Baseline characteristics and outcomes of patients with heart failure receiving bronchodilators in the CHARM programme
Abstract
Aims
Heart failure (HF) and chronic obstructive pulmonary disease are common partners. Bronchodilators are associated with adverse cardiovascular outcomes in patients with pulmonary disease. The outcome of patients with HF prescribed bronchodilators is poorly defined.
Methods and results
The Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme randomized 7599 patients with symptomatic HF to receive candesartan or placebo. The relative risk conveyed by bronchodilator therapy was examined using a multivariable Cox proportional hazards model. The prevalence of bronchodilator therapy was similar in patients with reduced and preserved systolic function (respectively, 8.7 vs. 9.2%, P = 0.46). Beta-blocker utilization was markedly lower in patients receiving bronchodilators compared with those without (overall 31.9 vs. 57.6%, P < 0.0001). Bronchodilator use was associated with increased all-cause mortality [HR 1.26 (1.09–1.45), P = 0.0015], cardiovascular death [HR 1.21 (1.03–1.42), P = 0.0216], HF hospitalization [HR 1.49 (1.29–1.72), P < 0.0001], and major adverse cardiovascular events [HR 1.32 (1.17–1.76), P < 0.0001]. The adverse outcomes were consistent in patients with reduced and preserved systolic function. No significant interaction was observed between bronchodilators and beta-blockade with respect to outcomes.
Conclusion
Bronchodilator use is a powerful independent predictor of worsening HF and increased mortality in a broad spectrum of patients with HF. Whether this relates to a toxic effect of bronchodilators, underlying pulmonary disease, or both is unclear and warrants further investigation.
Introduction
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are common partners with common problems.1 Remarkably few studies have addressed this intersection between cardiovascular and pulmonary disease. The combination presents diagnostic challenges,1 limits the use of beta-blockers,2 and is associated with worse survival.1 The causes of higher mortality have been studied in a very limited fashion.3 Use of bronchodilators, both beta-agonist and anticholinergic, is associated with adverse cardiovascular outcomes in patients with pulmonary disease.4–8 The prognosis of patients with HF prescribed bronchodilators is however ill defined.9,10 In particular, there is little information regarding the prevalence of bronchodilator use in HF with and without systolic dysfunction, or the relationship between bronchodilator use and outcomes. In the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme, candesartan significantly reduced cardiovascular deaths and hospital admissions for HF.11 The CHARM programme provides a unique opportunity to examine the prevalence and prognostic implications of bronchodilator use in a large cohort of patients with HF and wide range of left ventricular ejection fraction (LVEF).
Methods
Patients with symptomatic HF [New York Heart Association (NYHA) class II–IV] receiving standard therapy were enrolled into one of three parallel clinical trials according to LVEF and angiotensin converting enzyme inhibitor (ACE-I) treatment: LVEF ≤ 40% and not receiving an ACE-I due to previous intolerance (CHARM-Alternative); LVEF ≤ 40% receiving ACE-I treatment (CHARM-Added); and LVEF > 40% (CHARM-Preserved). There were 7599 patients randomized, 3803 receiving candesartan and 3796 placebo: 2028 in CHARM-Alternative, 2548 in CHARM-Added, and 3023 in CHARM-Preserved. Details of the rationale, methods, exclusion criteria, and main outcomes have been published previously.11–13 The study was approved by local Ethics Committees in all participating centres and all patients provided written informed consent.
The primary outcome in each trial was a composite of cardiovascular death or unplanned hospital admission for management of worsening HF. Secondary pre-specified endpoints and components included: cardiovascular death; hospital admission for HF; and a composite of cardiovascular death, hospital admission for HF, non-fatal myocardial infarction or non-fatal stroke. The primary outcome for the overall CHARM programme was all-cause mortality. The present study aims to assess the causal association between bronchodilator use and cardiovascular events in the cohorts with reduced (combined CHARM-Alternative/Added) and preserved (CHARM-Preserved) LV systolic function. Investigators at each participating centre employed a checkbox to record the use of bronchodilator therapy at baseline. The specific type of bronchodilator was not recorded. The medical history did not record either COPD or asthma. However, the CHARM enrolment criteria excluded patients with ‘severe obstructive pulmonary disease’.
All data analyses were performed independently by the Medical Statistical Unit at the London School of Hygiene and Tropical Medicine, London, UK. Baseline characteristics of patients prescribed bronchodilators were summarized by mean (standard deviation) for continuous variables and by frequency (percentages) for categorical variables. Means were compared using the Student t-test and proportions compared using the χ2 test. The impact of bronchodilator use was evaluated for predefined clinically relevant outcomes, including the primary outcome and other major cardiovascular events.
The estimated hazard ratios (HR) were adjusted for all important predictors of mortality and morbidity identified in the CHARM programme,14 including age, sex, diabetes mellitus, NYHA class, bundle branch block, body mass index, rest dyspnoea, current cigarette smoking, previous hospitalization for HF, first diagnosis of HF over 2 years ago, previous myocardial infarction, atrial fibrillation, heart rate, diastolic blood pressure, dependent oedema, pulmonary crackles, cardiomegaly, pulmonary oedema, mitral regurgitation, and candesartan treatment, using a multivariable Cox proportional hazards model. A two-tailed P-value of less than 0.05 was considered statistically significant. Data from the two studies of patients with reduced LVEF were combined, as this group was pre-specified as clinically important. For combined analysis of the three trials, statistical heterogeneity tests were performed for each endpoint. To identify the independent predictors of bronchodilator prescribing, a logistic regression model was employed with demographic and disease-related characteristics as potential predictors.
Results
Baseline characteristics
The findings from 7599 patients were analysed. The median duration of follow-up was 37.7 months. A detailed review of patients' baseline characteristics has previously been published.13 The baseline characteristics of patients receiving bronchodilators are displayed in Table Table 1. Six hundred and seventy-four patients (8.9%) were prescribed bronchodilators. The prevalence was similar in patients with reduced compared with preserved systolic function (respectively, 8.7 vs. 9.2%, P = 0.46).
| Characteristics, mean (SD) or n (%) | Preserved LVEF (n = 3023) | Reduced LVEF (n = 4576) | Overall (n = 7599) | |||
|---|---|---|---|---|---|---|
| Bronchodilator [n = 277 (9.2)] | No bronchodilator [n = 2746 (90.8)] | Bronchodilator [n = 397 (8.7)] | No bronchodilator [n = 4179 (91.3)] | Bronchodilator [n = 674 (8.9)] | No bronchodilator [n = 6925 (91.1)] | |
| Demographics | ||||||
| Age (years) | 67.7 (10.5) | 66.6 (11.1) | 66.5 (9.8) | 64.5 (11.1) | 67.0 (10.1) | 65.3 (11.1) |
| Female sex | 116 (41.9) | 1096 (39.9) | 92 (23.2) | 1096 (26.2) | 208 (30.9) | 2192 (31.7) |
| BMI | 30.4 (7.1) | 29.0 (5.6) | 27.8 (5.5) | 27.6 (5.1) | 28.9 (6.3) | 28.2 (5.3) |
| Smoking status | ||||||
| Current smoker | 37 (13.4) | 372 (13.5) | 72 (18.1) | 633 (15.1) | 109 (16.2) | 1005 (14.5) |
| Previous smoker | 151 (54.5) | 1221 (44.5) | 252 (63.5) | 2080 (49.8) | 403 (59.8) | 3301 (47.7) |
| Non smoker | 89 (32.1) | 1153 (42.0) | 73 (18.4) | 1466 (35.1) | 162 (24.0) | 2619 (37.8) |
| Medical history | ||||||
| Myocardial infarction | 111 (40.1) | 1229 (44.8) | 230 (57.9) | 2434 (58.2) | 341 (50.6) | 3663 (52.9) |
| Angina | 163 (58.8) | 1654 (60.2) | 234 (58.9) | 2301 (55.1) | 397 (58.9) | 3955 (57.1) |
| Stroke | 23 (8.3) | 245 (8.9) | 36 (9.1) | 359 (8.6) | 59 (8.8) | 604 (8.7) |
| Hypertension | 184 (66.4) | 1759 (64.1) | 202 (50.9) | 2041 (48.8) | 386 (57.3) | 3800 (54.9) |
| Diabetes mellitus | 91 (32.9) | 766 (27.9) | 116 (29.2) | 1190 (28.5) | 207 (30.7) | 1956 (28.2) |
| Atrial fibrillation | 85 (30.7) | 796 (29.0) | 114 (28.7) | 1088 (26.0) | 199 (29.5) | 1884 (27.2) |
| Prior HF hospitalization | 212 (76.5) | 1864 (67.9) | 312 (78.6) | 3038 (72.7) | 524 (77.7) | 4902 (70.8) |
| Cancer | 31 (11.2) | 195 (7.1) | 34 (8.6) | 253 (6.1) | 65 (9.6) | 448 (6.5) |
| Severity markers | ||||||
| Ejection fraction | 55.6 (10.1) | 53.9 (9.3) | 28.8 (7.6) | 28.8 (7.5) | 39.8 (15.8) | 38.8 (14.8) |
| NYHA II | 113 (40.8) | 1723 (62.7) | 113 (28.5) | 1467 (35.1) | 226 (33.5) | 3190 (46.1) |
| NYHA III | 157 (56.7) | 983 (35.8) | 259 (65.2) | 2586 (61.9) | 416 (61.7) | 3569 (51.5) |
| NYHA IV | 7 (2.5) | 40 (1.5) | 25 (6.3) | 126 (3.0) | 32 (4.7) | 166 (2.4) |
| Physical examination | ||||||
| Heart rate (b.p.m.) | 75.4 (12.7) | 70.9 (12.4) | 76.9 (13.2) | 73.6 (13.3) | 76.3 (13.0) | 72.5 (13.0) |
| Systolic BP (mmHg) | 134.8 (18.3) | 136.3 (18.5) | 127.1 (18.6) | 127.4 (18.8) | 130.3 (18.9) | 130.9 (19.2) |
| Diastolic BP (mmHg) | 77.3 (11.0) | 77.9 (10.7) | 74.2 (11.0) | 76.0 (10.7) | 75.5 (11.1) | 76.7 (10.7) |
| Elevated JVP | 111 (40.1) | 955 (34.8) | 149 (37.5) | 1393 (33.3) | 260 (38.6) | 2348 (33.9) |
| Peripheral oedema | 83 (30.0) | 752 (27.4) | 98 (24.7) | 921 (22.0) | 181 (26.9) | 1673 (24.2) |
| Pulmonary crepitations | 72 (26.0) | 418 (15.2) | 87 (21.9) | 655 (15.7) | 159 (23.6) | 1073 (15.5) |
| Pulmonary wheeze | 30 (10.8) | 51 (1.9) | 49 (12.3) | 100 (2.4) | 79 (11.7) | 151 (2.2) |
| Electrocardiogram | ||||||
| Atrial fibrillation | 43 (15.5) | 435 (15.8) | 61 (15.4) | 609 (14.6) | 104 (15.4) | 1044 (15.1) |
| Bundle branch block | 50 (18.1) | 384 (14.0) | 141 (35.5) | 1236 (29.6) | 191 (28.3) | 1620 (23.4) |
| Chest X-ray | ||||||
| Pulmonary oedema | 8 (2.9) | 74 (2.7) | 11 (2.8) | 118 (2.8) | 19 (2.8) | 192 (2.8) |
| Cardiomegaly | 55 (19.9) | 439 (16.0) | 115 (29.0) | 1058 (25.3) | 170 (25.2) | 1497 (21.6) |
| Concomitant therapy | ||||||
| Beta-blocker | 90 (32.5) | 1594 (58.0) | 125 (31.5) | 2394 (57.3) | 215 (31.9) | 3988 (57.6) |
| Carvedilol | 10 (3.6) | 194 (7.1) | 34 (8.6) | 742 (17.8) | 44 (6.5) | 936 (13.5) |
| Metoprolol | 37 (13.4) | 734 (26.7) | 50 (12.6) | 1124 (26.9) | 87 (12.9) | 1858 (26.8) |
| Bisoprolol | 9 (3.2) | 126 (4.6) | 8 (2.0) | 142 (3.4) | 17 (2.5) | 268 (3.9) |
| Atenolol | 23 (8.3) | 339 (12.4) | 19 (4.8) | 219 (5.2) | 42 (6.2) | 558 (8.1) |
| Other beta-blocker | 11 (4.0) | 205 (7.5) | 15 (3.8) | 172 (4.1) | 26 (3.9) | 377 (5.4) |
| Calcium channel blocker | 119 (43.0) | 825 (30.0) | 70 (17.6) | 528 (12.6) | 189 (28.0) | 1353 (19.5) |
| Amiodarone | 32 (11.6) | 214 (7.8) | 60 (15.1) | 457 (10.9) | 92 (13.6) | 671 (9.7) |
| Digoxin | 86 (31.0) | 756 (27.5) | 225 (56.7) | 2187 (52.3) | 311 (46.1) | 2943 (42.5) |
| ACE-inhibitors | 45 (16.2) | 531 (19.3) | 210 (52.9) | 2339 (56.0) | 255 (37.8) | 2870 (41.4) |
| Spironolactone | 43 (15.5) | 309 (11.3) | 91 (22.9) | 829 (19.8) | 134 (19.9) | 1138 (16.4) |
| Diuretics | 232 (83.8) | 2027 (73.8) | 366 (92.2) | 3661 (87.6) | 598 (88.7) | 5688 (82.1) |
Overall, a prior smoking history was more frequent in patients receiving bronchodilators (59.8 vs. 47.7%, P < 0.0001), although the proportion of current smokers was similar (16.2 vs. 14.5%, P = 0.24). No significant difference was observed in cardiovascular comorbidity between those prescribed and not prescribed bronchodilators, including history of myocardial infarction, angina, stroke, diabetes, hypertension, and atrial fibrillation. A greater proportion of patients receiving bronchodilators had previously been hospitalized for worsening HF (77.7 vs. 70.8%, P = 0.0001). These findings were consistent irrespective of reduced or preserved ejection fraction.
Patients prescribed bronchodilators had poorer functional status, as indicated by an increased prevalence of NYHA classification III to IV. Overall, in the reduced and preserved systolic function groups, clinical signs of HF were more common in those receiving bronchodilators. These included elevated jugular venous pressure, peripheral oedema, pulmonary crepitations, and wheeze. Mean ejection fraction was, however, similar comparing those with and without bronchodilators (overall 39.8% vs. 38.8% respectively, P = 0.10).
Beta-blocker utilisation was markedly lower in patients receiving bronchodilators compared to those without bronchodilator therapy: Overall 31.9 vs. 57.6%; Reduced LVEF: 31.5 vs. 57.3%; Preserved LVEF: 32.5 vs. 58.0% (all P < 0.0001). The proportion of patients receiving a beta-1 selective adrenoceptor blocker (metoprolol, bisoprolol, or atenolol) was similar in patients with and without bronchodilators (67.9 vs. 67.3%, P = 0.85). The use of amiodarone, digoxin, and calcium channel blockers was greater in those receiving bronchodilators, both overall and in patients with reduced or preserved ejection fraction. Treatment with bronchodilators was also associated with greater use of diuretic therapy, including spironolactone.
Independent predictors of use of bronchodilator therapy
Multivariable analysis of predictors of bronchodilator prescribing revealed smoking history to be the strongest independent determinant. After adjusting for baseline variables including demographics, aetiology of HF, and medical history, the odds of receiving bronchodilators for smokers were approximately twice those for non-smokers [odds ratio 0.47 (0.39–0.56), P < 0.0001]. Increasing age, body mass index, heart rate, blood pressure, and presence of ischaemic heart disease were also independent predictors of bronchodilator use.
Mortality
Bronchodilator therapy was independently associated with increased mortality. 32.6% of patients receiving bronchodilators died from any cause, compared with 23.3% of those without bronchodilators (Table Table 2). After adjusting for additional predictors of mortality, the risk of death was 26% higher in patients prescribed bronchodilators [adjusted HR 1.26 (95% CI 1.09–1.45)]. This higher risk of overall mortality reflected a higher incidence of both non-cardiovascular death [7.6 vs. 4.6%, HR 1.49 (1.10–2.01)] and cardiovascular death [25.1 vs. 18.6%, HR 1.21 (1.03–1.42)]. The higher risk of cardiovascular death was largely attributable to death due to progressive pump failure [9.8 vs. 5.8%, HR 1.40 (1.07–1.82)]. The risk of sudden death was not elevated after correcting for baseline differences between patients receiving and not receiving bronchodilators. The greater mortality associated with bronchodilator use was consistent in patients with reduced and preserved systolic function: all-cause mortality (HR 1.27 vs. 1.26, respectively); cardiovascular death (HR 1.22 vs. 1.23); and non-cardiovascular death (HR 1.57 vs. 1.35).
| Outcomes/systolic function | Bronchodilator (n = 674) | No bronchodilator (n = 6925) | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | P-value interactiona |
|---|---|---|---|---|---|---|---|
| Cardiovascular death or HF hospitalization | |||||||
| Overall | 303 (45.0) | 2157 (31.1) | 1.65 (1.47–1.87) | <0.0001 | 1.38 (1.22–1.56) | <0.0001 | 0.1452 |
| Reduced LVEF | 198 (49.9) | 1563 (37.4) | 1.52 (1.31–1.76) | <0.0001 | 1.32 (1.14–1.53) | 0.0003 | |
| Preserved LVEF | 105 (37.9) | 594 (21.6) | 2.00 (1.62–2.46) | <0.0001 | 1.52 (1.22–1.89) | 0.0002 | |
| All-cause mortality | |||||||
| Overall | 220 (32.6) | 1611 (23.3) | 1.53 (1.33–1.76) | <0.0001 | 1.26 (1.09–1.45) | 0.0015 | 0.4485 |
| Reduced LVEF | 155 (39.0) | 1195 (28.6) | 1.49 (1.26–2.12) | <0.0001 | 1.27 (1.07–1.51) | 0.0055 | |
| Preserved LVEF | 65 (23.5) | 416 (15.1) | 1.63 (1.26–2.12) | 0.0002 | 1.26 (0.95–1.65) | 0.1041 | |
| Non-cardiovascular death | |||||||
| Overall | 51 (7.6) | 320 (4.6) | 1.78 (1.32–2.39) | 0.0001 | 1.49 (1.10–2.01) | 0.0097 | 0.4383 |
| Reduced LVEF | 31 (7.8) | 199 (4.8) | 1.81 (1.24–2.64) | 0.0021 | 1.57 (1.07–2.31) | 0.0214 | |
| Preserved LVEF | 20 (7.2) | 12 (4.4) | 1.73 (1.08–2.78) | 0.0231 | 1.35 (0.82–2.22) | 0.2394 | |
| Cardiovascular death | |||||||
| Overall | 169 (25.1) | 1291 (18.6) | 1.47 (1.25–1.72) | <0.0001 | 1.21 (1.03–1.42) | 0.0216 | 0.7738 |
| Reduced LVEF | 124 (31.2) | 996 (23.8) | 1.43 (1.18–1.72) | 0.0002 | 1.22 (1.01–1.47) | 0.0412 | |
| Preserved LVEF | 45 (16.2) | 295 (10.7) | 1.59 (1.16–2.18) | 0.0038 | 1.23 (0.89–1.71) | 0.2171 | |
| Death due to HF progression | |||||||
| Overall | 66 (9.8) | 403 (5.8) | 1.84 (1.42–2.38) | <0.0001 | 1.40 (1.07–1.82) | 0.0128 | 0.6735 |
| Reduced LVEF | 49 (12.3) | 318 (7.6) | 1.77 (1.31–2.39) | 0.0002 | 1.39 (1.03–1.89) | 0.0328 | |
| Preserved LVEF | 17 (6.1) | 85 (3.1) | 2.08 (1.23–3.50) | 0.0059 | 1.51 (0.86–2.64) | 0.1526 | |
| Sudden death | |||||||
| Overall | 66 (9.8) | 577 (8.3) | 1.29 (1.00–1.66) | 0.0532 | 1.13 (0.87–1.46) | 0.3474 | 0.1283 |
| Reduced LVEF | 47 (11.8) | 462 (11.1) | 1.17 (0.87–1.58) | 0.3105 | 1.06 (0.78–1.43) | 0.7094 | |
| Preserved LVEF | 19 (6.9) | 115 (4.2) | 1.72 (1.06–2.79) | 0.0292 | 1.34 (0.81–2.22) | 0.2594 | |
| HF hospitalization | |||||||
| Overall | 225 (33.4) | 1450 (20.9) | 1.81 (1.57–2.09) | <0.0001 | 1.49 (1.29–1.72) | <0.0001 | 0.5480 |
| Reduced LVEF | 142 (35.8) | 1016 (24.3) | 1.66 (1.40–1.98) | <0.0001 | 1.43 (1.20–1.71) | 0.0001 | |
| Preserved LVEF | 83 (30.0) | 434 (15.8) | 2.14 (1.69–2.71) | <0.0001 | 1.59 (1.25–2.04) | 0.0002 | |
| CV death, HF hospitalization, non-fatal MI, non-fatal stroke | |||||||
| Overall | 317 (47.0) | 2372 (34.3) | 1.57 (1.39–1.76) | <0.0001 | 1.32 (1.17–1.76) | <0.0001 | 0.2775 |
| Reduced LVEF | 205 (51.6) | 1667 (39.9) | 1.47 (1.27–1.70) | <0.0001 | 1.28 (1.10–1.48) | 0.0010 | |
| Preserved LVEF | 112 (40.4) | 705 (25.7) | 1.78 (1.46–2.17) | <0.0001 | 1.43 (1.16–1.76) | 0.0007 | |
- a Interaction between bronchodilator (vs. no bronchodilator) and reduced LVEF (vs. preserved LVEF).
Cardiovascular morbidity and mortality
Bronchodilator therapy was an independent predictor of worse fatal and non-fatal cardiovascular outcomes in patients with HF (Table Table 2). Overall, the primary outcome of cardiovascular death or HF hospitalization occurred in 45.0% patients receiving bronchodilators as opposed to 31.1% of those without bronchodilators [adjusted HR 1.38 (1.22–1.56), P < 0.0001, Table Table 2]. The risk of hospitalization due to worsening HF associated with bronchodilators was likewise 49% higher [HR 1.49 (1.29–1.72), P < 0.0001]. Finally, the relative risk of sustaining a major adverse cardiovascular event (defined as cardiovascular death, HF hospitalization, non-fatal MI, or non-fatal stroke) was 32% higher in those receiving bronchodilators [HR 1.32 (1.17–1.76), P < 0.0001].
As with mortality, the association between bronchodilator therapy and adverse outcomes was consistent in patients with reduced and preserved systolic function. Risk of the primary endpoint, HF hospitalization, and major adverse cardiovascular events was greater in patients receiving bronchodilators, irrespective of LVEF. Formal statistical testing for an interaction confirmed no significant difference between the cohorts (Table Table 2).
Interaction between bronchodilators and concurrent beta-blockers
Bronchodilator use was associated with adverse outcomes regardless of concurrent beta-blocker therapy (Table Table 3). Among patients receiving beta-blockers, bronchodilator use (compared with no bronchodilator use) was associated with greater all-cause mortality [HR 1.32 (1.01–1.72) vs. 1.14 (0.96–1.36) in those not receiving a beta-blocker], cardiovascular death or HF hospitalization [HR 1.44 (1.15–1.80) vs. 1.22 (1.05–1.42)], and major adverse cardiovascular events [HR 1.33 (1.07–1.65) vs. 1.19 (1.03–1.38)]. No statistical interaction was observed between bronchodilator therapy and beta-blockade with respect to any pre-specified outcome.
| Outcome, beta-blocker | Bronchodilator | No bronchodilator | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | P-value interactiona |
|---|---|---|---|---|---|---|---|
| Cardiovascular death or HF hospitalization | |||||||
| Beta-blocker | 85 (39.5) | 1054 (26.4) | 1.73 (1.39–2.16) | 0.0000 | 1.44 (1.15–1.80) | 0.0015 | 0.2557 |
| No beta-blocker | 218 (47.5) | 1103 (37.6) | 1.22 (1.05–1.42) | 0.0080 | 1.22 (1.05–1.42) | 0.0080 | |
| All-cause mortality | |||||||
| Beta-blocker | 61 (28.4) | 773 (19.4) | 1.62 (1.25–2.10) | 0.0003 | 1.32 (1.01–1.72) | 0.0406 | 0.3804 |
| No beta-blocker | 159 (34.6) | 838 (28.5) | 1.14 (0.96–1.36) | 0.1282 | 1.14 (0.96–1.36) | 0.1282 | |
| Non-cardiovascular death | |||||||
| Beta-blocker | 15 (7.0) | 153 (3.8) | 1.99 (1.17–3.38) | 0.0112 | 1.48 (0.86–2.55) | 0.1547 | 0.5878 |
| No beta-blocker | 36 (7.8) | 167 (5.7) | 1.39 (0.96–2.01) | 0.0798 | 1.19 (0.78–2.32) | 0.1543 | |
| Cardiovascular death | |||||||
| Beta-blocker | 46 (21.4) | 620 (15.5) | 1.52 (1.13–2.06) | 0.0059 | 1.26 (0.93–1.71) | 0.1318 | 0.4942 |
| No beta-blocker | 123 (26.8) | 671 (22.8) | 1.09 (0.90–1.33) | 0.3629 | 1.09 (0.90–1.33) | 0.3629 | |
| Cardiovascular death, HF hospitalization, non-fatal MI, non-fatal stroke | |||||||
| Beta-blocker | 89 (41.4) | 1187 (29.8) | 1.60 (1.29–1.98) | 0.0000 | 1.33 (1.07–1.65) | 0.0115 | 0.4781 |
| No beta-blocker | 228 (49.7) | 1185 (40.3) | 1.19 (1.03–2.51) | 0.0154 | 1.19 (1.03–1.38) | 0.0154 | |
- a Interaction between bronchodilator (vs. no bronchodilator) and beta-blocker (vs. no beta-blocker).
Relationship between beta-blockers and mortality
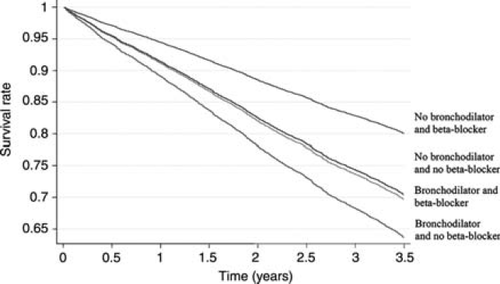
Survival was significantly better in patients receiving beta-blockers, irrespective of concurrent bronchodilator therapy (Figure Figure 1). Overall, the adjusted HR for mortality comparing patients with and without beta-blockade was 0.77 (0.70–0.85), P < 0.001. In patients receiving bronchodilators, 28.4% of those prescribed beta-blockers died, compared with 34.6% of those not prescribed beta-blockers [HR 0.87 (0.64–1.18), P = 0.354]. This relative risk of death in beta-blocker treated patients was also lower in those not receiving bronchodilators [19.4 vs. 28.5%, respectively, HR 0.76 (0.69–0.85), P < 0.001]. No interaction was observed between beta-blockade and bronchodilator therapy.
Discussion
Although the association between bronchodilator use (both beta-agonist and anticholinergic) and adverse cardiovascular events in patients with pulmonary disease is well recognized,4–8 the relationship between the use of these drugs and outcomes in patients with HF is uncertain.9,10 We describe several key findings. Bronchodilator use was associated with increased all-cause mortality, cardiovascular death, HF hospitalization, and major adverse cardiovascular events. The adverse outcomes were consistent in patients with reduced and preserved systolic function, and remained significant after comprehensive multivariate adjustment. Moreover, the magnitude of risk associated with bronchodilators was comparable to recognized predictors such as NYHA class, bundle branch block, ischaemic heart disease, heart rate, and blood pressure.14
Remarkably, few reports describe bronchodilator use in patients with HF. The prevalence in CHARM Overall (8.9%) was similar to that observed in a community HF clinic in the UK (12.1%).15 Although pulmonary disease is more common in patients with HF and preserved ejection fraction,1 the prevalence of bronchodilator use was similar in patients with reduced and preserved systolic function. Symptoms and signs of HF were more frequent in patients prescribed bronchodilators despite similar ejection fractions, as were prior hospitalizations for decompensated HF. The findings highlight the diagnostic dilemmas posed by the combination of HF and pulmonary disease.1 No qualitative symptoms are unique to HF.16
Signs are equally misleading. Although cor pulmonale is rare in patients with COPD,1,17,18 elevated jugular venous pressure is not. A comprehensive study investigated 405 elderly patients with stable COPD.18 Heart failure was diagnosed by an expert panel following chest radiography, electrocardiography, echocardiography, and pulmonary function tests. Nearly a quarter (23.3%) of the 322 patients with COPD in whom HF was excluded had a raised jugular venous pressure.19 A similar proportion of patients with COPD are reported to have mild pulmonary hypertension.17 Pulmonary disease therefore appears to worsen the clinical syndrome of HF.
The diversity and magnitude of adverse outcomes associated with bronchodilator therapy is surprising. Cohorts defined by bronchodilator prescription undoubtedly represent a heterogeneous group of patients: COPD, asthma, restrictive lung disease, and those misdiagnosed with airflow obstruction. The latter is common in patients with decompensated HF,20,21 in whom interstitial oedema causes airway compression and bronchial hyperresponsiveness.1,22 Non-cardiovascular deaths are inevitable in cohorts dominated by pulmonary disease. The excess cardiovascular mortality is more concerning. Bronchodilators were associated with a 40% higher risk of death due to progressive pump failure. The risk of hospitalization for worsening HF was likewise 49% greater.
Our findings corroborate and extend two prior studies examining patients with HF or left ventricular systolic dysfunction (LVSD) prescribed inhaled beta-agonists.9,10 In 1529 subjects with LVSD identified retrospectively through imaging records,9 all-cause mortality and HF hospitalization within 1 year increased with the average number of canisters dispensed per month. After covariate adjustment, the risk of HF admission was: 1.3 (0.9–2.0) (one canister per month); 1.7 (1.2–2.5) (two canisters per month); 2.0 (1.3–3.0) (≥3 canisters per month). Risk of death was similarly increased: 0.9 (0.5–1.6) (one canister per month); 1.4 (0.9–2.2) (two canisters per month); 2.0 (1.3–3.2) (≥3 canisters per month). However, the association was undermined by the indication for β-agonist use: increasing dyspnoea and resulting β-agonist prescription may simply have reflected worsening HF. Without markers of HF severity the multivariate model was unable to adjust for such confounding. A second case–control study observed a similar relationship between β-agonists and HF hospitalization in patients with existing HF.10 The risk remained significant after adjustment for age, cardiovascular comorbidity, presence of COPD and β-blocker prescription [OR 1.6 (1.0–2.7)].
The association between bronchodilators and worsening HF could be attributed to confounding by indication and the severity of underlying lung disease. Bronchodilators may simply be prescribed to patients with worse HF or airflow obstruction. However, unlike previous studies, the former confounder (severity of HF) is minimized by comprehensive adjustment incorporating measures of HF signs, symptoms, and functional class as well as history of HF hospitalization and ejection fraction. The latter confounder (severity of lung disease) is to some extent addressed in the current analysis as the CHARM enrolment criteria excluded patients with ‘severe obstructive pulmonary disease’. Furthermore, the recruitment bias inherent to clinical trials is also likely to have reduced inclusion of individuals with severe pulmonary disease. Nonetheless, infection is a recognized precipitant of HF decompensation and may have contributed to the marked increase in fatal and non-fatal pump failure.23,24
Heart failure is characterized by increased adrenergic drive. β1 and β2 Adrenoceptors mediate norepinephrine toxicity, fibrosis, and necrosis. Down-regulation of β1 receptors with preservation of the β2 subpopulation reduces the β1/β2 ratio.25 The inotropic responsiveness (and likewise vulnerability) of the failing myocardium to β2-agonists thereby assumes greater importance.26,27 Although the specific types and doses of bronchodilator were not recorded, inhaled β2-agonists are baseline therapy for both COPD and asthma. It is possible that bronchodilators compound maladaptive remodelling and further depress myocardial function. Two observations temper this argument. β2-Agonists exert numerous arrhythmic effects: tachycardia, hypokalaemia, QTc prolongation, disturbed autonomic modulation, and depressed heart rate variability.4,5,28,29 The lack of an associated increase in sudden cardiac death, particularly in those with LVSD, suggests systemic consequences are minimal. Secondly, if mediated by β-adrenergic stimulation, the adverse consequences would possibly be lessened by concurrent beta-blocker use. No such interaction was observed. However, the majority of patients received cardioselective β1-blockers, namely metoprolol or bisoprolol. Whether β1-blockade antagonises β2-mediated effects is unknown.
In three observational studies, beta-blockers were consistently associated with better survival in patients with HF and concurrent COPD.3,30,31 None reported the outcomes of patients receiving bronchodilators, in whom physicians may be wary of beta-blockade. We have now examined this important patient group. Formal testing revealed no significant interaction between bronchodilator use and the better survival associated with beta-blockade. As with previous reports,3,30,31 recruitment bias and the absence of pulmonary function data limit inference to patients with severe or reversible airflow obstruction. Moreover, residual confounding by the indication for beta-blockade is inescapable without random allocation of beta-blockers. The results must only be considered exploratory.
Several limitations must be acknowledged. Whether bronchodilators were prescribed for COPD, asthma, or alternative reasons is unknown. The specific type, administration route, dose, frequency, and duration of bronchodilator therapy were not recorded. This information would prove valuable in future studies of bronchodilator effects. Inhaled beta-agonists and anticholinergics, but not corticosteroids, are associated with adverse cardiovascular events in patients with COPD.6 The effect of different bronchodilators may likewise vary in patients with HF. However, their respective effects may inextricably merge with concurrent prescribing, particularly as inhaled short-acting β-agonists are recommended first line therapy.32 The prevalence of bronchodilator therapy was low relative to the prevalence of COPD in unselected cohorts.1 However, many patients with HF and COPD in recent studies have not been prescribed bronchodilators.15,33,34 Nevertheless, we must acknowledge that recruitment bias is problematic in any clinical trial, and that patients with severe pulmonary disease are likely under-represented in CHARM. The impact of patient selection on our results is difficult to predict. Finally, only a limited number of potential predictor variables were assessed, and the differences and associations found in this study may still be subject to unobserved confounding factors.
Conclusion
Bronchodilator use is a powerful independent predictor of worsening HF and increased mortality in a broad spectrum of patients with HF. Whether this relates to a toxic effect of beta-agonists (or other bronchodilators), underlying pulmonary disease or both is unclear. Bronchodilators improve symptoms, pulmonary function, quality of life, and frequency of exacerbations in patients with COPD.32 However, physicians should be alert to the fact that use of bronchodilators identifies a patient at increased risk of worsening HF and death.
There are many challenges for future research. The interaction between bronchodilators, pulmonary disease, and the syndrome of HF is complex. Studies need to compare different types and doses of bronchodilator while adjusting for the presence and severity of pulmonary disease. The present study may only be considered a first step in examining the association between bronchodilators and poor clinical outcomes. Only randomized controlled trials can conclusively prove or disprove the safety of bronchodilators in patients with HF.
Acknowledgements
The authors would like to thank Dr Francis Dunn for his valuable contribution to the study.
Conflict of interest: Drs J.J.V.M., M.A.P., K.S., C.B.G., S.Y., J.Ö., S.D.S., A.P.M., Dunn, M.C.P., and N.M.H. have received research grants, honoraria for lectures and/or consulting fees from a number of pharmaceutical companies manufacturing and selling inhibitors of the renin–angiotensin–aldosterone system, including AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Sanofi-Aventis, Merck, Novartis, Pfizer and Takeda Pharmaceutical Company. Dr E.L.M. is an employee of AstraZeneca, sponsor of the CHARM programme.
Funding
The CHARM programme was sponsored by AstraZeneca.




