Habitat Use and Fishery Dynamics of a Heavily Exploited Coastal Migrant, Spanish Mackerel
Abstract
Spanish Mackerel Scomberomorus maculatus support important commercial and recreational fisheries in the Gulf of Mexico (GOM). Although federally managed because of interstate migrations, harvest, essential fish habitat consideration, and management are largely state issues. Contrary to the accepted paradigm of a well-mixed stock from the GOM to the Atlantic, Spanish Mackerel have been suggested to exhibit reduced movement, and hence mixing, which could lead to areas of localized depletion within the larger stock. We addressed how local abundance patterns responded to changes in harvest pressure, evaluated migration patterns via examining temporal trends in abundance, and determined whether the spatial abundance patterns resembled habitat specialization or generalization. Abundance trends of Spanish Mackerel in Alabama have remained relatively constant from 2001 to 2011 despite a significant increase in commercial harvesting. Seasonal patterns of catch rates coincided with the proposed annual migration. We also observed adult Spanish Mackerel in low salinities (0–10‰) at the mouth of Mobile River during the fall when river discharge is relatively low. This extends the known range of estuarine habitat use by adult Spanish Mackerel. Because Mobile Bay includes various habitat types differing from surf zone and coastal ocean habitats typically inhabited by Spanish Mackerel, capturing Spanish Mackerel near the mouth of Mobile River provides evidence for a habitat generalist strategy. Based on a relatively constant abundance index and year-class strength, the stock seems to be relatively stable even after a drastic increase in commercial harvests in Alabama and heavy exploitation of age-1–3 Spanish Mackerel. The resilience of catch rate trends to changes in local harvest pressure as well as evidence of synchrony among areas in terms of migration suggest the Alabama fishery is influenced by population dynamics of a larger population.
Received July 3, 2014; accepted January 12, 2015
Understanding fish distributions and habitat use has long been a central focus in ecology and is critical not only for ecological research but also for effective resource management. In coastal habitats, management of resources is paramount for both the ecological health of the system as well as the economic health of the area. In the marine realm, management of mobile species is complex and management occurs at multiple governing levels (state, regional, national, and international). Species that cross these management boundaries further complicate the scenario, and a thorough understanding of their ecology and habitat use may provide insight for management decisions. Here, we explore three areas we view as essential for state and regional level management of fishery resources: the response of local abundance patterns to local harvest pressure, the potential for regional movement patterns, and the habitat preferences (specialist versus generalist) of exploited species.
Coastal migratory pelagic (CMP) fish are highly sought after by commercial and recreational fisheries and are an example of species that break the barriers of conventional management (state) boundaries. Coastal migratory pelagic fish are fast-swimming fishes that occupy relatively shallow coastal waters and generally migrate along coastlines. In the Gulf of Mexico (GOM), some examples of CMP species are Cobia Rachycentron canadum, Atlantic Tripletail Lobotes surinamensis, King Mackerel Scomberomorus cavalla, and Spanish Mackerel S. maculatus. Herein, we will focus on the Spanish Mackerel.
Spanish Mackerel have a wide distribution, occurring in coastal waters of the western Atlantic Ocean from the Gulf of Maine through the Yucatan Peninsula (Collette et al. 1978). This fish is considered to be a more pelagic or neritic-zone associated species, yet the use of more inshore and estuarine habitats is largely unknown. Larvae of Spanish Mackerel have been found in inshore waters of the GOM (McEachran et al. 1980), and juveniles probably migrate inshore since young of the year (age 0) have been collected in estuaries during flood tides (Peters and Schmidt 1997). It has also been suggested that estuaries may be used as nursery grounds for some age 0 (Godcharles and Murphy 1986). Apart from accounts of the species’ seasonal occurrence, adult Spanish Mackerel estuary use has not been fully elucidated. They are pelagic carnivores that usually feed on estuary-dependent fish like menhaden and anchovies (Godcharles and Murphy 1986; Finucane et al. 1990), so adults may enter estuaries to feed (Collette and Nauen 1983). Given their ecological and economic importance, a better understanding of estuarine habitat use could be beneficial for determining connectivity patterns and for making management decisions.
Estuarine habitats can constitute significant portions of the coastline in some areas. For example, the United States GOM coastline is approximately 2,624 km, but if bays and inland waters are included this number increases ca. 10-fold. Knowing how Spanish Mackerel may or may not interact with these estuarine systems would provide much-needed information about the species’ potential use of a large proportion of GOM habitat and allow for better-informed stock assessment and fishery management. Spanish Mackerel management occurs primarily at the state level, even though these fish are highly capable of quickly crossing boundaries; therefore, decisions in one state may have downstream consequences for others. Small-scale (state) distribution patterns and movements, however, are not well known. Furthermore, localized heavy fishing pressure could lead to areas of localized depletion if, in fact, movement patterns are also more localized. For example, the state of Alabama has a large harvest of Spanish Mackerel (even though the coastline is relatively small compared with that of other GOM states), and if Spanish Mackerel movement patterns are more localized, the depleted areas may not be readily replenished by fish from other areas. This unique situation provides a prime model to test for localized depletion effects in Alabama.
The most recent (2012) stock assessment for GOM Spanish Mackerel did not determine the status of the fishery because independent reviewers varied in opinions on the appropriateness of the assessment for making the determination (SEDAR 2013). The report noted the lack of fishery-independent data available for the assessment and stressed the need for better information on migration patterns as well as a comprehensive study of the stock structure. These issues are crucial for Spanish Mackerel management, especially since determining the spatial and temporal scales at which to manage a species depends in part on the understanding of fish movement patterns (Begg and Waldman 1999).
Given (1) the commercial and recreational importance of Spanish Mackerel, (2) the need for better assessment data, and (3) the lack of a full understanding of migration patterns (e.g., regional movement patterns) and habitat usage, we examined Spanish Mackerel catches in the GOM on two different spatial and temporal scales. Specifically, using data from long-term GOM harvests and a state-level scientific survey, we address three questions of general relevance to the many species that share the trans-management boundary condition of Spanish Mackerel: (1) how do local abundance patterns respond to changes in local harvest pressure, (2) is there evidence for regional migration, and (3) do Spanish Mackerel exhibit specialization in habitat utilization or are they habitat generalists? Combining information from long-term trends and fishery-independent catches in estuarine waters may provide information about the spatiotemporal variability in habitat use and the factors contributing to Spanish Mackerel distributions in the area.
METHODS
Review of Gulf of Mexico harvest history.
To assess the long-term exploitation of the Spanish Mackerel fishery resource, commercial and recreational landings data were obtained from National Marine Fisheries Service (NMFS) statistics. The commercial landings records date back to 1950 and represent multiple gear types, including hand lines, gill nets, rod and reel, cast nets, skimmer nets, haul seines, combined gears, etc. Recreational landings records date back to 1981 and are a result of the Marine Recreational Information Program (MRIP, started in 2007; formerly MRFFS (Marine Recreational Fisheries Statistics Survey, started in 1979) survey system, including catch-and-effort information from charter boats, private boats, beach or shore fishing, etc. All landings data, commercial and recreational, was analyzed as a time series for general trends in landings over time.
Examining local habitat use: study area.
Alabama's coastal waters include Mobile Bay, Perdido Bay, Mississippi Sound, and GOM waters along Alabama's shoreline. Mobile Bay is a highly stratified, shallow (~3 m) estuary resulting from the draining of the Mobile and Tensaw rivers into the northern end of the bay, which combined have the fourth largest river discharge in the United States (Schroeder 1978). Perdido Bay receives approximately 36 times less freshwater than Mobile Bay (USEPA 1999), but both drain large agricultural areas with high precipitation rates, averaging 165 cm/year (Robinson et al. 1996). Mississippi Sound, another shallow (~4 m) estuary, runs east-west along the southern coasts of Mississippi and Alabama, about 145 km in length. The Escatawpa, Pascagoula, Tchoutacabouffa, Biloxi, Wolf, and Jourdan rivers drain into Mississippi Sound. The southern boundary of the sound is made up of multiple barrier islands, separating the sound from the GOM.
Sampling design.
The Alabama Department of Conservation and Natural Resources, Marine Resources Division, established a gill-net sampling program in 2001 to monitor juvenile and adult finfish populations in Alabama's coastal waters. The coastal waters, excluding GOM waters, were subdivided into four major sampling areas, including Mobile Bay (north and south), Mississippi Sound, and Perdido Bay (Figure 1). Mobile Bay was subdivided based on evidence of a north-south salinity gradient within Mobile Bay; Mississippi Sound and Perdido Bay were not subdivided because of the lack of a salinity gradient (J. F. Mareska, unpublished data). The standardized methods currently used were adopted in 2004. Stratified random gill-net sampling occurred monthly from January to December, with the number of gill-net sets and their locations computer derived to maximize sampling efficiency. The number of sets ranged from 8 in winter months to 13 in summer months. The survey used two gill nets, identified by stretched-mesh size (small versus large), that were set separately but typically fished concurrently. The small mesh net was composed of five panels (8 × 150 ft) of graduated mesh sizes (750 ft TL), ranging from 2.0–4.0-in stretched mesh, and increasing in 0.5-in increments. The large mesh gill net had four panels (8 × 150 ft) of graduated mesh sizes (4.5–6.0 in), also increasing by 0.5-in increments, for 600 ft TL. For this study, only small mesh nets were analyzed because the vast majority of Spanish Mackerel catches (ca. 90%) occurred in the small mesh net.
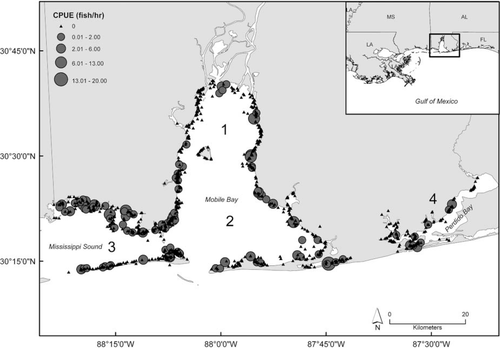
The sampling areas used for the fishery-independent gill-net survey conducted by the Alabama Department of Conservation and Natural Resources, Marine Resources Division, during 2001–2011. Numbers correspond to the four main sampling areas of (1) north Mobile Bay, (2) south Mobile Bay, (3) Mississippi Sound, and (4) Perdido Bay. A spatial bubble plot corresponding to Spanish Mackerel catch per unit effort (CPUE = fish/h) is overlaid.
Gill nets were set at various times during the day or night, and soak times were generally ≤ 1 h. Nets were set perpendicular or parallel to the shoreline, or when schooling was evident, a round haul or strike set was conducted. All captured finfish were identified to species and FL of up to 10 individuals of each species per mesh size was measured. For each gill-net set, water temperature, salinity, dissolved oxygen, depth, latitude, and longitude were also recorded.
Data analysis.
In order to analyze the annual abundance trend of Spanish Mackerel in Alabama's coastal waters from 2001 to 2011, first nominal CPUE (number of fish captured per hour soak time) was calculated. Due to the high percentage of zeroes in the catch data, we then used a delta-lognormal generalized linear model (GLM) to calculate a relative index of abundance by year, as described by Lo et al. (1992) and Ingram et al. (2010). The delta-lognormal approach adjusts for changes in catch rate data due to factors other than abundance changes so a relative index of abundance is obtained. This relative index is the result of 2 GLMs: the first estimates the probability of a positive observation, and the second models the catch rate when the catch is positive. The final standardized index is the product of the back-transformed effects of the two models. The SE and CV (100·SD/mean) were estimated by a jackknife routine on factors with more than two positive observations. Models were run using the R programming environment (R Development Core Team 2012). Because a large suite of potential explanatory variables were available in the data set, we first used a stepwise regression model to determine which physical variables explained variation in Spanish Mackerel CPUE so they could be included in the delta-lognormal GLM. We used a forward stepwise regression model with the stopping rule set to minimum corrected Akaike information criteria (AICc) in JMP 9.0. The following variables were statistically significant and therefore incorporated into the delta-lognormal GLM to obtain a relative abundance by year: temperature, salinity, depth, set type (parallel or perpendicular), and location (sampling area).
The spatiotemporal distribution of Spanish Mackerel CPUE within Alabama's coastal waters was further analyzed with nonparametric Wilcoxon tests followed by nonparametric multiple comparison tests because the data did not conform to the assumptions of parametric statistics, even after transformation. Spanish Mackerel CPUE was also analyzed for correlations with environmental variables as well as CPUE of other species (finfish and shrimp) captured during the survey period. Length data were used to assess the overall length-frequency distribution and to compare average lengths among years and areas with two separate one-way ANOVAs. When statistical significance was present, Tukey honestly significant difference post hoc tests were used to further describe the differences. Lastly, to assess the potential age structure of the local population, ages were assigned to Spanish Mackerel using the von Bertalanffy growth function with parameters suggested by SEDAR (2013; t0 = −0.5, K = 0.61, L∞ = 560.1). Any fish longer than L∞ for GOM Spanish Mackerel (n = 10 of 325 fish) were assigned the oldest recorded age-class (11 years; SEDAR 2013).
RESULTS
Long-Term Harvest Trends
Throughout the GOM, commercial landings of Spanish Mackerel generally increased from 1950 through the mid to late 1970s, but have since declined (Figure 2a). Gulf of Mexico recreational landings were comparable to commercial landings from 1981 to the mid-1990s, but have since surpassed commercial landings. Gulf of Mexico recreational landings have been slightly increasing, although variable, since 2005 (Figure 2a). In contrast with the Gulfwide commercial landings, Alabama's commercial landings have increased nearly 500% in the past 20 years (Figure 2b). Alabama's recreational landings, on the other hand, have been rather consistent since the mid-1980s. From 1995 to 1999, Alabama's recreational landings were comparable to commercial landings; however, they have been lower than commercial landings since 2000 (Figure 2b).
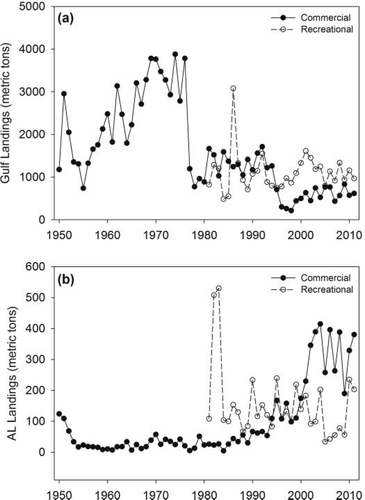
Commercial and recreational harvest trends for Spanish Mackerel (a) in the entire Gulf of Mexico and (b) in the state of Alabama.
Recent Trends in Alabama's Bays and Sounds
A total of 1,142 small mesh gill nets were set between 2001 and 2011, 104 of which caught Spanish Mackerel. Catch per unit effort ranged from 0 to 20 fish/h, but was clearly skewed toward lower catches: 98% of CPUEs were ≤ 2 fish/h. The highest proportion of positive sets (gill-net sets that captured Spanish Mackerel) occurred in the early years of the data set (2001–2003), beginning with 17% positive sets in 2001 and decreasing to 10–11% in 2002 and 2003. Post-2003, the proportion of positive gill-net sets rarely exceeded 9%. After standardizing the Spanish Mackerel CPUE with the delta-lognormal GLM, relative abundance appeared to remain fairly stable from 2001 to 2011 (Figure 3).
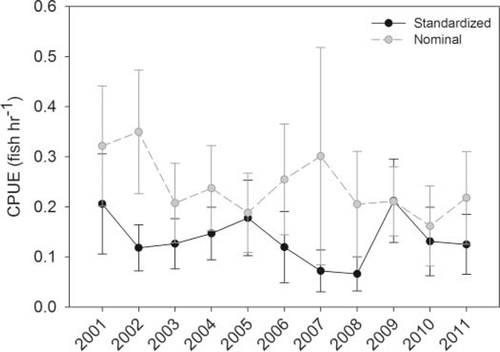
Nominal and standardized Spanish Mackerel CPUE (fish/h) for the fishery-independent gill-net survey conducted in 2001–2011.
Positive Spanish Mackerel catches were present in all sampled areas of Alabama's coastal waters, with higher CPUEs in Mississippi Sound and southern Mobile Bay (χ2 = 21.57, df = 3, p = 0.0001; 1, 4). Spanish Mackerel catches also occurred over wide ranges in environmental parameters: water temperatures of 15.5–34.0°C, salinities of 0–31‰, dissolved oxygen levels of 2.8–10.8 mg/L, and depths of 1.8–9.0 m. When gill nets did capture Spanish Mackerel, CPUE and depth were positively correlated (Spearman's ρ = 0.198; P = 0.0435); however, CPUE was not correlated with any other environmental parameter (temperature, salinity, dissolved oxygen). Moreover, Spanish Mackerel CPUE was also not correlated with CPUE of 26 of the 29 other species captured in gill nets in Alabama's bays and sounds (Table 1).
| Species | Spearman ρ | P > |ρ| |
|---|---|---|
| Atlantic Sharpnose Shark Rhizoprionodon terraenovae | 0.866 | 0.333 |
| Atlantic Bumper Chloroscombrus chrysurus | 0.703 | 0.052 |
| Atlantic Croaker Micropogonias undulatus | 0.137 | 0.288 |
| Black Drum Pogonias cromis | 0.275 | 0.362 |
| Bighead Searobin Prionotus tribulus | 0.726 | 0.165 |
| Blacktip Shark Carcharhinus limbatus | 0.738 | 0.155 |
| Bluefish Pomatomus saltatrix | 0.345 | 0.098 |
| Bull Shark Carcharhinus leucas | 0.318 | 0.539 |
| Cownose Ray Rhinoptera bonasus | 0.696 | 0.125 |
| Crevalle Jack Caranx hippos | 0.052 | 0.860 |
| Florida Pompano Trachinotus carolinus | 0.333 | 0.667 |
| Gafftopsail Catfish Bagre marinus | 0.080 | 0.753 |
| Gray Snapper Lutjanus griseus | 0.359 | 0.553 |
| Gizzard Shad Dorosoma cepedianum | 0.155 | 0.648 |
| Harvestfish Peprilus alepidotus | 0.422 | 0.050 |
| Ladyfish Elops saurus | 0.127 | 0.435 |
| Leatherjack Oligoplites saurus | 0.459 | 0.156 |
| Gulf Menhaden Brevoortia patronus | 0.240 | 0.052 |
| Pigfish Orthopristis chrysoptera | 0.291 | 0.526 |
| Pinfish Lagodon rhomboides | 0.431 | 0.017 |
| Red Drum Sciaenops ocellatus | 0.822 | 0.007 |
| Scaled Sardine Harengula jaguana | 0.555 | 0.076 |
| Hardhead Catfish Ariopsis felis | 0.182 | 0.161 |
| Sheepshead Archosargus probatocephalus | 0.503 | 0.204 |
| Shrimp (multiple species) | 0.858 | 0.006 |
| Silver Perch Bairdiella chrysoura | –0.291 | 0.312 |
| Skipjack Herring Alosa chrysochloris | 0.283 | 0.538 |
| Southern Kingfish Menticirrhus americanus | 0.102 | 0.550 |
| Southern Flounder Paralichthys lethostigma | 0.179 | 0.558 |
Spanish Mackerel catches generally occurred March through November (Figure 5a), with relatively higher catch rates in the spring and fall. In north Mobile Bay (Figure 5b), catch rates were greatest August through October. In south Mobile Bay (Figure 5c), Spanish Mackerel occurred April through October, with higher catch rates in the fall. Spanish Mackerel were caught in Mississippi Sound (Figure 5d) March through November, with highest rates in April and September. Lastly, Perdido Bay (Figure 5e) had relatively low catch rates all year, with two apparent pulses of Spanish Mackerel in April and September.
Although CPUE was low overall, FLs were measured for 278 fish. Fork length ranged from 195 to 635 mm (Figure 6a), averaging 407.4 ± 4.9 mm, and was positively correlated with gill-net mesh size (Spearman's ρ = 0.511; P < 0.0001). Length distributions were normally distributed for most years (2003–2010); however, 2001 and 2002 had more larger fish and the distribution for 2011 was nearly bimodal (Figure 7). The average length of Spanish Mackerel differed among years (F = 5.6919, P < 0.0001; Figure 6b), with an increasing trend from 2001 to 2003, a decline from 2003 to 2007, then an increase in 2008 followed by another decline. In regards to sampling area, average length of individuals was similar in north and south Mobile Bay and Mississippi Sound, but significantly smaller in Perdido Bay (F = 4.652, P = 0.003; Figure 4b).
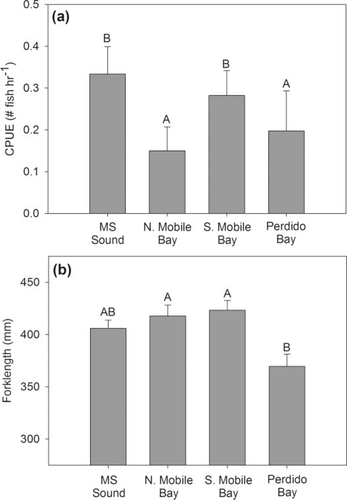
(a) Spatial variability, determined by nonparametric Wilcoxon tests, in Spanish Mackerel CPUE (fish/h) for the fishery-independent gill-net survey conducted by the Alabama Department of Conservation and Natural Resources, Marine Resources Division, during 2001–2011. (b) Spatial variability in average FL, determined by one-way ANOVA, for fish captured during the gill-net survey. Data are presented as mean ± SE.
Based on the assigned ages, the majority (89%) of the Spanish Mackerel collected by gill nets in this survey appear to be between 1 and 3 years old (Figure 6c). Cohorts can generally be tracked 3–5 years (e.g., age-0 in 2006 can be tracked through 2009), but the first and last years constituted a smaller proportion of the catch. For most years, age-2 fish were the dominant age-class, but a few years showed relatively strong age-1 classes: 2004–2007 and 2011.
DISCUSSION
Long-Term Harvest Trends in the Gulf of Mexico
Two different trends were visible in the Spanish Mackerel landings data from 1950 to 2011 for the GOM and Alabama. Commercial harvest in the GOM increased until the mid-1970s and then decreased, with lowest commercial harvests occurring after the mid-1990s. This is likely a result of state gill-net restrictions beginning in Texas, Louisiana, and Florida in the 1980s to mid-1990s. Contrary to the overall GOM commercial harvest, commercial harvest in Alabama was relatively stable through the mid-1990s but then increased dramatically. Alabama does not have a gill-net ban but rather places restrictions on net length and fishing licenses. The commercial gill-net fishery in Alabama has been landing 92–100% of Alabama's commercial harvest since 1990 and recently, Alabama's commercial landings have comprised nearly 60% of the entire GOM harvest.
With more than half the commercial harvest coming from one state with comparatively limited coastline, localized depletion may pose a serious problem if the fish resource cannot naturally replenish the area of localized depletion. For Spanish Mackerel, one essential need for management is characterizing movement patterns (SEDAR 2013) of adult fish that have recruited to the fishery. If recruitment of adult fish is more locally based, local exploitation effects would be of more concern than if the source of recruits is more Gulfwide. For example, localized (within one state) capture of a large proportion of the total commercial GOM landings could prove problematic if this species is locally recruited or moves on much smaller spatial scales (e.g., more onshore–offshore movements). An example of this can be seen in Florida before the gill-net restrictions of 1994 and Alabama after the restrictions in 1994, as Florida landings decreased dramatically and Alabama's landings comprise ca. 60% of the total commercial GOM landings. Based on the proposed migration pattern, it is possible that prior to Florida's gill-net restrictions Spanish Mackerel were harvested in Florida before reaching Alabama's coastal waters, hence the increase in Spanish Mackerel landings in Alabama post-1994. If recruitment to the states’ fisheries is from a larger spatial scale, extracted fish could be replaced from outside sources, possibly reducing some effects of heavy exploitation in specific locales. Based on the Spanish Mackerel's wide distribution and coastal migratory behaviors (Powell 1975; Collette et al. 1978; Sutherland and Fable 1980), presumably Spanish Mackerel should be able to replenish the area. Indeed, mitochondrial and nuclear DNA analyses suggest no differentiation between the GOM and the Atlantic (Buonaccorsi et al. 2001), suggesting nonlocalized recruitment to the fishery. Differences in hemoglobin phenotypes (Skow and Chittenden 1981), morphological characteristics (Collette and Russo 1984), and muscle enzyme loci (Nakamura 1987) have been reported, however, suggesting some restricted movement. Therefore, a better understanding of more localized movements is necessary to properly predict the effects of elevated harvest in Alabama.
Recreational landings, although variable, have remained relatively constant in the GOM and in Alabama, although an increasing trend is apparent since 2005 for Alabama's recreational harvest. This may be a result of fishermen shifting their targets to more inshore species as more restrictions are placed on offshore and reef fish species. Despite relatively constant recreational harvest in both Alabama and the GOM as a whole, the relationship between recreational and commercial harvests has changed since the mid-1990s. Recreational landings of Spanish Mackerel now exceed commercial landings in the GOM, but commercial landings exceed the recreational in Alabama. In this case, variations in gear type among states may provide some insight. Although gear type differences (i.e., gill net versus hook and line) may not necessarily lead to one fishery having greater harvest than another, this appears to be the case for the Spanish Mackerel fishery in Alabama. Where gill nets have either been banned or heavily restricted (Texas and Florida), commercial harvest may be expected to be lower than recreational harvests. Likewise, with commercial gill nets still in use in Alabama, it is not surprising that commercial harvests exceed recreational harvests.
Spatiotemporal Variation of Estuarine Habitat Use within Alabama
The relative abundance index for Spanish Mackerel in Alabama's coastal waters appears to remain relatively constant for 2001–2011, despite the magnitude of commercial landings in Alabama during this time. We did observe differences in catch rates among sampling areas, with generally higher rates in south Mobile Bay and Mississippi Sound, and lower rates in north Mobile Bay and Perdido Bay (1, 4). One possible explanation for this pattern is the salinity variation among areas. The northern reaches of Mobile Bay typically have lower salinities where freshwater, riverine influence is strong. Adult Spanish Mackerel presumably prefer more oceanic salinities (Godcharles and Murphy 1986), while juveniles can tolerate a wider range of salinities (less than 18‰, as noted by Springer and Woodburn 1960), so generally lower catch rates in areas of lower salinity are expected. (Note, however, that we did not find a significant effect of salinity on Spanish Mackerel catch rates). We also note that catch rates are generally lower along the northwestern shores of Mobile Bay, an area that tends to have lower salinity but also more industrialization and some armored shores (Douglass and Pickel 1999; Jones et al. 2009). Furthermore, relatively lower catch rates were also observed in Perdido Bay, and annual salinity regimes in Perdido Bay have been found to be similar to those in south Mobile Bay (Mareska, personal communication). This suggests the importance of other factors in driving Spanish Mackerel abundances and distributions; it does not appear that the variation in salinity alone drives the spatial variability in Spanish Mackerel catch patterns.
In fact, our results show that Spanish Mackerel use the entire estuary, regardless of salinity, and may be found in low-salinity areas near the river mouth (where salinities were recorded between 0‰ and 10‰). Large juvenile and adult Spanish Mackerel are not the only coastal migratory pelagic fish to be found near or within rivers—large juvenile and adult Atlantic Tripletail have been detected in the Ossabaw Sound estuary in Georgia from March through November when water temperatures were above 21°C, and they were observed as far as 33 km upstream (Streich et al. 2013). Furthermore, the Spanish Mackerel captured in this study were not all juvenile fish—sexually mature adult fish (up to 663 mm FL) were captured near the river mouth as well. One possibility for their presence in low-salinity areas may be foraging behavior. Previous studies have suggested the possibility of adults entering estuaries to feed (Collette and Nauen 1983) because Spanish Mackerel usually feed on estuary-dependent fish like menhaden and anchovies (Godcharles and Murphy 1986; Finucane et al. 1990). Interestingly, we did not find a correlation between catch rates of Spanish Mackerel and any known major prey items, including potential estuarine-dependent prey species. This indicates that habitat selection by Spanish Mackerel may not be based on prey availability. An alternative explanation for Spanish Mackerel presence in estuaries involves spawning behavior and nursery grounds. Exact spawning locations of Spanish Mackerel in the GOM are not known, but larvae have been found in inshore waters of the GOM (McEachran et al. 1980). Spanish Mackerel are batch spawners reported to have a protracted spawning season encompassing the summer months (McEachran et al. 1980; Collins and Stender 1987), and large adults were captured within the estuaries during this time. This suggests that the potential exists for some spawning to possibly take place near or within estuaries, although we do not have direct evidence for this. The presence of juveniles within Alabama's bays and sounds aligns with Godcharles and Murphy's (1986) suggestion that some young of the year may use estuary nursery grounds. Tagging Spanish Mackerel in highly stratified estuaries like Mobile Bay could provide a more thorough understanding of their residence times and use of estuarine habitats.
The temporal trend seen in Spanish Mackerel catches in Alabama's coastal waters is expected based on their migration pattern. Spanish Mackerel generally overwinter off southern Florida (Godcharles and Murphy 1986) but also off the east coast of Mexico (Sutherland and Fable 1980). In the eastern GOM, mackerel move northward in the late winter and early spring and continue northwest to the northern GOM coast (Sutherland and Fable 1980). They are most abundant in the northern GOM in the spring through fall (Powell 1975), and then migrate back southward in the fall (Sutherland and Fable 1980). Indeed, this is the general pattern observed in catch rates: highest in the spring through fall (Figure 5). Catch rates in Perdido Bay, with two distinct pulses in April and September, best reflect the migration pulses. It appears that Spanish Mackerel are caught in Perdido Bay during the migration north (in spring) and south (in fall), but spend the spring and summer months elsewhere. Mississippi Sound had a similar pattern, with relatively higher catch rates in the spring and fall, but Spanish Mackerel were also caught consistently throughout the spring and summer. North and south Mobile Bay had highest catch rates in the late summer and fall (August through October), but lacked the pulse in the spring. The higher catch rates in the fall coincide with seasonal low river discharge from June through November (Figure 5b; Kim and Park 2012), resulting in relatively higher salinities near the river mouth.

Temporal variability, determined by Wilcoxon tests, in Spanish Mackerel CPUE (fish/h) among (a) all sampling areas and (b–e) within each area. River discharge data are overlaid on (b) and was calculated as in Kim and Park (2012).
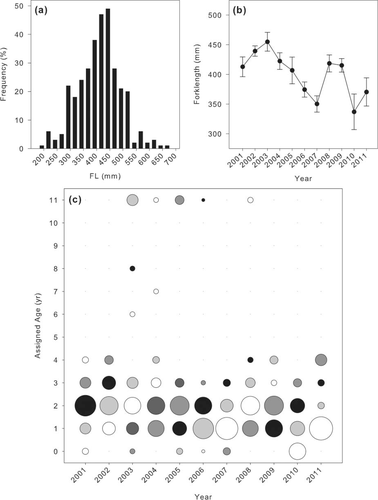
(a) The length-frequency distribution of captured Spanish Mackerel in Alabama's coastal waters, (b) the trend of average FL over time, and (c) the relative proportion of assumed ages (based on von Bertalanffy growth function parameters per SEDAR 2013) within each year (all bubbles within a given year sum to 100%). Black, white, and gray shading are aids for progressively tracking a cohort through time.
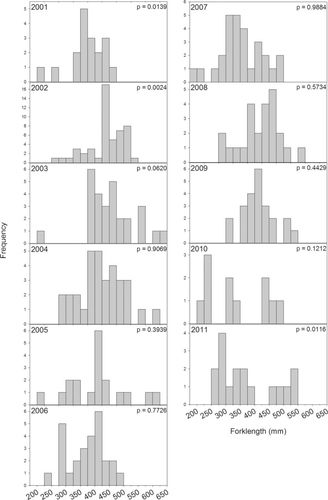
Length-frequency distributions of Spanish Mackerel captured by gill net in Alabama's coastal waters from 2001 to 2011. Note the scale differences in y-axes. P-values in the upper-right corner refer to the results of a Shapiro–Wilk goodness-of-fit test against a normal distribution.
The size of captured Spanish Mackerel also varied spatially and temporally. Average FL differed among sites, with fish captured in Perdido Bay smaller than those in the other three areas. A couple of different explanations may hold for this observation. First, the fish in Perdido Bay may be smaller because of food availability. Perdido Bay receives 36 times less freshwater (USEPA 1999) than Mobile Bay, and if Spanish Mackerel feed on estuarine-dependent species, Perdido Bay may not have prey abundances as high as Mobile Bay due to less freshwater input. Second, Spanish Mackerel from Perdido Bay may be smaller because they are younger (especially in the fall when these fish could have been spawned in the same year's spring or summer). Juvenile Spanish Mackerel have been captured entering estuaries on flood tides (Peters and Schmidt 1997), and Godcharles and Murphy (1986) suggested that some young of the year use estuaries as nursery grounds, so it is possible that there is an abundance of younger, smaller fish in Perdido Bay.
The average length of Spanish Mackerel captured in Alabama's coastal waters showed a marked decline from 2003 to 2007 (Figure 6), but has otherwise been fairly stable. An increase in catch of smaller, younger age-1 fish in those years compared with others (Figure 6c) is likely the cause of decline in average length. The same phenomenon occurred in 2010 and 2011 as well, where a significant proportion of the catch was age-1 fish as opposed to age-2 fish, which would result in smaller FLs. The reason for capturing more age-1 fish in these particular years is unclear.
The age composition of Spanish Mackerel captured in Alabama's coastal waters during the fishery-independent gill-net survey suggests that most captured fish are between 1 and 3 years old. This is consistent with previous reports that suggest approximately 90% of the population is between 0 and 4 years (Palmer et al. 2012). The small range in lengths, and therefore assumed ages, may be due to gear selectivity; in this case, the gear would be selecting for fish lengths that correspond to ages 1–3. However, we do not believe that gear selectivity is resulting in the small length–age range in this study. The gill net used in this study had variable-sized mesh that should allow capture of small and large fish. Although only the small mesh net was analyzed herein, the large mesh net did not capture many Spanish Mackerel (<10% of the catch). It is more likely that there are not many large fish in the population and, therefore, they are not captured by this survey.
CONCLUSIONS
The Spanish Mackerel is a coastal migratory pelagic species, typically completing its lifecycle off open beach waters and moving across boundaries of multiple management levels. There has been a lack of full understanding of regional movement patterns and the response of the fishery to localized heavy harvest as well as the extent of habitat specialist versus generalist characteristics of the species, all of which are important considerations for management. Here we report full estuarine habitat use by adult Spanish Mackerel; adults will travel into the northernmost reaches of the Mobile Bay estuary (near the river mouth) during the fall when river discharge is relatively low, suggesting a habitat generalist strategy in estuaries. In Alabama, most of the Spanish Mackerel harvested by the state's gill-net survey appear to be between the ages of 1 and 3, well below the maximum reported in the GOM (11 years). At the present time, recruitment to Alabama's fishery seems to be able to keep up with current harvests, as suggested by the stable standardized index of abundance within Alabama's waters over the past 10 years, despite the dramatic increase in commercial fishing harvests.
ACKNOWLEDGMENTS
The authors would like to thank the staff at the Alabama Department of Conservation and Natural Resources, Marine Resources Division, for the years of gill-net sampling and data collection in Alabama's coastal waters, as well as E. J. Dick (NMFS, Santa Cruz) for providing the R code for calculating a standardized index of abundance. We also thank the editor and two anonymous reviewers for comments that improved the quality of this manuscript.




