1.96 (0.45–8.52) | 0.37 | 5.88 (1.10–31.34) | 0.04 |
| Ischaemic | 0.73 (0.28–1.90) | 0.52 | 0.61 (0.24–1.58) | 0.31 |
| AS–P delay (per 1 ms) | 1.00 (1.00–1.01) | 0.38 | 1.01 (1.00–1.02) | 0.001 |
| Concordant LV lead | 3.55 (1.29–9.80) | 0.01 | 2.98 (1.10–8.12) | 0.03 |
| RV-HS | 0.96 (0.38–2.42) | 0.93 | 0.96 (0.39–2.38) | 0.93 |
| Multivariable | ||||
| QRS (per 1 ms) | – | – | 1.01 (0.98–1.03) | 0.60 |
| LBBB | – | – | 4.15 (0.57–30.36) | 0.16 |
| AS–P delay (per 1 ms) | – | – | 1.01 (1.00–1.02) | 0.006 |
| Concordant LV lead | 3.65 (1.30–10.23) | 0.01 | 4.22 (1.26–14.08) | 0.02 |
| RV-HS | 1.16 (0.44–3.09) | 0.76 | 1.27 (0.42–3.83) | 0.67 |
- a AS–P delay, left ventricular (LV) anteroseptal to posterior time difference in peak radial strain measured by speckle tracking radial strain (ST-RS) imaging; CI, confidence interval; Concordant LV lead, LV lead at the latest activated segment from ST-RS imaging; LBBB, left bundle branch block; LV, left ventricular; OR, odds ratio; RV-HS, right ventricular high posterior septal lead position.
Echocardiograpic reproducibility
The intra- and interobserver variability were, respectively, 0.4 ±1.7% (r = 0.93; P < 0.001) and −0.8 ±3.1% (r = 0.82; P = 0.004) for LVEF, 2.2 ±7.3 mL (r = 0.98; P < 0.001) and 6.9 ±13 mL (r = 0.96; P < 0.001) for LVESV, and −6.6 ±19.0 ms (r = 0.99; P < 0.001) and −13 ±26 ms (r = 0.98; P < 0.001) for AS–P delay from ST-RS imaging.
Discussion
The main findings in this study can be summarized as follows: (i) in this randomized study of RV-A vs. RV-HS lead placement in CRT, both RV lead positions demonstrated similar responder rates by using the endpoints LV reverse remodelling and LV reverse dyssynchrony; (ii) there was a statistically significant and similar improvement in NYHA function class, 6 min walking distance, LVEF, LV volume reduction, and LV dyssynchrony in the two RV lead positions; (iii) concordant LV leads at the LV segment with the latest mechanical activation, using ST-RS echocardiography, provided more responders in LV reverse remodelling and LV reverse dyssynchrony, greater improvement in LVEF, and more LV volume reduction from CRT; and (iv) discordant LV leads were more frequently observed in ischaemic cardiomyopathy compared with non-ischaemic cardiomyopathy.
Reverse remodelling defined as ≥15% reduction of LVESV is frequently used as an endpoint in CRT. It is associated with a favourable outcome in CRT, but the non-responder rate can be 40–50%.15 The benefits of CRT in congestive heart failure could possibly be explained by the improvement in LV contractile function and the decrease in the extent of LV dyssynchrony during follow-up. In a recent study, the reduced LV dyssynchrony provided by CRT, evaluated by speckle tracking echocardiography, was related to better outcome.20 Furthermore, a decrease in the magnitude of LV dyssynchrony has been demonstrated in LV reverse remodelling responders to CRT.2 The present study defined LV reverse remodelling responders as an AS–P delay reduction of ≥50% from a theoretical assumption, and this cut-off was able to predict LV reverse remodelling. However, the clinical implication of evaluating the decrease in LV dyssynchrony provided by CRT needs further investigation.
To our knowledge, this is the first prospective randomized study to evaluate the impact of a single alternative RV lead position to the conventional RV-A in CRT. In the present study, RV-HS demonstrated similar effects to RV-A in the clinical status, LV reverse remodelling, and LV reverse dyssynchrony after 6 months of CRT. Previous retrospective non-randomized studies have reported the haemodynamic effects of alternative RV lead positions in CRT. Riedlbauchová et al.6 studied 99 CRT recipients and found a greater LVEDD decrease 12 months after CRT in RV leads located in the RV mid septum (n = 74) compared with the RV-A (−3.4 ±6.5 mm vs. +1.7 ±6.4 mm; P = 0.004), but a similar increase in LVEF was observed [P = non-significant (NS)]. Khan et al.8 investigated 131 patients undergoing CRT with the RV lead positioned in RV-A (n = 76) and RV mid septum (n = 55), and demonstrated similar benefits effected by CRT. Haghjoo et al9 studied 73 patients with the RV lead positioned in the RV-A (n = 51) and RVOT (n = 23), with no differences in LV reverse remodelling. The results in the present study support the evidence from the aforementioned studies, that the additive effect of alternative RV lead position in CRT is limited.
The extent of LV reverse remodelling and long-term prognosis have recently been related to the LV lead position in CRT. Singh et al.12 studied 799 CRT recipients and demonstrated an increased risk for death (hazard ratio, 2.91; P = 0.004) in the apical compared with a non-apical LV lead position. Importantly, a non-apical LV lead location was used in the present study. Furthermore, several studies have demonstrated the heterogeneity in the LV contraction pattern in patients undergoing CRT by using different echocardiographic techniques.16,21,22 Subsequently, there has been interest in LV lead position individually targeted at the site of the latest mechanical LV activation identified by pre-operative imaging. Conventionally the LV lead is positioned in a lateral or posterolateral LV segment in CRT.23 By using ST-RS echocardiography, concordant LV leads at the segment with the latest mechanical activation and without low-amplitude radial strain in the segment containing the LV lead have demonstrated a superior response to CRT. Ypenburg et al.5 studied 257 patients undergoing CRT and demonstrated more reverse remodelling in concordant LV leads compared with discordant LV leads (LVESV, 189 ±83 mL to 134 ±71 mL; P < 0.001 vs. 172 ±61 mL to 162 ±63 mL; P = NS). The long-term survival was favourable in 397 CRT recipients with ischaemic cardiomyopathy without transmural scarring by using a cut-off for radial strain <16.5% (hazard ratio, 2.913; P < 0.001).3 To our knowledge, all previous studies on concordant LV leads evaluated by speckle tracking echocardiography are empirical data, without prospectively guided LV lead implantation. The present study demonstrates that it is feasible to position the LV lead selectively at the latest contraction LV segment and avoid segments with extensive transmural scarring, assessed by pre-operative ST-RS echocardiography. Concordant LV leads in the subgroup analysis demonstrated greater reverse remodelling provided by CRT as compared with discordant LV leads, and the results in this study confirm earlier retrospective findings. The larger reduction of LV dyssynchrony observed in concordant LV leads might contribute to a greater reverse remodelling effected by CRT.
Previous studies have reported concordant LV leads in 57–68% without guided LV lead implantation from ST-RS analysis.3–5 In the present study, concordant LV leads were obtained in 72% of the patients. As presented, the available coronary veins were the major cause of discordant LV leads. Furthermore, patients with the posterior segment identified as the targeted segment for the LV lead placement had a low concordant LV lead success rate. The present findings also demonstrate the limitations of selective site LV lead placement according to the latest activated LV segment, that it is not possible to achieve 100% LV lead concordance by using the transvenous CRT implant technique. The high occurrence of ischaemic cardiomyopathy in patients with discordant LV lead placement in our study is similar to previous findings5 and might be explained by increased scarring leading to a reduced number of coronary tributary veins available.
Several pre-implantation characteristics have been identified to influence the benefits of CRT.24 In this study, only 13% of the study population were women, which is lower than the number in large multicentre trials.1 In recent studies, a superior effect of CRT has been shown in women.25 All consecutive eligible patients referred to CRT at our centre during the inclusion period were selected in the present study, and no women were excluded. The effects demonstrated by CRT in the present study might be affected by the limited number of females in the study population. Different QRS morphologies have been evaluated in CRT recipients, and LBBB has been demonstrated to give a favourable outcome in CRT.26 The prevalence of LBBB morphology in the study population was 86%, but not different with respect to RV lead position or LV lead concordance. Furthermore, LBBB was not associated with more reverse remodelling at 6-month follow-up, but a longer observation period might be needed to identify any difference. Previous studies have demonstrated the increased effect of CRT in patients with non-ischaemic compared with ischaemic cardiomyopathy.24 Non-ischaemic cardiomyopathy was not predictive of LV reverse remodelling in the present study; however, it may be insufficiently powered to discriminate the benefits of CRT according to non-ischaemic HF. The need for and which method should be used to optimize the AV and VV interval in CRT recipients are still under debate, but optimization is at least recommended in patients that do not respond favourably to CRT.19 AV and VV optimization was performed in all patients, and was not different according to RV lead position.
Clinical implication
The current study demonstrates that the additive effect of an alternative RV lead position in CRT may be limited. Moreover, the presented results show that guided LV lead implantation from ST-RS echocardiography is feasible, and further supports the importance of obtaining concordant LV leads in CRT. A discordant LV lead position can be a confounding factor if not taken into account in future studies of alternative RV lead positions. The limitations of selective site LV lead positioning in the transvenous CRT implant procedure may be improved by coronary vein angioplasty during the implant procedure,27 targeted LV endocardial pacing,28 or limited lateral thoracomy29 to facilitate concordant LV lead placement. Larger prospective studies are needed to assess further the feasibility and the potential additive effect of echocardiography or other imaging modalities as a guide to the optimal LV lead placement in CRT. Furthermore, it is necessary to establish a consensus on how the echocardiographic LV segment can be transferred most correctly into standardized fluoroscopic views used during the CRT implantation procedure, as previous studies on LV lead concordance have used different approaches to define the LV lead position.3–5,30
Recently, CRT has become an indication for patients with mild symptomatic HF.1 Moreover, CRT has shown similar LV reverse remodelling in HF patients with LVEF >35%, and might also be a therapeutic option in this patient population.31,32 The influence of LV lead position in CRT as demonstrated in HF patients with LVEF ≤35% might also apply to patients with preserved LV systolic function, and could be implemented in future studies.
Study limitations
The present study may be limited by the relatively small study population from a single centre, and the sample size and power might be too small to identify any differences between the two RV lead placements in CRT. Moreover, the duration of the observation period might be insufficient to demonstrate possible advantages of one RV lead position. However, the prospective trial design, the similar patient population, and the targeted LV lead implantation by ST-RS echocardiography strengthen the results presented in this study.
Conclusions
The position of the RV lead in the RV-A or RV-HS resulted in similar benefits in the clinical status, LV contractile function, LV reverse remodelling, and reduction of LV dyssynchrony 6 months after CRT. Targeted LV lead implantation to the latest activated segment identified by ST-RS echocardiography was feasible in CRT. Concordant LV leads in CRT provided a larger increase in LVEF, superior LV reverse remodelling, and a greater reduction of LV dyssynchrony than discordant LV leads evaluated at 6-month follow-up.
Acknowledgements
Helse Vest, who supported this study, had no role in the study design, data collection or analysis, preparation of the manuscript, or publishing process.
Funding
Helse Vest, the Norwegian Research Council.
Conflict of interest: none declared.




