Impact of device-guided slow breathing on symptoms of chronic heart failure: a randomized, controlled feasibility study
Abstract
Aims
In many patients with chronic heart failure (CHF) even normal daily life activities cause dyspnoea and fatigue, well-being might be considerably improved by even a modest decrease in such symptoms. The aim of this study was to investigate if lowering breathing rate with the help of a respiratory modulation (RM) device could improve symptoms in patients with CHF.
Methods and results
Stable CHF patients with symptoms of dyspnoea were randomized to twice-daily 20 min sessions using an RM device or to music listening (ML) using a CD player, for a 4-week study period. Respiratory modulation guides the user to achieve a slow breathing rate (<10 breaths/min) while increasing exhalation time (Tex) relative to inhalation time (Tin). Lower breathing rate was accomplished by synchronizing respiratory movements with musical tones generated in response to breathing movements monitored with a belt-type sensor. Endpoints were reduced breathlessness and New York Heart Association (NYHA) class. Seventy-two patients (52 male, age 73 ± 11 years, NYHA 3.1 ± 0.9) were randomized and 65 completed the study (30 RM and 35 ML, respectively). There was no in-between group improvement in breathlessness and NYHA class. Patients in the RM group who displayed an average increase in Tex/Tin of >0.2 and a reduction in the average respiration rate during 30 sessions were considered responders. Responders reported reduced breathlessness (−0.86 ± 0.23 units, P < 0.005) and improved NYHA class (−0.64 ± 0.20, P < 0.01) compared with non-responders.
Conclusion
Device-guided RM might have the potential to relieve symptoms of heart failure in outpatients by changing their breathing pattern.
Introduction
The syndrome of chronic heart failure (CHF) is disabling and leads to decreased quality of life, frequent hospitalizations and poor survival.1 The patient's subjective response to the condition is associated with symptoms that reflect physical aspects as well as integrated aspects of lifestyle, anxiety, depression, and expectations of the patient.2–4 These symptoms, especially fatigue and dyspnoea, are important factors in the diagnosis of heart failure and assessment of severity.5,6 However, in large clinical trials for new treatments of CHF the primary endpoint is commonly to reduce morbidity and mortality while a measure of symptom relief is used as a secondary endpoint.7–9
Patients with CHF consider symptom relief to be of greater importance than prolonged life as an effect of their medication10 and the major reason for seeking care in this population is symptoms of dyspnoea.11–13 Definitions of dyspnoea are often a mixture of the true symptom, what patients say they are feeling, and physical signs, such as what the clinician observes about the patient, e.g. ‘exhibits laboured breathing’.
Although new onset of dyspnoea in CHF reflects worsening heart failure and should be treated with conventional drug treatment, there are some patients who, despite optimal treatment, continue to experience chronic dyspnoea even at rest or at low levels of exercise. Accordingly, these patients might need alternative treatment options directed specifically at their main symptom, dyspnoea. Breathing differs from other vital functions in that it is regulated not only by automatic centres located in the brainstem but also by voluntary signals initiated in the cortex.
Breathing control can easily be performed with a newly developed device that, in a few minutes, will teach a person how to breathe slowly and deeply. Device-guided breathing (DGB) exercises have been found to give a feeling of relaxation and well-being, as well as to affect cardiovascular reflexes, reduce sympathoadrenergic activity and decrease blood pressure in patients with hypertension or CHF.14–16
The aim of the present pilot study was to explore whether slow and deep breathing could improve symptoms in patients with CHF.
Methods
Patients
Patients diagnosed with stable, New York Heart Association (NYHA) class II–IV CHF, with persistent symptoms of breathlessness despite optimal pharmacological treatment were included in the study. Patients were excluded if they were diagnosed with a disease that might interfere with their ability to perform DGB (psychiatric illness, chemical dependency, unstable angina pectoris, or chronic obstructive pulmonary disease), their survival was expected to be shorter than the study duration, they had poor communication skills or ability to follow instructions, were unwilling to participate or were participating in another study.
Breathlessness was assessed on a 5 point Likert scale. If the Likert score was 2 or higher at rest, the patient was considered eligible to participate in the study.
All patients gave written consent to participate in the study. The investigation conformed to the principles outlined in the Declaration of Helsinki and the regional ethical review board in Gothenburg approved the study protocol. After an initial pilot phase during which study logistics were tested, the main study started in 2007 and ended in 2009.
Study design and method of respiration modulation
After inclusion in the study, patients were randomized either to the intervention arm or the control arm in an open trial design. The control patients listened to music for 20 min twice a day using a CD player with earphones. While listening to the music, the patients were asked to rest comfortably in a sitting position and avoid talking or engaging in other activities.
The patients in the intervention arm were asked to do a 20 min, twice-daily session of DGB using a commercially available device (RESPeRATE®, InterCure Ltd, Lod, Israel). The device provides a way of slowing the respiratory rate (RR) in an effortless manner by generating musical tones in response to the patient's own breathing movements.17 Breathing movements were recorded in real time via a belt-type sensor placed around the chest or upper abdomen. The data were processed and stored in a battery-operated, computerized device the same size as a portable CD player. The device also generated musical tones that the patient listened to with headphones. During an initial phase of 1 min the patient's spontaneous breathing pattern was identified, then based on this information, the respiratory modulation (RM) phase was initiated using different musical tones for inhalation and exhalation. The goal was to progressively slow the respiration rate to <10 breaths per min15 in an individualized way, which was accomplished by continuous feedback to the device about the patient's RR during the session. In addition to slowing the RR, the musical tunes provided support to increase the exhalation time (Tex) relative to the inhalation time (Tin).17 The device could be programmed to shut-off automatically after a pre-selected time interval which in this study was 20 min. All patients received detailed instructions from a nurse about how to apply and use the DGB exercises before the first session.
During each session, variables that characterized performance such as RR, and duration of inhalation and exhalation, were calculated as a running average over 5 breaths and stored as one value every minute. In addition, the device calculated and stored the relative time when breathing movements were synchronized with the guiding tones. Patients in the intervention arm and control arm completed a 4-week study period. At the end of the 4-week period, data from the control and treatment arms were downloaded to a computer at the research centre.
Measured variables, data analysis and statistical methods
All patients underwent evaluation of NYHA class, N-terminal pro brain natriuretic peptide (NT-proBNP) levels, cuff blood pressure and self-rated sleep quality at baseline and end of treatment. Furthermore, self-reported breathlessness and fatigue were recorded on a 5 point Likert scale at the same time points. Left ventricular ejection fraction and information about smoking habits were collected at baseline.
Predefined endpoints included baseline-to-end changes in NYHA class, breathlessness and fatigue. Measurements associated with the use of DGB were number and duration of completed sessions used as an expression of compliance and performance variables that included RR, exhalation (Tex), and inhalation (Tin) times and an exhalation/inhalation ratio (Tex/Tin). Averages and average change in these variables from the start to the end of the session (ΔRR, ΔTex, ΔTin, and Δ(Tex/Tin), respectively) were applied. Averages were then calculated over the maximum number of sessions available, starting from the third session to allow the patients to reach stable performance.
We postulated that the individualized way of modulating breathing with DGB was associated with the effect on symptoms. Therefore, a major goal of the analysis was to establish a criterion based on performance data that could identify DGB ‘responders’ vs. ‘non-responders’. Responders were expected to reduce breathlessness significantly more than non-responders. This approach of finding ‘explanatory variables’ after patients have been randomized has been used previously.17,18
Appropriate use of DGB during a session should result in a reduction in breathing rate and an increase in the relative duration of exhalation. Based on this, a variable threshold (Thresh) for Δ(Tex/Tin) was selected and compared with the change of breathlessness in responders (defined by Δ(Tex/Tin)>Thresh) vs. non-responders (defined by Δ(Tex/Tin)≤Thresh) using t-test for independent samples. The final threshold of ≥0.2 was related to the smallest P-value.
Data are reported as actual numbers or mean ± standard deviation for the whole group in the tables and as change from baseline in the figures. Differences were considered significant if P < 0.05.
SPSS Statistics 18 (IBM Corporation, Armonk, NY, USA) was used for the analysis of the raw data from the RM device while Microsoft Excel 2010 was used to derive mean, SD, median, intra quartile ranges, and P-values from the patient characteristics, symptoms, signs, and derived respiratory measurements.
Results
Patients
Seventy-two patients were included, of these 35 were randomized to use DGB to slow RR. Thirty patients in the intervention arm and 35 patients in the control arm completed the 4-week study period. There was no difference in baseline characteristics between the two groups (Table 1).
| Intervention, DGB (n = 30) | Control (n = 35) | P-value | |
|---|---|---|---|
| Age (year) | 73 ± 11 | 73 ± 10 | 0.794 |
| Male/female (n) | 22/8 | 24/11 | 0.680 |
| LVEF (%)a | 30 (25–41) | 35 (25–50) | 0.164 |
| Systolic blood pressure (mmHg) | 123 ± 17 | 129 ± 22 | 0.268 |
| Diastolic blood pressure (mmHg) | 75 ± 11 | 75 ± 10 | 0.963 |
| Heart rate (bpm) | 67 ± 11 | 65 ± 10 | 0.482 |
| Waist/bottom ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.864 |
| NT-proBNP (pg/mL)a | 1980 (944–3088) | 1310 (674–2890) | 0.325 |
| Good sleep quality (n) | 24 | 23 | 0.271 |
| Hours sleep at night (h) | 6.3 ± 1.4 | 6.2 ± 1.6 | 0.783 |
| Tobacco use, yes/no/previously (n) | 3/14/13 | 4/10/21 | 0.373 |
| Diuretics (n) | 24 | 29 | 0.772 |
| Beta-blocker (n) | 29 | 32 | 0.389 |
| ACE-inhibitor (n) | 15 | 16 | 0.735 |
| ARB (n) | 16 | 17 | 0.778 |
- a DGB, device-guided breathing; n, number; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro brain natriuretic peptide; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
- a Data are expressed as median (lower, upper quartile).
Seven patients did not complete the study, of these four patients withdrew consent due to other illnesses, living too far away or psychological/memory reasons. Two patients reported not liking the respiration modulation system; one did not like the music and one did not like the breathing exercise and subsequently withdrew from the study. One patient could not be reached at time for follow-up and was excluded due to non-compliance.
The DGB exercises were well tolerated and patients performed a median of 48 successful DGB sessions during the 4-week period. Of the 30 patients in the intervention arm, 25 performed 30 or more DGB sessions during the study. One patient reported breathlessness and need for rest for 4–5 min after the DGB sessions. No other adverse events related to the RM procedure were reported during the study.
Respiratory modulation
Between-group comparisons showed no difference in changes in self-reported symptoms or NYHA class from baseline to the end of the study (Table 2, Figure 1).
| Baseline | 4 weeks | |||||
|---|---|---|---|---|---|---|
| Intervention, DGB (n = 30) | Control (n = 35) | P-value | Intervention, DGB (n = 30) | Control (n = 35) | P-value | |
| NYHA class | 2.9 ± 0.4 | 2.7 ± 0.5 | 0.141 | 2.6 ± 0.8 | 2.6 ± 0.7 | 0.850 |
| Breathlessness (scale 1–5) | 3.1 ± 0.9 | 2.9 ± 0.9 | 0.409 | 2.7 ± 1.2 | 2.7 ± 1.0 | 0.986 |
| Fatigue (scale 1–5) | 3.1 ± 1.0 | 3.0 ± 1.1 | 0.612 | 2.6 ± 1.0 | 2.8 ± 1.0 | 0.446 |
| NT-proBNP (pg/mL)a | 1980 (944–3088) | 1310 (674–2890) | 0.325 | 1980 (559–3690) | 1340 (533–3245) | 0.592 |
| Heart rate (bpm) | 67 ± 11 | 65 ± 10 | 0.482 | 64 ± 10 | 63 ± 8 | 0.821 |
| Systolic blood pressure (mmHg) | 123 ± 17 | 129 ± 22 | 0.260 | 124 ± 21 | 129 ± 20 | 0.339 |
| Diastolic blood pressure (mmHg) | 75 ± 11 | 75 ± 10 | 0.963 | 74 ± 10 | 73 ± 10 | 0.650 |
- a DGB, device-guided breathing; NYHA, New York Heart Association; NT-proBNP, N-terminal pro brain natriuretic peptide.
- a Data are expressed as median (lower, upper quartile).

Patients in the intervention arm improved self-reported symptoms and NYHA class significantly after 4 weeks of treatment compared with baseline (Figure 1); however, patients in the control arm did not improve. No changes in NT-proBNP levels, heart rate, or blood pressure were seen in either group (Table 2).
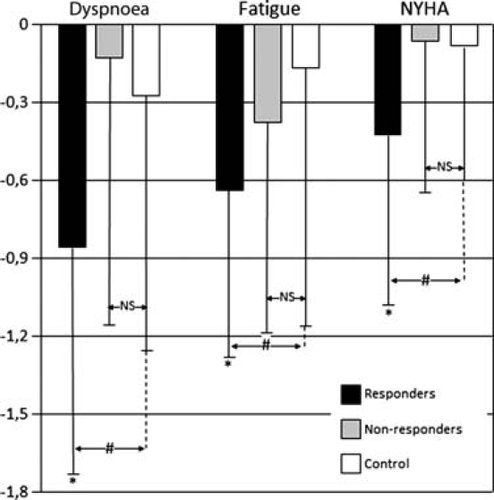
Analysis by responders (n = 14) in the intervention arm showed significant improvement in self-reported symptoms and NYHA class after 4 weeks of treatment in comparison with non-responders or controls (Table 3, Figure 2). Responders and non-responders did not differ in any clinical or demographic variable or in the number of sessions performed (data not shown).
| Responders (n = 14) | Non-responders (n = 16) | |||||
|---|---|---|---|---|---|---|
| Baseline | 4 weeks | P-value | Baseline | 4 weeks | P-value | |
| NYHA class | 2.9 ± 0.3 | 2.5 ± 0.7 | 0.028 | 2.8 ± 0.5 | 2.8 ± 0.9 | 0.669 |
| Breathlessness (scale 1–5) | 3.4 ± 0.7 | 2.5 ± 1.0 | 0.003 | 2.9 ± 1.0 | 2.8 ± 1.3 | 0.633 |
| Fatigue (scale 1–5) | 3.4 ± 0.7 | 2.7 ± 0.7 | 0.002 | 2.9 ± 1.2 | 2.6 ± 1.2 | 0.083 |
- a NYHA, New York Heart Association.
Discussion
Device-guided breathing by musical tones did not improve symptoms in patients with heart failure compared with patients who only listened to music. However, patients in the DGB group who increased their exhalation/inhalation time ratio, improved symptoms of dyspnoea significantly.
Dyspnoea is a complex symptom and may have several origins even in the same patient; the causes of dyspnoea may be interrelated thus making multiple treatments both an option as well as a necessity. Bernardi et al.19 showed that dyspnoea and rapid breathing in patients with CHF are partly caused by a sensitized chemoreflex response that result from vasoconstriction and slower circulation through chemoreflex areas in relation to pulmonary congestion. Other groups have reported that decreased strength in inspiratory muscles might contribute to dyspnoea.20–22
The American Thoracic Society has proposed a broad definition of dyspnoea that stresses on the subjective origin of the symptom:
‘Dyspnea is a term used to characterize a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioural responses'.23
Previous studies have shown that a reduced RR in patients with CHF will increase oxygen saturation, improve ventilation/perfusion efficacy and exercise tolerance, and reduce sympathetic activation in an acute setting.15,16,19. In addition, a study using DGB in a home setting showed improvement in exercise capacity and ventricular function.14 The study also reported anecdotal evidence of a reduction in dyspnoea. However, patients in the study did not have persistent dyspnoea at the time of inclusion and thus no direct measurement of symptoms was performed during the study.14
In the present study, the improvement in symptoms might result from the positive effect on baroreflex sensitivity.16 In addition, improved thoracic muscle strength22 and better coordination between the diaphragm and inhalation/exhalation movements might also contribute. However, the present study was not designed to evaluate these factors and thus their impact can only be speculated on at this point.
In patients with CHF even normal daily activities can cause dyspnoea and fatigue as patients get close to their cardiopulmonary reserve limit. Thus, quality of life might be considerably improved by even a modest decrease in such symptoms as dyspnoea and fatigue and a small improvement in exercise tolerance.
Slow breathing guided by a device is an easy, non-invasive, and non-pharmacological technique to improve patient symptoms that can be performed anywhere with a quiet rest area. Other advantages are that it does not require complex equipment and physical impairment is no contraindication. The preset requirement of twice-daily sessions of 20 min each was not followed rigorously in this study, but despite this lack of stringent regulation, a positive effect on symptoms could be achieved. The recommended number of sessions per day and the length of sessions required to reach an optimal effect will have to be studied further in this group of patients before a more conclusive statement can be made. Patients’ experiences of the device and willingness to adhere to it have been investigated in patients with hypertension, many of whom expressed the intention of keeping up with the treatment for >4 months.24 The possibility for patients to manage their own treatment, choose the time of treatment, perceive the effect of the treatment immediately, and use a treatment with no side effects may explain the high level of adherence to DGB.24
Responders to DGB were identified using a simple criterion based on the exhalation/inhalation time ratio and the RR during each session. We suggest that in the present study, the increased exhalation/inhalation ratio at slow breathing reduced sympathetic activity and increased perfusion of the abdominal muscle while minimizing breathing efforts.25,26 Non-responders seemed to be less adherent to the necessary breathing techniques, thus future studies should allow for interventions to improve RM techniques to minimize non-performance and/or selection of other treatment strategies at an early stage.
Limitations
Seven patients did not complete the study, as would be expected in this very sick and elderly group of patients. Only two patients withdrew consent due to not liking the music or the breathing exercise.
Four weeks of DGB improved symptoms in half of the patients who underwent the exercise; however, the study design did not follow the patients for long enough to assess the sustainability of the results. In addition, no intervention to improve adherence to DGB was performed in the non-responder group as the results from the RM were not available during the course of the study. If the breathing technique had been improved in the non-responders this might have affected the results. The responders had more symptoms at baseline; however, there was no difference in clinical signs between the groups that could explain this finding. Finally, we have no information about breathing patterns in the control group and any potential relationship with reduced symptoms.
Conclusion
Device-guided breathing may have the potential to relieve symptoms of heart failure in outpatients by changing their breathing pattern.
Acknowledgements
The authors acknowledge support from the Swedish Heart and Lung Foundation and the Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Sweden. We would also like to thank Benjamin Gavish for valuable advice and help on data interpretation.
Funding
This work was supported by funding from the Swedish Heart Lung Foundation.




