Prognostic importance of natriuretic peptides and atrial fibrillation in patients receiving cardiac resynchronization therapy
Abstract
Aims
The aim of this study was to investigate the prognostic value of natriuretic peptides and atrial fibrillation (AF) on response to cardiac resynchronization therapy (CRT) and mortality.
Methods and results
This study included 338 consecutive CRT patients. Response to CRT was defined as a reduction in left ventricular end-systolic volume of ≥15% in the absence of death at 6-month follow-up. During follow-up (27 ± 19 months), 139 patients (41%) had AF, being new onset in 40 patients (21%). Forty-two patients (12%) had permanent AF. Response to CRT was observed in 168 of 302 patients (56%): 60 of 123 patients (43%) with AF vs. 108 of 179 patients (60%) without AF (P = 0.047). Low baseline atrial natriuretic peptide (ANP) [odds ratio for log2 ANP 0.49, 95% confidence interval (CI) 0.35–0.68, P < 0.001] and large left ventricular end-systolic volume (odds ratio for every 50 mL 1.40, 95% CI 1.09–1.79, P = 0.009) were independent predictors of response. Neither the presence of AF nor the increase in AF burden independently predicted response. Ninety patients (27%) died; 50 patients (36%) with AF vs. 40 patients (20%) without AF (log rank P = 0.029). Important predictors of all-cause mortality were new-onset AF (hazard ratio 8.11, 95% CI 3.31–19.85, P < 0.001), permanent AF (hazard ratio 3.19, 95% CI 1.61–6.30, P = 0.001), and baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) (hazard ratio for log2 NT-proBNP 0.77, 95% CI 0.66–0.90, P = 0.001).
Conclusion
In patients treated with CRT, lower ANP and larger left ventricular end-systolic volume were independent predictors of response. New-onset AF, permanent AF, and NT-proBNP were independently associated with increased all-cause mortality.
Introduction
Atrial fibrillation (AF) is often present in patients with heart failure, and its incidence increases with the severity of heart failure.1 Cardiac resynchronization therapy (CRT) is an accepted non-pharmacological therapy for patients with severe heart failure.2–4 Many patients receiving CRT therefore have or develop AF.5 Atrial fibrillation may interfere with CRT, because it can decrease effective biventricular pacing due to a too fast intrinsic ventricular response.6 It is still uncertain whether AF patients benefit from CRT to the same extent as patients with sinus rhythm.7–12 Natriuretic peptides such as atrial natriuretic peptide (ANP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are increased in heart failure and are strong predictors of mortality.13,14 Plasma levels of natriuretic peptides are correlated with the extent of left ventricular dysfunction and may be affected by AF.15 The influence of ANP and NT-proBNP in addition to AF on response to CRT has not been studied before. Therefore, it was our aim to investigate the prognostic values of ANP and NT-proBNP in addition to AF on response to CRT and on mortality.
Methods
Patient population and study protocol
Consecutive patients who received a CRT device in the University Medical Center Groningen from January 2001 to July 2009 were included in this single-centre prospective observational study. Eligibility criteria for CRT were based on the standard guidelines and included New York Heart Association functional class III or IV despite optimal pharmacological treatment, left ventricular ejection fraction ≤35%, left ventricular end-diastolic diameter ≥55 mm, and QRS duration ≥130 ms. Significant dyssynchrony was not a pre-requisite for CRT implantation, nor was the presence of sinus rhythm. Our CRT protocol has been described before.11,16 Coronary angiography was performed prior to implantation. At baseline and 6-month follow-up patient history, physical examination, treadmill cardiopulmonary exercise testing, 12-lead electrocardiogram, transthoracic echocardiography, and radionuclide ejection fraction were examined and blood samples were collected. Baseline data were collected at hospital admission prior to CRT implantation. Atrial natriuretic peptide and NT-proBNP were analysed with enzyme-linked immunosorbent assays using commercially available kits (R&D systems, Minneapolis, MN, USA). Patients gave written informed consent for the biomarker analyses. All patients were seen at the outpatient department and for CRT interrogation at baseline and 6-monthly thereafter. At each CRT interrogation, data were stored both on computer disc and in a computerized medical record database of the University Medical Center Groningen. To increase response rates, patients underwent atrioventricular delay optimization at 2 weeks post-implantation. Effective biventricular pacing was assessed by performing treadmill cardiopulmonary exercise tests in addition to monitoring device counters. Imperfect biventricular stimulation during exercise testing was defined as heart rates exceeding the upper rate of the device at moderate exercise levels, defined as 25% of the maximal achieved exercise duration.16 Atrial fibrillation was carefully monitored during follow-up and was treated aggressively with rhythm control including institution of amiodarone and electrical cardioversion if required. In case of unsuccessful rhythm control, AF was accepted and rate control therapy was instituted aiming at effective biventricular pacing. Atrioventricular node ablation for permanent AF was performed only if pharmacological rate control therapy failed.
Echocardiographic evaluation
Transthoracic echocardiography was conducted using a commercially available echocardiographic system (VIVID 7, General Electric Vingmed Ultrasound, Milwaukee, WI, USA). Images were obtained from the parasternal (long- and short-axis) and apical (two- and four-chamber) views and were digitally stored for offline analysis (Echopac 6.1, General Electric Vingmed Ultrasound). Left ventricular end-diastolic and end-systolic volumes were measured with the modified biplane Simpson method using the apical two- and four-chamber views. Ventricular dyssynchrony was assessed by inter-ventricular mechanical delay (IVMD), i.e. right ventricular pre-ejection time subtracted from left ventricular pre-ejection time, and septal-to-lateral delay using tissue Doppler imaging. An IVMD >40 ms was considered indicative of inter-ventricular dyssynchrony, a septal-to-lateral delay >60 ms of intraventricular dyssynchrony. PA-TDI interval, the time between initiation of the electrocardiographic P-wave in lead II to the A′ wave on the lateral left atrial tissue Doppler tracing, was analysed in sinus rhythm.17
Definitions of atrial fibrillation and response
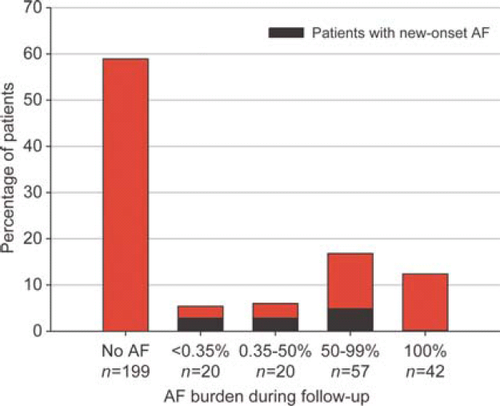
Atrial fibrillation was defined as any AF episode lasting at least 30 s as verified by electrocardiogram, Holter recording, or device interrogation. History of AF was defined as a history of documented AF before implantation. Atrial fibrillation during follow-up was defined as any documented AF episode occurring during follow-up. New-onset AF was defined as AF during follow-up occurring in patients who did not have a history of AF. Paroxysmal AF was defined as AF terminating spontaneously, persistent AF as AF lasting >7 days or requiring termination by cardioversion, and permanent AF as longstanding AF in which cardioversion has failed or has been foregone, i.e. if AF was accepted.18 Atrial fibrillation burden was determined by the device and was defined as the proportion of time in AF. Average AF burden during overall follow-up was categorized into four groups: <0.35% AF burden, i.e. minor paroxysmal AF; 0.35–50% AF burden, i.e. moderate paroxysmal AF; 50–99% AF burden, i.e. long-lasting AF; and, additionally, 100% AF burden, i.e. permanent AF during total follow-up (sinus rhythm was never observed).19 This categorization of AF burden was pre-defined.
Response to CRT was the primary endpoint of the study. Response was defined as a reduction in left ventricular end-systolic volume of 15% or more in the absence of death at 6 months in accordance with current literature20,21 and was determined by an independent examiner who was blinded to the clinical response of the patient. All-cause mortality during total follow-up was the secondary endpoint of the study and was carefully documented during follow-up. The duration of follow-up was computed from the time of CRT implantation until all-cause mortality or until the date when the last follow-up data were obtained, as appropriate.
Statistical analysis
Baseline descriptive statistics are presented as mean ± standard deviation or median (inter-quartile range) for continuous variables and numbers with percentages for categorical variables, as required. We evaluated differences between groups using χ2 test and Fisher's exact test for categorical data, and Student's t-test and Mann–Whitney U test for continuous data, dependent on whether data were normally distributed. To compare data within patient groups, paired Student's t-test was used for normally distributed data and Wilcoxon signed rank test for not normally distributed data. Box plots were used to represent biomarker levels: boxes symbolize medians and inter-quartile ranges, whiskers correspond to 5th/95th percentiles. Spearman's correlations were used for correlations between biomarkers and clinical variables. Cumulative event proportions were calculated using Kaplan–Meier analysis. The log-rank test was used to compare groups. Logistic regression analysis was conducted to evaluate predictors of response. Cox proportional hazards regression analysis was conducted to evaluate predictors of all-cause mortality. All baseline variables were included in the regression analyses including echocardiographic parameters and biomarkers. Multivariate analyses were performed using all variables with P < 0.1 in univariate analysis. A stepwise approach was used. The final multivariate models included all variables with P < 0.05. Interactions were investigated. The predictive value of the natriuretic peptides regarding response and all-cause mortality was additionally evaluated using the c-statistic, a generalization of the area under the receiver operator characteristic curve. In all statistical analyses, P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 338 CRT patients were included. Mean age was 65 ± 11 years and 145 patients (43%) had a previous history of AF (Table 1).
| AF during follow-up (n = 139) | Sinus rhythm during follow-up (n = 199) | Total population (n = 338) | P-value | |
|---|---|---|---|---|
| Age, years | 65 ± 11 | 65 ± 11 | 65 ± 11 | 0.88 |
| Male sex, n (%) | 109 (78) | 141 (71) | 250 (74) | 0.12 |
| History of AF, n (%) | 99 (71) | 46 (23) | 145 (43) | <0.001 |
| Type of AF, n (%) | ||||
| Paroxysmal AF | 13 (9) | 28 (14) | 41 (12) | 0.19 |
| Persistent AF | 45 (32) | 17 (9) | 62 (18) | <0.001 |
| Permanent AF | 41 (30) | 1 (1) | 42 (12) | <0.001 |
| Total AF duration, months | 48 (13–114) | 39 (11–120) | 48 (13–115) | 0.81 |
| AF at implantation, n (%) | 66 (48) | 8 (4) | 74 (22) | <0.001 |
| Current AF duration, months | 13 (5–52) | 9 (1–10) | 12 (4–39) | 0.02 |
| Non-ischaemic cardiomyopathy, n (%) | 71 (51) | 98 (49) | 169 (50) | 0.83 |
| Ischaemic cardiomyopathy, n (%) | 68 (49) | 102 (51) | 170 (50) | 0.67 |
| Previous myocardial infarction | 53 (38) | 83 (42) | 136 (40) | 0.51 |
| Previous cardiac surgery, n (%) | 54 (39) | 69 (35) | 123 (36) | 0.43 |
| Hypertension, n (%) | 56 (40) | 85 (43) | 141 (42) | 0.70 |
| Diabetes, n (%) | 32 (23) | 44 (22) | 76 (23) | 0.82 |
| NYHA class for heart failure, n (%) | 0.73 | |||
| II | 15 (11) | 17 (9) | 32 (10) | |
| III | 114 (82) | 165 (83) | 279 (83) | |
| IV | 10 (7) | 17 (9) | 27 (8) | |
| Systolic blood pressure, mmHg | 117 ± 19 | 118 ± 20 | 118 ± 20 | 0.61 |
| Diastolic blood pressure, mmHg | 72 ± 11 | 71 ± 11 | 71 ± 11 | 0.38 |
| Body mass index, kg/m2 | 27 ± 4 | 27 ± 5 | 27 ± 5 | 0.63 |
| Peak VO2, mL/min/kg | 15.0 ± 9.5 | 15.2 ± 4.5 | 15.1 ± 17.1 | 0.82 |
| Electrocardiogram | ||||
| AF/AFL, n (%) | 63 (45) | 8 (4) | 71 (21) | <0.001 |
| Heart rate, b.p.m. | 76 ± 14 | 74 ± 15 | 75 ± 15 | 0.45 |
| QRS duration, ms | 163 ± 31 | 164 ± 28 | 164 ± 30 | 0.76 |
| Medication, n (%) | ||||
| Beta-blocker | 103 (74) | 166 (83) | 269 (80) | 0.04 |
| ACEi/ARB | 130 (94) | 180 (91) | 310 (92) | 0.31 |
| Diuretic | 128 (92) | 182 (92) | 310 (92) | 0.84 |
| Digoxin | 36 (26) | 25 (13) | 61 (18) | 0.002 |
| Amiodarone | 32 (23) | 40 (20) | 72 (21) | 0.52 |
| Statin | 80 (58) | 106 (53) | 186 (55) | 0.44 |
| Nitrate | 20 (14) | 38 (19) | 58 (17) | 0.26 |
| Oral anticoagulation | 125 (90) | 143 (72) | 268 (79) | <0.001 |
| Aspirin | 27 (19) | 52 (26) | 79 (23) | 0.15 |
| Echocardiographic parameters | ||||
| LA size, parasternal, mm | 52 ± 9 | 47 ± 7 | 49 ± 8 | <0.001 |
| LA size, length, mm | 72 ± 10 | 64 ± 9 | 67 ± 10 | <0.001 |
| LA size, width, mm | 53 ± 9 | 49 ± 8 | 51 ± 8 | <0.001 |
| LA volume index, mL/m2 | 52 ± 20 | 41 ± 14 | 45 ± 17 | <0.001 |
| RA size, length, mm | 63 ± 10 | 57 ± 9 | 59 ± 10 | <0.001 |
| RA size, width, mm | 49 ± 8 | 45 ± 8 | 47 ± 8 | <0.001 |
| Septum, mm | 10 ± 2 | 9 ± 2 | 9 ± 2 | 0.49 |
| Posterior wall, mm | 9 ± 2 | 9 ± 2 | 9 ± 2 | 0.03a |
| LV end-diastolic volume, mL | 248 ± 102 | 266 ± 93 | 259 ± 97 | 0.14 |
| LV end-systolic volume, mL | 192 ± 85 | 209 ± 78 | 202 ± 81 | 0.09 |
| LV ejection fraction, % | 24 ± 9 | 24 ± 14 | 24 ± 12 | 0.91 |
| Mitral valve regurgitation, n (%) | 43 (31) | 48 (24) | 91 (27) | 0.17 |
| Tricuspid valve regurgitation, n (%) | 12 (9) | 15 (8) | 27 (8) | 1.00 |
| IVMD >40 ms | 39 (28) | 75 (38) | 114 (34) | 0.10 |
| Septal-to-lateral delay >60 ms | 38 (27) | 84 (42) | 122 (36) | 0.054 |
| PA-TDI interval, ms | 159 (126–186) | 139 (120–161) | 140 (120–167) | 0.007 |
| Laboratory values | ||||
| eGFR, mL/min/1.73 m2 | 59 ± 22 | 62 ± 21 | 61 ± 22 | 0.17 |
| ANP, pg/100 μL | 100 (59–165) | 83 (43–120) | 88 (51–144) | 0.03 |
| NT-proBNP, ng/L | 1855 (882–3245) | 1145 (494–2642) | 1399 (649–3014) | 0.004 |
- a Data expressed as mean ± standard deviation or median (inter-quartile range), as required.
- b ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; ANP, atrial natriuretic peptide; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IVMD, inter-ventricular mechanical delay; LA, left atrial; LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association functional class; RA, right atrial; VO2, aerobic capacity.
- a 9.36 ± 1.78 mm for AF during follow-up vs. 8.95 ± 1.57 mm for sinus rhythm during follow-up.
Atrial fibrillation during follow-up
Atrial fibrillation was present in 139 patients (41%) during a mean follow-up of 27 ± 19 months (inter-quartile range 13–37 months) (Table 1). New-onset AF developed in 40 of 193 patients (21%), which was persistent in 20 patients and paroxysmal in 20 patients (of whom 19 patients had an AF burden <0.35%). Electrical cardioversion was conducted in 15 new-onset AF patients, and amiodarone was instituted in 12 patients. At the end of follow-up, 30 of the 40 new-onset AF patients (75%) were in sinus rhythm: 13 of the 20 persistent AF patients (65%) and 17 of the 20 paroxysmal AF patients (85%).
Atrial fibrillation patients more often had heart rates exceeding the upper rate of the device at moderate exercise than sinus rhythm patients (12% of AF patients vs. 4% of sinus rhythm patients, P = 0.02). In these patients, beta-blockers were first increased to allow optimal biventricular pacing. In 10 patients (3%), it was necessary to perform atrioventricular node ablation. Distribution of AF burden during the total follow-up is shown in Figure 1.
Response to cardiac resynchronization therapy
Of the 302 patients in whom left ventricular end-systolic volumes could be assessed, 168 patients (56%) were responders to CRT at 6-month follow-up: 60 of 123 patients (43%) with AF during follow-up vs. 108 of 179 sinus rhythm patients (60%) (P = 0.047). Nine of the 10 patients (90%) who had undergone atrioventricular node ablation were responders. Independent predictors of response to CRT were lower baseline ANP levels and larger left ventricular end-systolic volume (Table 2). The c-statistic for ANP was 0.68. Neither presence of AF nor increasing AF burden was an independent predictor of response.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Permanent AF in history | 0.43 (0.22–0.85) | 0.015 | ||
| Systolic blood pressure/10 mmHg | 1.21 (1.06–1.37) | 0.003 | ||
| Diastolic blood pressure/10 mmHg | 1.24 (1.01–1.52) | 0.038 | ||
| Beta-blocker | 2.57 (1.42–4.67) | 0.002 | ||
| LA size, length/10 mm | 0.77 (0.61–0.99) | 0.038 | ||
| LA volume index/10 mL/m2 | 0.85 (0.72–0.997) | 0.046 | ||
| LV end-diastolic volume/50 mL | 1.22 (1.06–1.40) | 0.005 | ||
| LV end-systolic volume/50 mL | 1.34 (1.13–1.58) | 0.001 | 1.40 (1.09–1.79) | 0.009 |
| Septal-to-lateral delay >60 ms | 1.71 (1.03–2.82) | 0.037 | ||
| Log2 ANP, pg/100 μLa | 0.56 (0.41–0.76) | <0.001 | 0.49 (0.35–0.68) | <0.001 |
| Log2 NT-proBNP, ng/La | 0.96 (0.82–1.12) | 0.96 | ||
- a AF, atrial fibrillation; ANP, atrial natriuretic peptide; CI, confidence interval; LA, left atrial; LV, left ventricular.
- a Biomarkers are log2 transformed, e.g. an odds ratio of 0.49 of log2 ANP implies that a doubling of any ANP value corresponds to an odds ratio of 0.49.
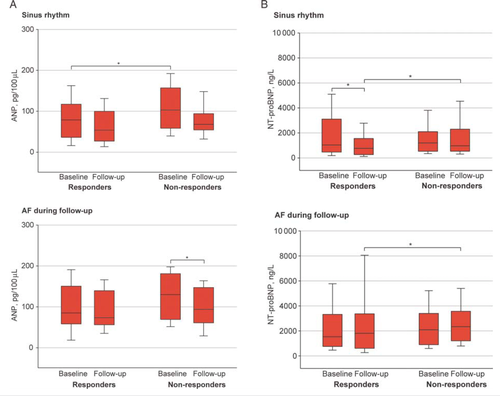
A significant decrease in left atrial volume was observed at 6 months only in responders with sinus rhythm (40 ± 13 mL/m2 at baseline vs. 34 ± 11 mL/m2 at 6 months, P = 0.003) but not in responders with AF (51 ± 18 mL/m2 at baseline vs. 48 ± 15 mL/m2 at 6 months, P = 0.08). Patients with AF during follow-up had higher baseline ANP levels than sinus rhythm patients (Table 1). Non-responders had higher baseline ANP levels than responders only in sinus rhythm patients, and ANP decreased at 6 months only in non-responders with AF during follow-up (Figure 2). Baseline ANP was correlated with increased left atrial volume index (r = 0.402, P < 0.001) and increased left ventricular end-systolic volume (r = 0.219, P = 0.044) only in sinus rhythm patients. In patients with AF during follow-up, baseline ANP was correlated with increasing AF burden (r = 0.409, P = 0.038), but not with left atrial or ventricular volumes. Baseline NT-proBNP levels overall were higher in patients with AF (Table 1). A decrease in NT-proBNP levels was only observed in responders with sinus rhythm (Figure 2). In patients both with and without AF, non-responders had higher follow-up NT-proBNP levels.
Of all patients who were in sinus rhythm during the first 6 months of follow-up, 21 of 82 non-responders (26%) developed AF during further follow-up when compared with 23 of 141 responders (16%) (P = 0.09).
All-cause mortality
During follow-up, 90 patients (27%) died: 50 of 139 patients (36%) with AF vs. 40 of 199 patients (20%) without AF during follow-up (log rank P = 0.029). Two of the 10 patients (20%) who had undergone atrioventricular node ablation died during follow-up. Independent predictors of mortality were new-onset AF during the first 6 months, permanent AF (100% AF burden) during total follow-up, higher baseline NT-proBNP levels, ischaemic cardiomyopathy, and lower systolic blood pressure (Table 3). The c-statistic for NT-proBNP was 0.66.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age/10 years | 1.24 (1.01–1.51) | 0.038 | ||
| Male gender | 1.74 (1.02–3.00) | 0.044 | ||
| AF during follow-up | 1.59 (1.05–2.41) | 0.030 | ||
| New-onset AF | 4.43 (2.12–9.27) | <0.001 | 8.11 (3.31–19.85) | <0.001 |
| Permanent AF (100% AF burden) | 2.34 (1.32–4.16) | 0.004 | 3.19 (1.61–6.30) | 0.001 |
| Non-ischaemic cardiomyopathy | 0.59 (0.38–0.89) | 0.013 | 0.46 (0.26–0.81) | 0.007 |
| Systolic blood pressure/10 mmHg | 0.70 (0.61–0.79) | <0.001 | 0.77 (0.66–0.90) | 0.001 |
| Peak VO2, mL/min/kg | 0.91 (0.85–0.97) | 0.002 | ||
| Beta-blocker | 0.57 (0.36–0.90) | 0.016 | ||
| Oral anticoagulation | 2.13 (1.13–4.00) | 0.019 | ||
| LA size, parasternal/10 mm | 1.40 (1.08–1.08) | 0.011 | ||
| LA size, length/10 mm | 1.54 (1.25–1.89) | <0.001 | ||
| LA volume index/10 mL/m2 | 1.24 (1.11–1.39) | <0.001 | ||
| RA size, length/10 mm | 1.41 (1.15–1.74) | 0.001 | ||
| Septum/10 mm | 0.31 (0.10–0.98) | 0.047 | ||
| eGFR/10 mL/min/1.73 m2 | 0.85 (0.76–0.94) | 0.003 | ||
| Log2 ANP, pg/100 μLa | 1.42 (1.11–1.81) | 0.005 | ||
| Log2 NT-proBNP, ng/La | 1.45 (1.24–1.70) | <0.001 | 1.27 (1.07–1.51) | 0.006 |
- a AF, atrial fibrillation; ANP, atrial natriuretic peptide; CI, confidence interval; LA, left atrial; LV, left ventricular; eGFR, estimated glomerular filtration rate; LA, left atrial; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RA, right atrial.
- a Biomarkers are log2 transformed, e.g. a hazard ratio of 1.27 of log2 NT-proBNP implies that a doubling of any NT-proBNP value corresponds to a hazard ratio of 1.27.
Discussion
We found that lower baseline ANP, but not the presence of AF or increasing AF burden, was an independent predictor of response to CRT. New-onset AF, permanent AF, and high baseline NT-proBNP were associated with increased all-cause mortality.
Prognostic value of atrial natriuretic peptide regarding response to cardiac resynchronization therapy
The response rate to CRT of 56% in our study was similar to response rates seen in other CRT studies using the same definition.21 Low baseline ANP predicted response. Atrial natriuretic peptide is predominantly produced in the atria, but especially in heart failure the ventricles also contribute to ANP secretion. Atrial fibrillation instead of sinus rhythm may further increase ANP levels in heart failure patients.15 Low ANP may reflect a haemodynamic status still sensitive for reverse remodelling and thus for response to CRT. The role of ANP regarding response in CRT patients has barely been studied before. In two small studies, response to CRT was associated with a decrease in ANP.22,23 In one study, low baseline ANP was also a predictor of response.23 It is unknown, however, whether these studies included patients with AF, which is a relevant issue. We observed that baseline ANP was correlated with increasing left atrial volume and left ventricular end-systolic volume in sinus rhythm patients, while in patients with AF, ANP only correlated with increasing AF burden. Unlike the two previous studies, ANP did not decrease in responders, but it decreased in non-responders with AF during follow-up. This ANP decrease in AF patients may be explained by depleting ANP levels during longstanding AF, which is seen after an acute surge of ANP levels at the start of AF.24
Influence of atrial fibrillation on response to cardiac resynchronization therapy
Previous results concerning the influence of AF on response are conflicting. Several studies have demonstrated comparable clinical response to CRT in sinus rhythm and permanent AF patients, in whom 100 and 15% of patients, respectively, had undergone atrioventricular node ablation.7,12 In other studies, only permanent AF patients with atrioventricular node ablation showed similar clinical response to CRT when compared with sinus rhythm patients.25,26 In contrast, more non-response has been observed among AF patients despite the fact that atrioventricular node ablation was present in 57% of patients.9 In our study, AF during the first 6 months was not an independent predictor of response even though a small number of patients underwent atrioventricular node ablation. This may in part be explained by the aggressive treatment of AF in our centre,27–29 as demonstrated by the large proportion of new-onset AF patients who were in sinus rhythm during follow-up. On the other hand, successful CRT may prevent or reduce AF, as we also observed a trend that responders less often developed AF during follow-up.
Atrial fibrillation and mortality in cardiac resynchronization therapy patients
Both new-onset and permanent AF predicted mortality in our study. It is still uncertain whether AF influences mortality in CRT patients. Atrial fibrillation may increase mortality by further deterioration of heart failure and induction of ventricular arrhythmias.30–32 In AF patients without heart failure, mortality is high in new-onset and permanent AF.33 In CRT studies AF during follow-up and permanent AF have been associated with adverse prognosis.9,12,34 Other studies have demonstrated comparable survival between permanent AF and sinus rhythm patients,10,35,36 although survival was better in case of atrioventricular node ablation.10,36 Survival may have increased in our permanent AF patients if atrioventricular node ablation had been performed more often. On the other hand, the acceptance of AF may reflect the patients’ overall condition, making permanent AF a marker of more advanced underlying disease instead of a direct cause of increased mortality.37
Although AF independently predicted mortality, it was not an independent predictor of response. This apparently discrepant finding might be explained by the difference in follow-up duration between response, defined at 6 months, and all-cause mortality, analysed at 27 months. Six months may be too short for AF to influence response to CRT, which is supported by the observation that AF patients did not have a reduction in left atrial volume and ANP levels at 6 months despite the presence of response.
Limitations
This is an observational study, which means that no randomization took place; it was not powered on mortality; and causality could not be determined. The compilation of the multivariate models of response and all-cause mortality may have been different with a larger sample size. Response to CRT may have differed if atrioventricular node ablation had been performed in more AF patients. Some patients may have been diagnosed with new-onset AF due to the monitoring capacities of the device while they could have had unrecognized AF before implantation. Atrial fibrillation during follow-up was defined as AF being present anytime during the total follow-up period, which means that some patients did not have AF during the first 6 months. Natriuretic peptide levels may fluctuate according to the presence of AF. This means that the value of natriuretic peptide levels determined at 6 months and changes in these levels between baseline and 6 months in the context of ‘AF during follow-up’ should be interpreted with caution as there may have been an interaction with the presence or absence of AF. The study population is relatively large and the follow-up duration is substantial, and we deliberately chose all-cause mortality as one of the outcome parameters because it is a hard endpoint. Our study bears an important message, but the non-randomized observational design precludes definite conclusions. Our study generates new hypotheses, namely that ANP may be used in the selection of candidates for CRT and that early detection and treatment of new-onset AF may improve outcome in CRT, which should be investigated in future studies.
Conclusion
In patients treated with CRT, lower ANP and larger left ventricular end-systolic volume, but not the presence of AF or increasing AF burden, were independent predictors of response. However, new-onset AF, permanent AF, and higher baseline NT-proBNP were associated with increased all-cause mortality.
Funding
This study was supported by unrestricted educational grants from Medtronic, Biotronik, and St Jude Medical. V.G. reports receiving grant support from Medtronic, Biotronik, and St Jude Medical.
Conflict of interest: V.V. reports receiving consulting fees from Medtronic and Biotronik, and lecture fees from Medtronic. V.G. reports receiving lecture fees from Medtronic.




