Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: results from a multicentre international randomized trial (PREVENT-HF)
Abstract
Aims
Previous experimental and clinical studies have consistently suggested that right ventricular (RV) apical pacing has important adverse effects. Ventricular pacing (VP), however, is required, and cannot be reduced in many patients with atrioventricular (AV) block. The PREVENT-HF study was an international randomized trial that explored differences in left ventricular (LV) remodelling during RV apical vs. biventricular (BIV) pacing in patients with AV block.
Methods and Results
Patients with an expected VP prevalence ≥80% were assigned to RV apical or BIV pacing. The primary endpoint was the change in LV end-diastolic volume (EDV) >12 months. Secondary endpoints were LV end-systolic volume (ESV), LV ejection fraction (EF), mitral regurgitation (MR), and a combination of heart failure (HF) events and cardiovascular hospitalizations. Overall, 108 patients were randomized (RV: 58; BIV: 50). Intention to treat and on-treatment analyses revealed no significant differences in any of the outcomes. Analysis of covariance (ANCOVA) difference for treatment according to randomization (in mL): LVEDV −3.92 (−18.71 to 10.85), P= 0.6; LVESV −1.38 (−12.07 to 9.31), P= 0.80; LVEF 2.47 (−3.00 to 7.94), P= 0.37. Analysis of covariance difference for the on-treatment analysis: LVEDV −4.90 (−20.02 to 10.22, PP= 0.52; LVESV −6.45 (−17.28 to 4.38), P= 0.24, LVEF 2.18 (−3.37 to 7.73), P= 0.44. Furthermore, secondary endpoints did not differ significantly.
Conclusion
This study did not demonstrate significant LV volume differences >12 months between RV apical and BIV pacing for AV block. Thus, BIV pacing cannot be recommended as a routine treatment for AV block in these patients. However, the results encourage and inform the design of subsequent larger trials with higher power for detecting small volume changes. ClinicalTrials.gov Identifier: NCT00170326.
See page 599 for the editorial comment on this article (doi: 10.1093/eurjhf/hfr058)
Introduction
In the USA, the annual number of first pacemaker implants is 990 for every million inhabitants. This corresponds to an extrapolated 288 000 first implants.1 According to the Association of Pacemaker Manufacturers,2 the current annual number of pacemaker implants in Europe is 355 000, which corresponds to 878 implants for every million inhabitants. The indication for pacing in about half of all first pacemaker implants is the permanent or intermittent compromise of atrioventricular (AV) conduction.3 These patients require at least intermittent ventricular pacing (VP). In up to 50% of patients with AV block, VP can largely be prevented;4 thus, the annual number of pacemaker patients with unpreventable VP exceeds 88 000 across Europe and 72 000 in the USA. This implies that a considerable patient population may experience adverse effects with conventional right ventricular (RV) apical pacing, even after implementing various algorithms to reduce unnecessary VP.
Over the past several years, there has been growing concern over the significant undesired effects of RV pacing, including left ventricular (LV) desynchronization,5,6 proarrhythmia,7 and promotion of heart failure (HF).8–11 However, it remains debatable whether pacing-related ventricular dyssynchrony can translate into significant LV structural changes and produce important clinical sequelae in an average pacemaker population with compromised AV conduction. Alternatively, biventricular (BIV) pacing has been shown to attenuate ventricular dyssynchrony and to improve symptoms and prognosis in patients with severe systolic HF and prolonged QRS duration.12,13 The favourable effect of BIV pacing in cardiac resynchronization trials gave rise to the hypothesis that the BIV pacing configuration might better attenuate ventricular dyssynchrony compared with RV pacing for AV block. Changes in LV volumes have been shown to be among the most powerful prognostic factors in patients with and without chronic HF.14 This international randomized study was conducted to explore LV remodelling and clinical events with BIV vs. RV pacing >12 months in patients with a pacemaker indication and high anticipated need for VP.
Methods
Patients were enrolled at 14 European centres (five in Spain, two in Italy, and seven in Germany). The steering committee (see Appendix) designed the trial. The pacemaker manufacturing company, Medtronic, funded the trial and provided logistic support. Study follow-up data and adverse events were documented on electronic case report forms and transferred to a central data server in Spain. Echocardiographic readings were carried out by a single, experienced, core laboratory examiner (J.J. Gomez Doblas, Malaga, Spain). The PREVENT-HF study has been filed at the United States National Institute of Health ClinicalTrials.gov registry (ClinicalTrials.gov Identifier: NCT00170326).
Patients
Eligible patients were aged at least 18 years and met Class I or IIa implantation criteria for pacemaker stimulation according to the guidelines of the American College of Cardiology/American Heart Association.15 To ensure that only individuals with presumably unpreventable VP entered the study, the need for future VP had to be at least 80% (estimated by the enrolling investigator) prior to inclusion.
Patients were excluded from study participation when they exhibited advanced HF (New York Heart Class III or IV) prior to the development of the pacing indication, a myocardial infarction or cardiac surgery during the preceding 3 months, future need for revascularization within 3 months, hypertrophic cardiomyopathy, constrictive pericarditis, aortic stenosis, poor echocardiographic window, a previously implanted pacemaker or defibrillator, pregnancy, life expectancy <1 year, or no signed informed consent.
Randomization and implantation
Eligible patients underwent an internet-based 1:1 hardware randomization and were assigned to receive either a conventional DDD(R) RV pacemaker or a BIV pacemaker system. Randomization was stratified according to the presence or absence of atrial fibrillation (AF). In the absence of AF, the treatment groups were balanced in each centre; in the presence of AF, the randomization was not stratified by site. Patients with an additional indication for an implantable cardioverter-defibrillator (ICD) received either a standard dual-chamber device or a BIV ICD, according to the assigned treatment arm. Patients with permanent AF and a symptomatic slow ventricular response received either a single-chamber VVIR pacemaker or a single-chamber ICD in the RV pacing group, or an atrio-BIV pacemaker or ICD system with plugged atrial port in the BIV pacing group. The patients were blinded to the treatment assignment.
The investigators placed RV leads at the RV apex and LV leads via coronary sinus tributaries on the LV lateral wall. When the placement of a transvenous LV lead was unacceptable, investigators were instructed to finalize the procedure as a routine dual-chamber implantation instead of establishing a BIV configuration with open surgery and an epicardial lead. This resulted in a hardware cross-over. For the primary analysis, patients were allocated to the randomized treatment arm; for the on-treatment analysis, patients were classified according to the de facto pacing configuration.
Investigators were encouraged to perform a post-operative optimization procedure by a method selected by the site investigator.
Medical treatment
Appropriate concomitant medical therapies were pre-specified. Angiotensin-converting enzyme (ACE) inhibitors and beta blockers (initiated after implant) were given to patients with an EF <40%; spironolactone and diuretics were given when overt HF developed (New York Heart Class III and IV); digoxin was given for symptoms that persisted despite the medication-mentioned above; and oral anticoagulants were given for paroxysmal or permanent AF.
Follow-up visits
Patients were assessed at a baseline visit, during admission for the implantation, prior to hospital discharge, and at 6 and 12 months. Each visit included clinical assessments of adverse experiences, hospitalizations, and the concomitant medication. A 12-lead electrocardiogram was recorded at each visit, and a chest X-ray was performed after implant and at 12 months. The device interrogation information was saved to diskette at the beginning and end of each visit, and the stored files were transmitted to the central server via an internet connection.
Echocardiography
Comprehensive echocardiographic examinations were conducted prior to hospital discharge and at the 6-and 12-month visits. A pre-specified protocol was always performed by the same site-based examiner on the same echocardiography machine. The data were shipped to the echocardiography core laboratory. The examiner was blinded to the amount of time after treatment and the treatment assignment. Left ventricular volumes were assessed with the bi-plane Simpson method. The intra-observer variability of LV volume measurements was 3.2%.
Study endpoints
The primary endpoint of PREVENT-HF was the change in LV end-diastolic volume (LVEDV) after 1 year, and RV apical pacing was compared with BIV pacing. Secondary endpoints included changes in LV end-systolic volume (ESV) and LVEF; the development or worsening of mitral regurgitation (MR); and a combination of endpoints, including cardiac mortality, new development or worsening of symptomatic HF (according to the European Society of Cardiology HF guidelines),16 and hospitalization due to cardiovascular causes. All events were reviewed and classified by two steering committee members (M.S. and X.N.) in a blinded fashion.
Statistical analysis and sample size computation
The primary endpoint was compared between groups with the analysis of covariance (ANCOVA), which took into account baseline values. Primary and secondary endpoints were compared between groups with the Student's t-test or χ2 test, as appropriate. Survival analyses and log rank testing were conducted to evaluate the combined clinical secondary endpoints and the incidence of HF events. A principal analysis was carried out for the assigned groups, and the last valid results were carried over in incomplete cases. In view of the liberal handling of hardware cross-overs, the steering committee decided, in accordance with the statistical advisor, to also perform all analyses according to the actually implemented pacing configuration. Differences in the change of MR between treatment groups were tested with the Cochran Armitage test for trends. A P≤ 0.05 was considered significant.
Assuming a standard deviation of 30 mL in the primary endpoint (based on the control group from the SOLVD echo substudy)17 and a bilateral α = 0.05, we calculated that we could obtain an 80% power for detecting a difference of 18 mL by analysing 88 individuals (44 per treatment group). Subgroup analyses were performed according to the presence or absence of AF, baseline QRS duration, and the initial LVEF.
Results
Study population
A total of 108 patients were randomized to receive RV apical (n= 58) or BIV pacing (n= 50). Baseline characteristics were similar between groups (Table 1). Patients had a nearly normal average LVEF at baseline. Arterial hypertension and coronary artery disease were highly prevalent, but a history of AF was infrequently present. Because impaired AV conduction was an inclusion criterion, the QRS duration at baseline was slightly longer than normal values in both groups. The majority of patients reported symptoms of mild HF prior to the development of the pacemaker indication.
| RV pacing group n = 58 | BIV pacing group n = 50 | P-value | |
|---|---|---|---|
| Age (years) | 69.5 ± 8.2 | 71.6 ± 9.3 | 0.21 |
| Male sex (%) | 44 (76) | 34 (68) | 0.40 |
| Pre-enrolment LVEF (%) | 54.9 ± 12.9 | 57.5 ± 11.8 | 0.33 |
| NYHA class I (%) | 22 (38) | 24 (48) | 0.23 |
| Hypertension (%) | 34 (59) | 30 (60) | 0.60 |
| CAD (%) | 18 (31) | 16 (32) | 0.99 |
| Valvular disease (%) | 8 (14) | 4 (8) | 0.37 |
| History of AF (%) | 6 (10) | 5(10) | 0.99 |
| SBP (mmHg) | 141.7 ± 24.4 | 139.1 ± 23.6 | 0.59 |
| Body weight (kg) | 79.9 ± 12.6 | 78.7 ± 13.1 | 0.62 |
| QRS width (ms) | 123.9 ± 30.0 | 120.9 ± 31.8 | 0.61 |
| Heart rate (bpm) | 54.8 ± 15.7 | 61.2 ± 20.9 | 0.32 |
| PM dependenta (%) | 13 (22) | 10 (20) | 0.99 |
- a RV, right ventricular; BIV, biventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; CAD, coronary artery disease; AF, atrial fibrillation; SBP, systolic blood pressure; PM, pacemaker.
- b Values represent means±standard deviations or the number of patients and (%).
- a Typically reflecting complete and permanent AV block.
Medication
Concomitant medications did not differ between groups. After pacemaker implantation, beta blockers were prescribed to 52% of patients, an ACE-inhibitor or angiotensin receptor blocker to 85%, an aldosterone receptor antagonist to 10%, and a calcium channel blocker to 15% of study patients.
Drop-out patients
After randomization, six patients received implants outside the protocol specification for a variety of reasons, including patients’ preference and administrative issues. These patients were dropped out of the study. In addition, six patients received implants according to the randomization arm, but the follow-up did not adhere to the protocol; therefore, no follow-up data were transmitted. Baseline characteristics did not differ significantly between the 12 drop-out patients (RV group: 8; BIV group: 4) and the 96 remaining patients with follow-up data.
Hardware cross-over patients
In accordance with the study protocol, eight patients (16% of the BIV group) received a conventional RV pacing system despite randomization to BIV pacing. In addition, due to patient preferences, seven study participants (12% of the RV arm) received BIV pacemakers, despite randomization to the RV pacing system. For these seven patients, the LV lead was deactivated, and they were paced at the RV apex during the entire study period.
Primary endpoint
In order to ensure that the echo examiner could not identify the length of time after the procedure, the echo study evaluations were started late in the course of the study. It was then recognized that, due to protocol non-compliance and technical issues (i.e. missed examinations and lost or damaged echo tapes), only 75 patients (RV group: 40; BIV group: 35) had analysable paired echo data. Of the 35 patients in the BIV group, 6 (17%) had received a RV apical pacemaker and were sorted to RV treatment for the on-treatment analysis.
Analysis according to assigned treatment
The LVEDV showed a slight and non-significant increase after 12 months of pacing in both groups. There were no significant differences between groups (Table 2).
| According to assigned treatment | ||||||
|---|---|---|---|---|---|---|
| n | RV pacing group | n | BIV pacing group | ANCOVA effect (mL) (95% CI) | P-value | |
| LVEDV PHD (mL) | 40 | 101.50 ± 41.70 | 35 | 97.20 ± 49.43 | −3.92 (−18.71 to 10.85) | 0.60 |
| LVEDV 12 months (mL) | 40 | 104.40 ± 36.39 | 35 | 99.43 ± 30.21 | ||
| On-treatment | ||||||
| n | RV pacing group | n | BIV pacing group | ANCOVA effect (mL) (95% CI) | P-value | |
| LVEDV PHD (mL) | 46 | 100.93 ± 40.34 | 29 | 97.21 ± 52.69 | −4.90 (−20.02 to 10.22) | 0.52 |
| LVEDV 12 months (mL) | 46 | 104.33 ± 35.19 | 29 | 98.52 ± 30.95 | ||
- a RV, right ventricular; BIV, biventricular; LVEDV, left ventricular end-diastolic volume; ANCOVA, analysis of covariance; PHD, prior to hospital discharge.
- b Values represent means±standard deviations, unless indicated otherwise.
On-treatment analysis
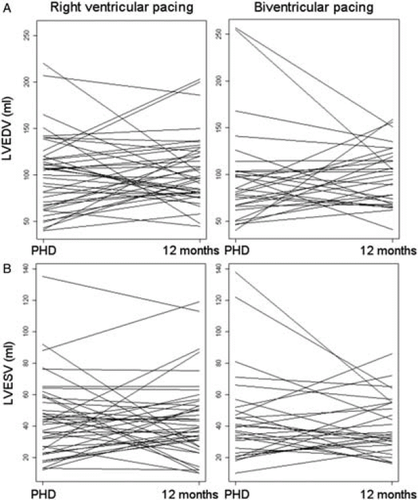
The LVEDV showed a slight increase in both groups. The ANCOVA treatment effect of 4.90 mL in favour of BIV treatment was not statistically significant, and it fell far below the anticipated difference (Table 2, Figure 1).
Secondary echocardiographic endpoints
Analysis according to assigned treatment
The left ventricular end-systolic volume (LVESV) was nearly unchanged in both groups, and was not significantly different between groups. The average LVEF was normal and nearly unchanged in both groups. The ANCOVA revealed no significant treatment effect (Table 3).
| According to assigned treatment | ||||||
|---|---|---|---|---|---|---|
| n | RV pacing group | n | BIV pacing group | ANCOVA effect (mL) (95% CI) | P-value | |
| LVESV PHD (mL) | 40 | 44.65 ± 25.13 | 35 | 41.26 ± 27.83 | −1.38 (−12.07 to 9.31) | 0.80 |
| LVESV 12 months (mL) | 40 | 44.69 ± 25.29 | 35 | 42.20 ± 23.65 | ||
| LVEF PHD (%) | 40 | 55.58 ± 14.03 | 35 | 59.65 ± 12.25 | 2.47 (−3.00 to 7.94) | 0.371 |
| LVEF 12 months (%) | 40 | 56.24 ± 14.50 | 35 | 60.08 ± 9.61 | ||
| On-treatment | ||||||
| n | RV pacing group | n | BIV pacing group | ANCOVA effect (mL) (95% CI) | P-value | |
| LVESV PHD (mL) | 46 | 42.72 ± 24.34 | 29 | 43.62 ± 29.57 | −6.45 (−17.28 to 4.38) | 0.24 |
| LVESV 12 months (mL) | 46 | 45.90 ± 27.64 | 29 | 39.76 ± 17.95 | ||
| LVEF PHD (%) | 46 | 57.65 ± 14.35 | 29 | 57.20 ± 11.67 | 2.18 (−3.37 to 7.73) | 0.44 |
| LVEF 12 months (%) | 46 | 57.25 ± 13.87 | 29 | 59.27 ± 10.15 | ||
- a RV, right ventricular; BIV, biventricular; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; ANCOVA, analysis of covariance; PHD, prior to hospital discharge.
- b Values represent means ± standard deviations, unless otherwise indicated.
On-treatment analysis
Changes in the LVESV and LVEF were not significantly different between treatment groups. With RV apical pacing, LVESV increased slightly, and with BIV pacing, LVESV decreased slightly, but neither change was significant. The ANCOVA revealed no significant differences between groups. The LVEF was preserved with RV apical pacing and increased slightly with BIV pacing, but no statistically significant treatment effect was observed (Table 3, Figure 1).
Subgroup analyses of the primary endpoint
None of the pre-specified subgroup analyses revealed a significant difference regarding the primary endpoint. A baseline LVEF ≤50 vs. >50% was not associated with a significant differential treatment effect, although small numerical changes were observed, with ANCOVA effects of −10.5 mL [95% confidence interval (CI) −33.13 to 12.13 mL] vs. 2.60 mL (95% CI −5.34 to 10.53 mL), respectively. A history of AF vs. no history had no significant association with diastolic LV volume changes, with ANCOVA treatment effects of −14.95 mL (95% CI −51.23 to 21.32 mL) vs. 0.72 mL (95% CI −7.49 to 8.93 mL), respectively. Baseline QRS durations of <120 vs. ≥120 ms did not influence the treatment effect, with ANCOVA effects of (95% CI −13.58 to 10.71 mL) vs. −3.49 mL (95% CI −15.79 to 8.81 mL), respectively.
Mitral regurgitation (qualitative assessment and on-treatment analysis)
There were few patients with relevant MR and reasonably measurable MR jet areas. Therefore, a reliable quantitative analysis of MR could not be undertaken. The results that were obtained by qualitative assessment of MR are outlined in Table 4. The Cochran Armitage test for trends revealed no significant difference between treatment groups.
| RV pacing group | BIV pacing group | P-value (Cochran Armitage test for trends) | |
|---|---|---|---|
| Worsening MR (%) | 47 | 32 | 0.26 |
| Unchanged MR (%) | 29 | 36 | |
| Improved MR (%) | 24 | 32 |
- a RV, right ventricular; BIV, biventricular; MR, mitral regurgitation.
Combined secondary clinical endpoints (on-treatment analysis)
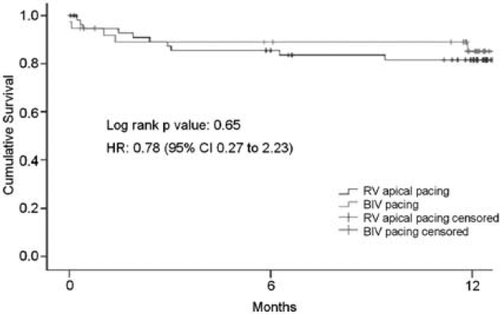
The combined clinical endpoints comprised cardiac mortality, new development or worsening of HF, and hospitalization due to cardiovascular causes. These endpoint events occurred in 13 patients on RV pacing and 9 patients on BIV pacing. Survival analysis revealed no significant difference between treatment groups (Figure 2).
Worsening or new development of heart failure
Heart failure events did not differ significantly between groups. Eight and three events of new or worsening HF occurred with RV and BIV pacing, respectively. The analysis of survival free of a first HF event showed a non-significant difference in favour of BIV pacing (Figure 3).

Adverse events
Adverse events occurred slightly more frequently in the BIV than in the RV on-treatment group (Table 5). However, the two groups displayed different distribution patterns of adverse events. One patient in the RV pacing group died prior to the 6-month follow-up from an unknown cause. Patients on RV pacing had more incident HF events and more atrial lead issues compared with patients on BIV pacing. However, patients on BIV pacing had more events due to LV lead problems (phrenic nerve stimulation) and more events that appeared to be unrelated to pacemaker treatment (e.g. renal failure late during the study period, malignancy, peripheral artery disease, and angina) compared with patients on RV pacing. There was no significant difference between groups in the analysis of survival free of a first adverse event (P= 0.24)
| RV pacing group (number of events) | BIV pacing group (number of events) | |
|---|---|---|
| Related to atrial leads | 3 | 1 |
| Related to RV leads | 1 | 0 |
| Related to LV leads (e.g. phrenic nerve stimulation, exit block) | 1 | 3 |
| Procedure related (pneumothorax, vagal reaction) | 1 | 1 |
| Worsening or new development of HF | 8 | 3 |
| Arrhythmias | 1 | 3 |
| Other (e.g. malignancy, collapse, angina, renal failure, and peripheral artery disease) | 2 | 10 |
| Death | 1 | 0 |
| Total | 18 | 21 |
- a RV, right ventricular; BIV, biventricular; LV, left ventricular.
Discussion
Main findings
The main finding of the PREVENT-HF study was that, among patients without advanced clinical HF and with a high expected need for VP, BIV pacing did not show a significant gross benefit compared with RV apical pacing with regard to LV volumes and LVEF after 12 months. In addition, the combined secondary endpoint did not differ significantly between treatment groups. It is important to note, however, that considerably larger samples and longer follow-up periods will likely be required to detect small LV volume changes and long-term differences in clinical outcomes. The substantial number of hardware cross-overs reflects the technical complexity involved in BIV pacing and emphasizes that this must be taken into account when considering this treatment option.
Previous studies
Although RV apical pacing is a well-tested method for treating bradycardia due to compromised AV conduction, consistent findings from several retrospective or non-pre-specified analyses suggest that dyssynchrony-related electromechanical,18 pathophysiological, and histological 19 alterations could indeed have important clinical implications. Several post hoc analyses of pacemaker and defibrillator trials 8,9,20,21 and of registry data 22 have suggested the hypothesis that RV apical pacing may promote clinical HF events. This hypothesis was strengthened by one multicentre randomized trial on HF and mortality that compared single- vs. dual-chamber ICDs.10,11 Right VP was also shown to promote atrial arrhythmias in one single-centre randomized trial of atrial vs. dual-chamber pacing 23 and in a multicentre trial on the effect of VP prevention.7 The extent of pacing-induced dyssynchrony, progressive worsening of the LV, and symptomatic HF appear to be influenced by pre-existing LV compromise and the intrinsic or paced ventricular QRS duration.24
The subsequent question as to whether adverse effects of RV apical pacing on LV remodelling can be prevented by the more complex BIV pacing configuration remains to be answered. A small single-centre randomized study in patients with AV block and severe systolic HF 25 demonstrated improved outcomes with BIV pacing compared with RV apical pacing in this population. The recently published PACE trial compared BIV and RV pacing in a mixed pacemaker population.26 The PACE study showed preservation of LV function and favourable systolic LV remodelling with BIV, but it has been criticized for the inclusion of many patients with sinus node dysfunction.27 Another study showed improved walking capacity with BIV pacing vs. RV pacing after AV nodal ablation for AF and refractory fast conduction.28 However, it is unclear as to whether the favourable results of these previous trials in specific subpopulations hold true for the average pacemaker population with AV block.
Study population
In contrast to previous trials on VP and HF, the PREVENT-HF study cohort was pre-specified to have impaired AV conduction and a high expected need for VP. Furthermore, the population of the PREVENT-HF study corresponded well to populations from previous pacemaker studies in terms of the pre-ponderantly normal LVEF, the mean age of ~70 years, the presence of coronary artery disease in about one-third, and the prevalence of arterial hypertension in nearly two-thirds of patients.20
Changes in left ventricular volumes and left ventricular ejection fraction
Neither the analysis according to treatment assignment nor the on-treatment analysis showed significant differences in the primary endpoint (LVEDV) between the groups. This finding was in accordance with the results of the recently published PACE trial. The PACE authors also reported a non-significant LVEDV difference of 5.2 mL between RV and BIV pacing after 12 months in a patient cohort with preserved systolic LV function and a pacemaker indication due to sinus node dysfunction or AV block.26 The non-significant ANCOVA treatment effect of the present study was ~ −4 mL, which is far below the 18 mL difference estimated in the sample size calculation. This indicates that changes in LVEDV are not expected to be substantial after 1 year of BIV or RV pacing for AV block in a population with preserved LV function. In contrast, a previous study 25 demonstrated an LVEDV difference of in patients with reduced LVEF and AV block; moreover, a treatment effect of was reported after cardiovascular resynchronization therapy (CRT) in patients with intraventricular conduction delay and severely compromised LVEF.29 Even patients with less depressed baseline LVEF and intraventricular conduction delays demonstrated favourable LV remodelling with CRT.30
Furthermore, the PREVENT-HF study could not detect a significant difference in LVESV changes between RV and BIV pacing groups after 12 months. The LVESV can be considered a more sensitive remodelling endpoint in the context of device therapy. This negative result contradicts the findings of the PACE trial,26 where LVESV constituted the primary endpoint and was significantly diminished with BIV pacing. Notably, the non-significant numerical effect on LVSEV detected in the PREVENT-HF study (on-treatment ANCOVA difference −6.45 mL) was similar in magnitude to the BIV treatment effect on LVESV reported by the PACE study (−8.1 mL). However, in contrast to the PACE study, this effect was far from statistically significant in the PREVENT-HF study.
Likewise, the ANCOVA analysis revealed no significant difference in the LVEF between the treatment groups after 1 year. In this study, we detected a non-significant ANCOVA effect that was less than three absolute percentage points; in contrast, the PACE study published a considerably larger and statistically significant absolute LVEF difference of 7.4%.26
To date, two small studies have been published on LV volume evolution during RV vs. BIV pacing in patients without advanced systolic HF. Apart from the PACE study, which compared RV apical and BIV pacing, a small single-centre study examined RV outflow tract pacing vs. BIV pacing for advanced AV block.31 That study found a preserved LVEF with BIV pacing, but RV pacing produced a significant systolic 7 mL enlargement and a modest, but significant 2.5% LVEF deterioration after 12 months. Hence, despite the electromechanical disadvantages of RV pacing, BIV pacing provided only limited, if any, benefit in terms of LV volumes >12 months in the absence of systolic HF.
Based on an expert consensus, the current European guidelines on device therapy in HF 32 have already classified BIV pacing as a class II indication in AV block and severe HF. Despite the lack of conclusive data from large multicentre trials, the use of BIV pacing systems has increased for patients with AV block and advanced HF. This may have contributed to the rising number of BIV implants over the past few years.33 The present results do not justify the routine use of BIV pacing for treating bradycardia in non-HF patients.
Combined clinical endpoint and heart failure events
The combined secondary endpoint included the new development or worsening of HF, cardiovascular death, and hospitalizations for cardiovascular causes. Survival free of secondary endpoint events did not significantly differ between the treatment groups. A weak trend was observed for the HF component of the combined endpoint; HF tended to occur more frequently with RV than with BIV pacing. Interestingly, the secondary hospitalizations due to LV lead issues showed a trend in the opposite direction. Thus, the combination reflected a possible net clinical benefit for the study patients, but the importance of the single components may have been be less perceptible.
In a recent pacemaker registry,34 >25% of patients were reported to develop clinical HF over a median follow-up of 7.8 years. Hence, it is possible that a treatment benefit with regard to clinical HF may develop over the long term; in contrast, LV lead problems are likely to aggregate within the first post-operative year. Many patients with pacemakers who have a normal baseline LVEF are likely to experience decades of VP; thus, the 12-month follow-up of the PREVENT-HF study must be considered short term. Although the observed incidence of HF events was not significantly different between treatment groups in this study, the long-term evaluation may show a clinical benefit of BIV pacing in larger populations with AV block; these long-term studies are currently under way.35,36
Adverse events
Adverse events occurred slightly more frequently on BIV compared with RV pacing. As expected, LV lead issues contributed to the disadvantage of BIV treatment. Unexpectedly, events most likely unrelated to pacemaker therapy (malignancy, renal failure during late follow-up, and peripheral artery disease) seemed to affect more patients in the BIV arm, and atrial lead problems occurred more often in the RV arm. After a BIV system has been successfully implanted, the treatment success may not be limited by adverse events related to the pacing system. However, there was a high cross-over rate in this study due to failed LV lead placements during a single intervention. This reflects the considerable complexity of this treatment option. However, current technical advances and increasing operator experience are likely to improve the success rate of lateral coronary venous lead implantation.
Study limitations
The PREVENT-HF study was not able to demonstrate a possible treatment effect on LV volumes. The effect on LV volumes may have been much smaller than anticipated, and the limited sample size may have been inadequate to achieve the required statistical power. In view of this, the PREVENT-HF results cannot be used for the definite disqualification of BIV pacing for AV block. Larger, clinically oriented trials with extended follow-up periods are needed for a conclusive appraisal of the usefulness of this treatment for AV block.
Conclusion
Over 12 months, or the short term in the context of cardiac pacing, no significant differences in LV volume changes or LV function were observed between BIV and RV apical pacing in routine patients with AV block who had pre-dominantly preserved LVEF at the time of pacemaker implantation. In patients with normal LV function, prophylactic BIV pacing for AV block is likely to show small, if any, differential treatment effects on LV remodelling >12 months compared with previously described effects of BIV antibradycardia pacing in patients with severe systolic HF25 or a typical CRT cohort.29 Future trials on LV volume effects in a standard pacemaker population with normal LV function should enrol a considerably larger population.
At present, the BIV pacing method, which is quite complex and resource consuming, cannot be recommended as a routine option to treat AV block in patients with preserved LV function. A conclusive appraisal of the usefulness of BIV pacing for AV block in these patients awaits the results of larger trials with clinical primary outcomes.35,36
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The study was funded by the pacemaker manufacturing company, Medtronic.
Conflict of interest: M.S. receives research support and honoraria for educational activities from the pacemaker manufacturers Biotronik, Boston Scientific, Medtronic, and Sorin Group. X.N. is an employee of Medtronic. U.K.H.W. has received study grants, is a member of the speaker's bureau and is currently conducting research for Medtronic. None of the other authors report any conflicts.
APPENDIX
Steering Committee and statistical advisor
E. de Teresa, Malaga, Spain (international principle investigator); J. Alzueta, Malaga, Spain; J.J. Gomez-Doblas, Malaga, Spain (Echo Core Lab); I. Fernández-Lozano, Madrid, Spain; X. Navarro, Madrid, Spain (Medtronic Iberica); F. Navarro-López, Barcelona, Spain; G. Lamas, Miami (Fl), USA; M. Stockburger, Berlin, Germany (principle investigator for the German study group); and E. Cobo, Barcelona, Spain (statistical advisor).
Participating centres (number of enrolled patients)
M. Stockburger, Charité, Campus Virchow-Klinikum, Berlin, Germany (30); U. Wiegand, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Germany (17); J. Fernández de la Concha, Hospital Infanta Cristina, Badajoz, Spain (12); M. Desaga, Klinikum Dachau, Germany (7); I. Fernandez Lozano, Hospital Puerta de Hierro, Madrid, Spain (6); H. Nägele, St. Adolf-Stift, Reinbek, Germany (5); A. Barrera, Hospital. Clínico U. Virgen de la Victoria, Málaga, Spain (4); M. Winterhalter, Unfallkrankenhaus Berlin, Germany (4); T. Lawo, Berufsgenossenschaftliche Kliniken Bergmannsheil, Bochum, Germany (3); S. Spencker, Charité, Campus Benjamin Franklin, Berlin, Germany (3); J.G. Martinez, Hospital General, Alicante, Spain (2); L. Mont, Hospital Clinic i Provincial, Barcelona, Spain (1); M.V. Pitzalis, Azienda Ospedaliera Policlinico, Bari, Italy (1); and A. Curnis, Ospedale Civile, Brescia, Italy (1).




