Exercise training reduces serum capacity to induce endothelial cell death in patients with chronic heart failure
Abstract
Aims
Physical training improves endothelial function and exercise capacity in patients with heart failure (HF). Serum from patients with cardiovascular diseases increases apoptosis of human endothelial cells suggesting the importance of humoral factors in the progression of the disease. We evaluated whether exercise training influences the apoptotic capacity of serum from patients with chronic HF (CHF).
Methods and results
The study included 39 patients with HF (NYHA II) and 10 age-matched healthy controls. Patients were allocated to either a structured programme of exercise training (24 patients) or standard care (15 patients). Human umbilical vein endothelial cells (HUVECs) were incubated with a medium containing 20% serum obtained before and after either a 3-week exercise training programme or standard care. At baseline, serum from patients with CHF induced a higher degree of lactate dehydrogenase (LDH) release and apoptosis in HUVECs compared with healthy controls (43 ± 1.5 vs. 16 ± 1.1%, P< 0.001 and 67 ± 5.4 vs. 23 ± 5.8%, P< 0.001, respectively). Exercise training significantly increased performance in the 6 min walking test (+34.7%) and reduced the ability of serum to induce LDH release and apoptosis of HUVECs. The reduction of apoptosis after exercise training correlated with the improvement in functional capacity. The expression of the apoptosis markers Bax and Caspase-3 was significantly reduced in HUVECs exposed to serum collected after exercise training. Circulating tumour necrosis factor-alpha, matrix metalloproteinase-1 (MMP-1), and tissue inhibitor of metalloproteinase-1 (TIMP-1) levels were significantly reduced by exercise training and the MMP-9/TIMP-1 ratio increased.
Conclusion
A short term in-hospital structured cardiovascular training programme reduces the ability of serum-derived factors to induce endothelial cell death in patients with CHF.
Introduction
The prevalence of chronic heart failure (CHF) is progressively and rapidly increasing due to improved survival of patients with acute cardiac events and the increased prevalence of risk factors in the general population. Patients with CHF have a significant limitation of exercise capacity and several studies have shown that physical training in combination with optimal medical therapy, has positive effects on physical capacity and quality of life in patients with CHF.1
Patients with heart failure (HF) have an impaired endothelial function.2 A large body of evidence supports a positive role of exercise training in improving endothelial function in subjects with cardiovascular risk factors,3–5 established coronary artery disease (CAD)6–8 and HF.3,7,9–12 Several studies have demonstrated that the release of nitric oxide (NO) in response to acetylcholine is reduced in CHF and it is associated with impaired endothelium-dependent dilation.13–15 Patients with CHF also show high circulating levels of pro-inflammatory cytokines16,17 which are involved in disease progression.18 Exercise training induces increased NO bioavailability through increased activity/expression of endothelial Nitric Oxide Synthase (eNOS) and/or diminished NO catalysis.19 Furthermore, regular exercise promotes an antioxidant state and exercise training has a beneficial effect on inflammatory cytokines which are potent inducers of endothelial cell apoptosis.20 Pro-inflammatory cytokines induce the synthesis of matrix-degrading enzymes such as the matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs),19 which play a pivotal role in left ventricular (LV) remodelling and dysfunction.21,22 Exercise training has also been shown to modulate circulating levels of MMPs.23
Cultured human umbilical vein endothelial cells (HUVECs) have a marked sensitivity to the toxic effects of oxidative stress and pro-apoptotic factors24,25 and they are a valuable model in which to study the ex vivo endothelial effects of serum. Previous studies have shown that serum from patients with severe CHF (NYHA IV) or with acute coronary syndromes, reduces eNOS expression and induces apoptosis in cultured HUVECs from healthy donors (HD).26,27 Most cardioactive drugs interfere with the induction of apoptosis in endothelial cells, and may therefore provide a novel therapeutic target in patients with CHF.28
We hypothesized that exercise training would reduce vascular inflammation, and as a consequence, the ability of serum to induce apoptosis in patients with CHF. To this end, we studied the effect of an in-hospital exercise training programme on the proinflammatory cytokine content of serum from patients with CHF and on its capacity to trigger apoptosis in cultured endothelial cells ex vivo.
Methods
Study population and blood sampling
The study population structured programme consisted of 39 patients with stable CHF (NYHA class II) due to CAD and 10 age-matched controls with no signs and/or symptoms of either CHF or CAD and with an estimated risk of future events of <10% according to the European Society of Cardiology SCORE charts for low-risk countries.
Patients were selected from a consecutive cohort of patients referred for cardiac rehabilitation at the IRCCS San Raffaele Institute in Rome. Patients were included if they had been diagnosed with stable CHF due to ischaemic heart disease for >1 year, had a left ventricular ejection fraction ≤40%, were in NYHA functional class II, had been clinically stable for the past 3 months and receiving optimal HF treatment with no changes for at least 3 months. Patients were excluded as follows: acute myocardial infarction or unstable angina in the past 3 months, decompensated heart failure, complex ventricular arrhythmias, angina, or any neurological or orthopaedic conditions which would limit the ability to undergo exercise training. Age- and sex-matched healthy volunteers were recruited from outpatients undergoing regular cardiovascular checkups. All patients gave written informed consent to the study, which was approved by the Local Ethics Committee. Patients were allocated in a 3:2 ratio to a structured programme of exercise training (24 patients) or to standard care with no exercise training (15 patients).
At baseline all patients and controls underwent a full cardiovascular examination, blood sampling, and an echocardiogram. A repeat blood sample was obtained at the end of the study. Blood samples were collected in tubes which did not contain anticoagulant. The tubes were kept at room temperature (RT) for 1 h to allow coagulation and then centrifuged at 4°C for 10 min at 1700 g within 1.5 h after collection. Serum was collected into aliquots and stored at −80°C. All patients underwent a 6 min walking test at baseline and at study end. An electrocardiogram exercise test was performed if deemed necessary by the physician.
Assessment of physical rehabilitation and functional capacity
Patients underwent an in-hospital exercise training programme, in accordance with current recommendations.29 Specifically, each exercise session started with calisthenics followed by 30 min of aerobic exercise on a bicycle ergometer with incremental stationary workload. The exercise protocol included two sessions per day for 6 days each week over a 3-week period. Training intensity was graded according to 60–80% of the maximum heart rate attained in the initial incremental exercise test performed using an electrically braked bicycle ergometer. The exercise test started with 1 min of unloaded pedalling and increased by 10 W every minute until exhaustion (defined as the inability to keep the pedalling rate steady at 60 r.p.m.). Heart rate (HR) was tracked using a Polar chest-belt HR monitor (Polar Electro Oy), which simultaneously controlled the computer-driven workload of the bicycle ergometer (Bikerace, Technogym) to maintain the target HR.
Functional capacity was assessed by means of a 6 min walking test (6 MWT). Patients were asked to walk at their own maximal pace along a 100 m hospital corridor. Every minute a standard phrase of encouragement was spoken. Patients were allowed to stop if signs or symptoms of significant distress occurred (dyspnoea, angina), although they were instructed to resume walking again as soon as possible.30
Cell culture
Human umbilical endothelial cells (Lonza, San Diego, CA, USA) were grown in EBM-2 medium supplemented with hEGF, h-FGF-B, VEGF, ascorbic acid, hydrocortisone, R3-IGF-1, eparin, gentamicin, and 2% FBS (EGM-2, Lonza) at 37°C in an atmosphere of 95% air–5% CO2. HUVECs at passage 6 were cultured for 18–24–48 h in the presence of 20% serum from HD (used as control), serum from stable CHF patients on a structured programme of exercise training at admission (HFA-E) and discharge (HFD-E) and serum from stable CHF patients allocated to standard care without exercise training, at admission (HFA) and discharge (HFD).
Quantitative assessment of cell death by lactate dehydrogenase release
Lactate dehydrogenase (LDH), which is released from dying cells, was detected using the LDH cytotoxicity detection kit (Roche Diagnostics GmbH, Penzberg, Germany) according to the manufacturer's instructions. Maximum LDH release was assessed by adding a lysis solution to cells. The results are expressed as the percentage of LDH released in each experimental condition over the maximum LDH release.
Quantitative assessment of apoptosis by ELISA
For quantification of apoptosis, fragmented DNA was measured by sandwich Enzyme-Lynked ImmunoSorbent Assay (ELISA) with antihistone-coated microtiter plates and peroxidase-conjugated anti-DNA antibodies using a Cell Death detection kit (Roche, Japan) according to the manufacturer's instructions. Briefly, after exposure to serum (48 h), cells were washed in phosphate buffer solution (PBS), incubated in lysis buffer for 30 min at RT and then centrifuged at 12 000 r.p.m. for 10 min. The supernatants were transferred onto a streptavidin-coated microplate and incubated with biotin-conjugated anti-histone and peroxidase-conjugated anti-DNA monoclonal antibodies for 2 h at RT. After washing, substrate solution was added to each well for 15 min. Absorbance was measured at 405 nm. The results were expressed as percentage of DNA fragmentation compared with a positive control (included in the assay kit).
Quantitative assessment of apoptosis by flow cytometry
After experimental treatment, cells were collected and fixed in ice-cold 70% ethanol for 30 min at 4°C, washed with PBS and incubated for 1 h at 37°C in the dark with 1 mL of PBS containing propidium iodide (PI) (50 µg/mL) and RNAse (5 µg/mL). DNA staining by PI was measured by acquiring 104 events/sample using a FACSCalibur Flow Cytometer (Becton Dickinson, Milan, Italy). Data were analysed using Cell Quest software. Cells undergoing apoptosis were expressed as the percentage of hypodiploid cells (sub-G1 population) over total cells.
Apoptosis was assessed by flow cytometric analysis of cells after double staining with fluorescein isothiocyanate (FITC)-conjugated Annexin V and PI using the human Annexin V-FITC Kit (Bender MedSystems, Vienna, Austria) following the manufacturer's instructions. Double negative healthy cells accumulate in the lower left quadrant. Early apoptotic cells exposing phosphatidyl serine residues on the extracellular portion of cell membrane but maintaining plasma membrane integrity result Annexin V+PI− and are displayed in the lower right quadrant. Late apoptotic and necrotic cells, due to increased plasma membrane permeability, result Annexin V+PI+ and are shown in the upper right quadrant.
Western blot analysis
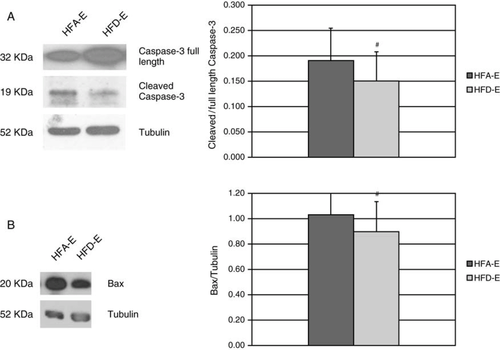
Cells were incubated for 24 h with 20% serum from HFA-E and HFD-E. Total cell extracts were obtained by lysing the cells in RIPA lysis buffer (50 mM Tris-HCl pH 7.6, 100 mM NaCl, 2 mM EDTA, 1 mM MgCl2, 1 mM CaCl2, 1% Triton X-100, 10% glycerol, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM sodium ortovanadate, 5 mM sodium pyrophosphate, and protease inhibitors). Protein concentration was determined by the Bicinchoninic Acid method, using known concentrations of Bovine Serum Albumin as a standard curve. Thirty microgram of proteins from each lysate were separated on a 10% SDS/PAGE gel and transferred to nitrocellulose membranes. Western blotting was performed by using anti-human Bax (Cell Signaling 1:1000), Caspase-3 (Upstate 1 µg/ml), Cleaved Caspase-3 (Asp175) (Cell Signaling 1:1000), and Tubulin (Santa Cruz Biotechnology 1:1000) as primary antibodies. The specific signal was detected with enhanced chemiluminescence (ECL, Pierce) and quantified by densitometry. Results were normalized for Tubulin.
Serum measurement of matrix metalloproteinases, tissue inhibitor of matrix metalloproteinases and inflammatory cytokines
Serum concentrations of cytokines, MMPs, and tissue inhibitor of MMPs (TIMP-1) in HF patients were measured by ELISA using the following commercially available kits: MMP-1, MMP-9 (Calbiochem), TIMP-1 (Chemicon), tumour necrosis factor-alpha (TNF-α, R&D Systems), and following the manufacturer's instructions.
Statistical analysis
Data are reported as the mean ± SD. Within-group changes in the reported variables after the exercise training programme were evaluated by paired t-test or Wilcoxon signed rank test for non-normally distributed variables. The differences were considered statistically significant at P ≤ 0.05. Relations between variables were assessed by Pearson product-moment correlation or Spearman's rank test for non-normally distributed data. All analyses were performed using a commercially available statistical package (SPSS for Windows 12.0, Chicago, IL, USA).
Results
Baseline clinical features of the study patients and the controls are shown in Table 1. All CHF patients were on optimal medical therapy and no significant change in medication was performed during the study period. All patients allocated to exercise training completed the 3-week in-hospital rehabilitation programme. In these patients, functional capacity measured by 6 MWT increased from 319 ± 16 to 489 ± 21 m (P< 0.001, Figure 1). No change in exercise capacity was observed in patients allocated to standard care. Resting HR decreased by 9% (from 66.2 ± 9 to 60.3 ± 10 bpm, P= 0.034), and a non-significant decrease in systolic (7%, from 123.5 ± 28 to 115 ± 0.4, P= 0.09) and diastolic BP (4%, from 84.1 ± 15 to 80.6 ± 13, P= 0.13) was found for patients in the exercise training group compared with the usual care group.
| Exercise training group (n=24) | Standard care group (n=15) | Healthy controls (n=10) | |
|---|---|---|---|
| Age (years) | 66 ± 10 | 67 ± 12 | 66 ± 8 |
| Male/female | 19/5 | 12/3 | 7/3 |
| Hypertension | 17 (71%) | 9 (60%) | 2 |
| Diabetes | 11 (46%) | 4 (26%) | 1 |
| Dyslipidaemia | 14 (58%) | 8 (53%) | 4 |
| Atrial fibrillation | 6 (25%) | 3 (20%) | 0 |
| LV ejection fraction (%) | 35.2 ± 8 | 34.6 ± 7 | 62 ± 4 |
| LV end-diastolic diameter (mm) | 62.4 ± 30 | 61.1 ± 25 | 47 ± 29 |
| Therapy | |||
| ACE inhibitors/ARBS | 22 | 13 | 2 |
| Beta-blockers | 21 | 12 | 1 |
| Diuretics | 15 | 9 | – |
| Aldosterone antagonists | 11 | 7 | – |
| Digoxin | 6 | 4 | – |
| Antiplatelet agents | 22 | 15 | – |
| Anticoagulants | 7 | 3 | – |
| Statins | 16 | 11 | 3 |
- a Data are expressed as mean ± SD. No significant differences were found between the exercise training and standard care groups.
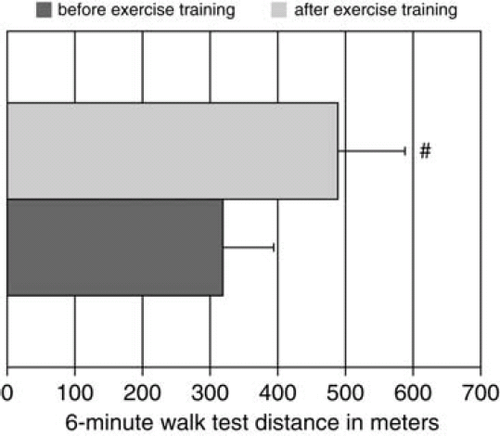
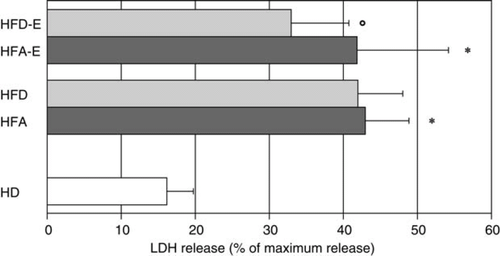
Intrinsic capacity of serum from chronic heart failure patients to induce lactate dehydrogenase release
In order to assess the influence of exercise training on endothelial cells we exposed HUVECs to serum from HD, serum from CHF patients undergoing exercise training at admission (HFA-E) and at discharge (HFD-E) and serum from stable CHF patients allocated to standard care at admission (HFA) and at discharge (HFD). Serum from CHF patients at admission showed an increased capacity to induce LDH release from the endothelial cells compared with serum obtained from HD (HFA 43 ± 1.5% and HFA-E 42 ± 2.2% vs. HD 16 ± 1.1%; P< 0.001 for both HFA and HFA-E vs. HD, Figure 2). Serum-induced endothelial cell death did not change significantly in CHF patients allocated to standard care; however, it was significantly reduced in CHF subjects allocated to exercise training (HFA-E vs. HFD-E, 42 ± 2.2 vs. 33 ± 1.4%; P< 0.01).
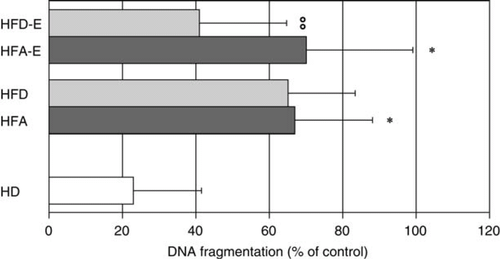
Pro-apoptotic capacity of serum from chronic heart failure patients
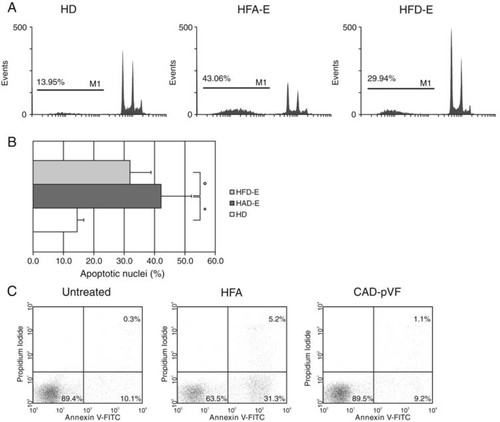
Given the ability of serum from CHF patients to induce endothelial cell death, as indicated by LDH release, we studied the contribution of apoptosis to this process. Programmed cell death of HUVECs exposed to serum in vitro was studied by assessing DNA fragmentation either by ELISA (‘Cell Death Assay') or by intracellular staining with PI and by flow cytometric analysis of cells stained with Annexin V and PI. Serum from CHF patients showed an enhanced pro-apoptotic capacity compared with serum from HD as assessed by Cell Death Assay (HFA 67 ± 5.4 and HFA-E 70 ± 5.6 vs. HD 23 ± 5.8%; P< 0.001 for both HFA and HFA-E vs. HD, Figure 3). In addition, the capacity of serum to trigger endothelial cell apoptosis in vitro was significantly reduced after physical activity (HFA-E 70 ± 5.6 vs. HFD-E 41 ± 4.5%, P< 0.001), while it remained unchanged in the untrained subjects (HFD). The induction of apoptosis by HFA serum was further confirmed by intracellular staining with PI (HFA-E 42.14 ± 1.9 vs. HD 14.2 ± 0.7% P< 0.001), as well as the positive effect of physical training (HFA-E 42.14 ± 1.9 vs. HFD-E 31.9 ± 1.4%, P< 0.001) (Figure 4, Panels A and B). To further confirm that HUVECs exposed to HFA underwent apoptosis, staining with Annexin V and PI was performed (Figure C). Increased Annexin V single positive and Annexin V+PI+ were observed after treatment with HFA serum but not with serum obtained from patients with CAD and similar co-morbidities but with preserved ventricular function (CAD-pVF) (Figure C).
The serum-induced activation of the apoptotic pathway in HUVECs was also investigated by measuring Caspase-3 activation and Bax expression. Exercise training down-regulated protein expression of the cleaved (active) form of Caspase-3 in HUVECs (Figure A). Similarly, the expression of the proapoptotic Bax protein was significantly reduced in HUVECs treated with HFD-E (Figure B).
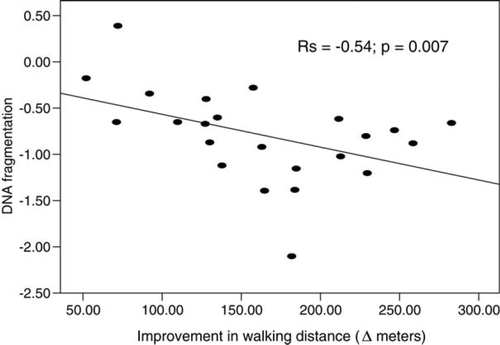
The reduction of the pro-apoptotic potential of CHF serum due to exercise training correlated with the improvement in distance walked at 6 MWT (Figure 6).
Serum content of pro-inflammatory factors
In order to assess the efficacy of our exercise training programme in reducing the systemic inflammatory state of CHF patients, we measured serum concentrations of important mediators of LV remodelling leading to HF, such as TNF-α, MMP-1, MMP-9, and the MMP1/9 inhibitor TIMP-1. Training reduced concentrations of circulating TNF-α, MMP-1, and TIMP-1 while increasing MMP-9/TIMP-1 ratio (Table 2).
| HFA-E | HFD-E | |
|---|---|---|
| TNF-α (pg/mL) | 3.7 ± 1.67 | 2.3 ± 1.65# |
| MMP-1 (ng/mL) | 12.46 ± 6.9 | 8.36 ± 4.6# |
| MMP-9 (ng/mL) | 302.5 ± 52.8 | 321.7 ± 81.6 ns |
| TIMP-1 (ng/mL) | 306.2 ± 140.2 | 202.2 ± 79.8* |
| MMP-9/TIMP-1 | 1.05 ± 0.6 | 2.01 ± 0.7* |
- a TNF-α, tumour necrosis factor-alpha; MMP-1, matrix metalloproteinase-1; MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase-1. Data are expressed as mean ± SD. #P< 0.05, *P< 0.01.
Discussion
The present study shows for the first time that a structured exercise rehabilitation programme reduces the ability of serum to induce endothelial cell apoptosis in patients with CHF and that this effect correlates with the degree of exercise improvement. The study also confirms the effect of exercise training in reducing circulating concentrations of TNF-α and MMP1.
Regular physical activity is a key intervention in the prevention and treatment of cardiovascular diseases. Several clinical studies have demonstrated a significant reduction of morbidity and mortality among individuals with an active lifestyle as compared with sedentary controls, independently of the presence of HF.31 These effects were not confirmed by the ‘HF-ACTION' study investigators, most probably because of poor compliance. In the present study, we used an in-hospital rehabilitation programme in order to achieve a homogeneous intervention in our patients with CHF of ischaemic origin. The decision to study patients in NYHA class II instead of patients with a more severe degree of CHF, was taken in order to have a group of patients undergoing exercise training of similar intensity and duration.
Exercise training has been shown to augment endothelial NO-dependent vasodilatation in both large and small vessels32,33 in several animal and human studies. Our study shows for the first time that cardiovascular rehabilitation reduces the ability of serum-contained factors from patients with CHF to induce endothelial cell death ex vivo, suggesting a possible mechanism besides the effect on NO production for the improvement in endothelial function with exercise.
Cell death may occur via two different mechanisms: apoptosis (programmed cell death) and necrosis. The consequences of necrotic and apoptotic cell death in vivo and in vitro are different. In necrosis in vivo, the cytoplasmic membrane lyses and intracellular content is released, resulting in inflammation,34 whereas in apoptosis the cellular content is safely sealed within the dying cells until phagocytosis occurs. However, in cultured cells the phagocytic step after apoptosis does not occur and apoptotic cells undergo secondary necrosis, releasing their contents into the surrounding medium.35 Thus, although it is generally assumed that LDH efflux in vivo represents necrotic cell death, measurement of LDH in vitro, represents overall cellular death, rather than necrotic death only. Biochemical hallmarks of apoptosis such as the activation of specific proteases (caspases) and oligonucleosomal DNA fragmentation do not occur in necrotic cells.36 The effect of serum taken from CHF patients before and after the exercise training programme on cultured HUVECs was tested by analysing both apoptosis and necrosis, using LDH release for necrosis detection, and DNA fragmentation, Annexin V staining and pro-apoptotic protein expression for apoptosis detection. Our results clearly show that serum from patients with CHF induces a higher degree of endothelial cell necrosis and apoptosis ex vivo, compared with serum from healthy controls.
Chronic HF patients have a high prevalence of comorbidities such as hypertension, diabetes, and dyslipidaemia, which can affect the overall pro-inflammatory capacity of serum, both in the presence of normal and abnormal ventricular function. However, we did not detect enhanced induction of apoptosis in HUVECs exposed to serum from patients with stable CAD and similar comorbidities but preserved ventricular function, suggesting that the enhanced capacity of HFA serum to induce necrosis and apoptosis of HUVECs is possibly related to the presence of congestive HF.
We found a decreased capacity of HFD-E compared with HFA-E for triggering apoptosis in HUVECs, as shown by the expression of two key pro-apoptotic proteins (cleaved Caspase-3 and Bax), suggesting that cardiovascular rehabilitation may reduce serum levels of toxic and/or increase concentration of protective factors.
TNF-α is known to be a major mediator in the pathophysiology of CHF37 and in the induction of cell death. We found a significant reduction of serum TNF-α concentration with exercise training suggesting a possible effect of this cytokine on programmed cell death.
The MMPs are a family of endopeptidases capable of degrading all components of the extracellular matrix. Matrix metalloproteinases and TIMPs play a pivotal role in LV remodelling and dysfunction21,22 and are involved in flow mediated remodelling in large vessels38 and neointima formation. The reduction of serum TIMP-1 observed after the training programme might enhance MMP-9 activity which in turn mediates extracellular matrix degradation, fosters angiogenesis, promotes tissue revascularization,39 and collateral vessel development.40 The MMP9/TIMP-1 ratio in patients with CAD is predictive of all-cause and cardiac mortality.41 We observed a significant reduction of MMP-1 and TIMP-1 with a significant increase in the MMP-9/TIMP-1 ratio, supporting the beneficial effect of exercise training on the inflammatory regenerative milieu.
Finally, we found a negative correlation between the reduction in DNA fragmentation of HUVECs by HFA serum and changes in the distance walked at 6 MWT, suggesting that the observed effects on cell apoptosis and necrosis may have functional clinical correlates.
A limitation of the study was that the prescription of exercise training intensity was subjective and not based on a cardiopulmonary exercise test. Also the assessment of improvement in exercise capacity was evaluated only by 6 MWT. However, as the study was performed in a single centre and the 6 MWT administered by the same therapists, who were blinded to clinical data, it is extremely unlikely that this could have affected the results.
In conclusion our findings indicate that an exercise training programme with controlled compliance improves endothelial health and enhance functional capacity by reducing circulating factors which may trigger major cardiovascular events in patients with CHF of ischaemic origin.
Funding
This work was supported by the Italian Ministry of Health, Ricerca Corrente 2005–2007.
Conflict of interest: none declared.




