Does Whirling Disease Mediate Hybridization between a Native and Nonnative Trout?
Abstract
The spread of nonnative species over the last century has profoundly altered freshwater ecosystems, resulting in novel species assemblages. Interactions between nonnative species may alter their impacts on native species, yet few studies have addressed multispecies interactions. The spread of whirling disease, caused by the nonnative parasite Myxobolus cerebralis, has generated declines in wild trout populations across western North America. Westslope Cutthroat Trout Oncorhynchus clarkii lewisi in the northern Rocky Mountains are threatened by hybridization with introduced Rainbow Trout O. mykiss. Rainbow Trout are more susceptible to whirling disease than Cutthroat Trout and may be more vulnerable due to differences in spawning location. We hypothesized that the presence of whirling disease in a stream would (1) reduce levels of introgressive hybridization at the site scale and (2) limit the size of the hybrid zone at the whole-stream scale. We measured levels of introgression and the spatial extent of hybridization between Rainbow Trout and Westslope Cutthroat Trout in four disease-positive streams and six disease-negative streams within the Blackfoot River basin of Montana. In addition to disease status, we considered habitat quality, stream slope, distance from the confluence, temperature, and elevation. Whirling disease presence was not associated with either the level of introgression at a site or the size of the hybrid zone. Temperature, elevation, and stream slope were all influential in determining levels of introgression at the site scale. Stream slope was the most influential factor determining the size of the hybrid zone, as longer, steeper streams contained smaller hybrid zones. Stream slope is a driver of many habitat characteristics that may provide refuge from invasive species in the coming decades. Although the multispecies interactions examined in this study did not alter the impacts of invasion on native species, community assemblages will continue to change with the spread of nonnative species, requiring continued assessment to determine their impacts on native species.
Received May 15, 2014; accepted January 7, 2015
Freshwater ecosystems are highly imperiled, exhibiting the greatest number of threatened and endangered species as well as the highest rates of species extinction worldwide (Pimm and Raven 1995; Ricciardi and Rasmussen 1999; Burkhead 2012). Anthropogenic degradation of habitat has caused fragmentation of aquatic populations, loss of critical habitat, and subsequent loss of biodiversity (Dudgeon et al. 2006). In addition, both climate change and human activities are facilitating the spread of nonnative species (including but not limited to protozoans, plants, and animals) across freshwater ecosystems at alarming rates (Walther et al. 2002; Strayer and Dudgeon 2010). This spread of nonnative species creates novel species assemblages in which nonnative species interact with native species as well as other nonnatives. Novel interactions between multiple nonnatives may have varied effects on the viability of native species across the landscape. Nonnatives may negatively impact one another through competition or predation (Simberloff and Von Holle 1999; Braks et al. 2004) or through commensal or mutualistic interactions that increase the spread and intensity of their individual impacts (Ricciardi 2001). In some cases, the presence of multiple nonnatives may amplify the negative impacts on native species (Ross et al. 2004; Johnson et al. 2009). Furthermore, the occurrence and impact of nonnative species may differ across the landscape due to natural variation in abiotic conditions that favor certain species over others. Nonetheless, interactions between nonnative species are explored less frequently than the negative impacts of nonnative species on the native community (Simberloff and Von Holle 1999). We need to better understand how interactions between multiple nonnative species affect the native species community, how landscape factors may alter interactions between native and nonnative species, and how such interactions influence our conservation strategies (Lindenmayer et al. 2008; Hobbs et al. 2009).
The persistence of native Westslope Cutthroat Trout Oncorhynchus clarkii lewisi is threatened by loss of habitat from human activities and by hybridization with nonnative Rainbow Trout O. mykiss (Shepard et al. 2005). Studies have shown that the proportion of Rainbow Trout alleles present in a population sample (i.e., introgression) varies with distance from the source of Rainbow Trout alleles and is altered by tributary characteristics (e.g., elevation, flow regime, and temperature) and human disturbances (e.g., stocking of Rainbow Trout, logging, agricultural practices, and grazing practices; Hitt et al. 2003; Weigel et al. 2003; Heath et al. 2009; Muhlfeld et al. 2009b; Rasmussen et al. 2010; Kovach et al. 2011). However, research has not explored whether the presence of additional nonnative species may alter the landscape-level gradients associated with hybridization both within and among watersheds.
The unintentional spread of parasites has impacted wildlife populations globally, and differences between native and nonnative species in terms of their vulnerability to disease may be a mechanism influencing the spread of nonnative species (Moyle and Light 1996; Peterson and Fausch 2003). For example, Fausch (2007) hypothesized that whirling disease (WD) has limited the invasion of Rainbow Trout in the United Kingdom. The causative agent of WD is the myxosporean parasite Myxobolus cerebralis, which is endemic to Eastern Europe. Human-facilitated transport of infected fish after World War II contributed to the global spread of the parasite, causing outbreaks that have decimated wild fish populations across multiple continents (Bartholomew and Reno 2002). Myxobolus cerebralis requires two hosts to complete its life cycle: oligochaete worms Tubifex spp. and salmonid fishes (Hedrick and El-Matbouli 2002). Young fish with substantial skeletal cartilage (<9 weeks posthatch) are the most susceptible to infection (Ryce et al. 2005). Infection can lead to substantial cartilage destruction, resulting in whirled swimming patterns, skeletal deformities, reduced growth rates, and death (MacConnell and Vincent 2002).
Salmonid species in the genus Oncorhynchus appear to be among the most susceptible to WD, but susceptibility varies depending on the species. Vincent (2002) found that Rainbow Trout suffered higher infection rates and severity than various Cutthroat Trout subspecies when exposed to WD in a laboratory setting. In many wild populations, Rainbow Trout may also be more vulnerable than Westslope Cutthroat Trout due to differences in their spawning location (Pierce et al. 2009). The rate of M. cerebralis infection decreases predictably in an upstream direction, presumably due to the reduction in habitat (i.e., slow-moving water with fine sediment) for the oligochaete hosts (De la Hoz and Budy 2004; Hallett and Bartholomew 2008), and Westslope Cutthroat Trout spawn higher in tributaries than Rainbow Trout (Pierce et al. 2007, 2009; Muhlfeld et al. 2009a; Buehrens et al. 2013). Thus, in addition to lower susceptibility, Westslope Cutthroat Trout likely experience a lower level of exposure to M. cerebralis than Rainbow Trout.
Research has yet to explore the susceptibility of Rainbow Trout × Westslope Cutthroat Trout hybrids to WD, but hybridization between other salmonid species has been examined. Wagner et al. (2002) found that F1 hybrids of moderately susceptible Brook Trout Salvelinus fontinalis and mildly susceptible Lake Trout Salvelinus namaycush showed intermediate susceptibility compared with parental strains. Therefore, Rainbow Trout × Westslope Cutthroat Trout hybrids may be more susceptible to WD than pure Westslope Cutthroat Trout due to their Rainbow Trout ancestry. Hybrids may also be more vulnerable than Westslope Cutthroat Trout due to differences in spawning habitat and rearing of hybrids in warmer, lower-elevation areas (Muhlfeld et al. 2009a). If the hybrid offspring of Rainbow Trout and Westslope Cutthroat Trout are more susceptible to WD, then we would expect the presence of WD to reduce the survival of Rainbow Trout and hybrids and to subsequently alter the spatial patterns of introgression between the two species.
Our research objective was to determine whether WD is associated with introgressive hybridization between Westslope Cutthroat Trout and Rainbow Trout in streams of the Blackfoot River basin, west-central Montana. We focused on the following questions: (1) does WD interact with physical and environmental variables (e.g., elevation, temperature, stream slope, distance from the source of Rainbow Trout alleles, and habitat quality) to alter introgressive hybridization between Rainbow Trout and Westslope Cutthroat Trout at the site scale, and (2) how are these variables (landscape characteristics, habitat quality, and WD) associated with the spatial extent of introgression within a stream?
Overall, we expected introgression to decline with increases in elevation, distance from the confluence, and stream slope and to be lower in areas with higher habitat quality and cooler temperatures. We also expected the presence of WD in a stream to interact with landscape characteristics and habitat quality variables by increasing the effects of these variables on the level of introgression and the spatial extent of hybridization in disease-positive streams.
METHODS
Study Area
The Blackfoot River is a free-flowing, fifth-order tributary of the upper Columbia River and drains a 5,998-km2 watershed through 3,038 km of perennial streams. The river lies in west-central Montana and flows westward 212 river kilometers from the Continental Divide to its confluence with the Clark Fork River at Bonner, Montana. Beginning in 1902, Rainbow Trout were heavily stocked throughout Montana's streams and rivers, including the Blackfoot River basin. Detailed records documenting the location and volume of stocking events throughout Montana were not well kept; however, the stocking of all trout in streams and rivers ceased in 1974 to encourage wild fish production (Zachheim 2006).
Our study focused on 10 tributaries located in the lower half of the Blackfoot River basin (Figure 1) where nonnative Rainbow Trout are present and express both resident and fluvial life histories (Pierce et al. 2009). Native Westslope Cutthroat Trout are present basinwide but are most prevalent in streams of the middle to upper elevations, such as the upper reaches of tributaries to the main stem, and throughout the upper basin (Pierce et al. 2008). Despite intensive stocking of Rainbow Trout throughout the Blackfoot River watershed through the early 1970s, hybridization between Rainbow Trout and Westslope Cutthroat Trout has been detected most commonly in the lower watershed but rarely in the upper basin (Pierce et al. 2005, 2008). Other salmonid species present in the basin include native Bull Trout Salvelinus confluentus and Mountain Whitefish Prosopium williamsoni as well as nonnative Brook Trout and Brown Trout Salmo trutta. Whirling disease was detected in the Blackfoot River basin during initial testing in 1998 (Pierce and Podner 2006), 4 years after Montana's first documented outbreak in the Madison River (Vincent 1996).
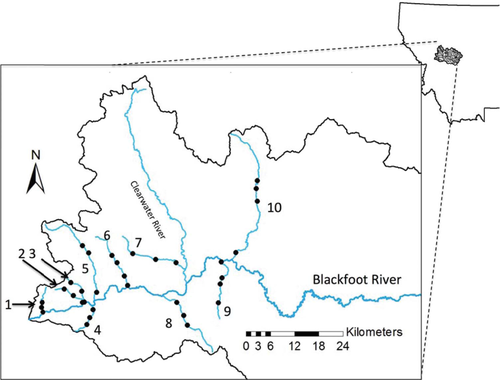
Sampling locations used to evaluate introgression between Rainbow Trout and Westslope Cutthroat Trout in the Blackfoot River basin of west-central Montana. Points indicate sampling locations; numbers correspond to the map codes defined in Table 1. The lowermost site in each stream was also the sentinel cage exposure site for whirling disease assessment.
| Map code | Stream | Stream_km | Elevation (m) | Delta elevation (m) | Slope | Bank_zone | Temp_zone (°C) | Temp range | Total stream length (km) |
|---|---|---|---|---|---|---|---|---|---|
| Disease-negative streams | |||||||||
| 1 | Johnson Gulch | 2.89 | 1,177 | 171 | 0.14 | 11.33 | 10.2 | 9.8–10.7 | 7.7 |
| 2 | West Twin Creek | 5.22 | 1,424 | 388 | 0.112 | 11.5 | 10.5 | 8.8–12.2 | 8.9 |
| 3 | East Twin Creek | 6.58 | 1,429 | 391 | 0.084 | 10.75 | 10.7 | 9.7–11.7 | 8.9 |
| 4 | Bear Creek | 5.4 | 1,350 | 311 | 0.079 | 9.33 | 11.3 | 10–12.6 | 9.1 |
| 5 | Gold Creek | 14.7 | 1,344 | 299 | 0.036 | 10.0 | 11.9 | 10.7–13.2 | 29.2 |
| 6 | Blanchard Creek | 8.7 | 1,433 | 261 | 0.028 | 9.25 | 13.7 | 12.5–15 | 20.7 |
| Disease-positive streams | |||||||||
| 7 | Belmont Creek | 7.18 | 1,330 | 263 | 0.046 | 8.5 | 12.0 | 11.4–12.5 | 16.5 |
| 8 | Elk Creek | 11.63 | 1,275 | 158 | 0.028 | 10.0 | 13.9 | 12.5–15.4 | 21.8 |
| 9 | Chamberlain Creek | 5.44 | 1,292 | 105 | 0.039 | 7.5 | 12.8 | 11.7–13.9 | 16.9 |
| 10 | Monture Creek | 26.42 | 1,341 | 140 | 0.023 | 9.0 | 11.6 | 9.5–13.7 | 46.0 |
Stream Selection
For the last two decades, Montana Fish, Wildlife, and Parks (MFWP) has conducted sentinel cage exposures with hatchery Rainbow Trout to monitor the presence and severity of WD in streams throughout the Blackfoot River basin. Exposures in the present study followed the methods of Pierce et al. (2009); we included all basin-fed streams in the watershed that (1) were sites of known hybridization between Rainbow Trout and Westslope Cutthroat Trout, (2) were repeatedly monitored for WD within 4.5 km of the confluence (median distance = 0.7 km), and (3) were monitored for WD at least once between 2004 and 2008 (see Supplementary Table S.1 in the online version of this article; Pierce et al. 2001, 2008; Pierce and Podner 2006).
In our study, we assumed that a stream was disease-negative (i.e., WD was not present) if no infection was detected in sentinel-caged fish for all tests conducted in that stream. We categorized streams as disease-positive if at least 70% of caged fish had an infection severity greater than 3 on the MacConnell–Baldwin rating scale and if the mean infection severity for all exposed fish was higher than 3. This level of disease severity is considered high enough to influence fish survival and to have population-level effects based on laboratory experiments and case studies (Vincent 2002; Granath et al. 2007). For example, Granath et al. (2007) found that declines in wild Rainbow Trout were associated with increasing infection severity (>2.5) of trout held in sentinel cages throughout the drainage. Six disease-negative streams and four disease-positive streams met the criteria for inclusion in our study (i.e., streams with known hybridization between Oncorhynchus species and where repeated tests documented either no detection of WD or the presence of high-severity infection).
Site-Scale Data Collection
Within each stream, we sampled three to four locations between 2009 and 2011 to determine the level of introgression between Rainbow Trout and Westslope Cutthroat Trout (Figure 1; Table S.1). Two sites were sampled again in 2013 to increase the sample sizes. The lowermost sampling site in each stream corresponded to the location of sentinel cage exposures for that stream. Sites were spaced roughly 1.3–16.2 km apart (median = 3 km) in order to describe the longitudinal pattern of introgressive hybridization. The uppermost sampling site targeted areas where we expected to find little to no introgression between Rainbow Trout and Westslope Cutthroat Trout (i.e., <5% Rainbow Trout alleles within a sample of fish) based on phenotypic indicators and initial analyses of our genetic samples collected in 2009. If we did not achieve our goal during the first sampling visit, we returned to the watershed to sample a site further upstream. We used 5% introgression as a threshold for defining the end of the hybrid zone. This threshold allows for the occurrence of natural polymorphisms, which may otherwise alter the detection of nonhybridized populations in these systems (Allendorf et al. 2012), yet it is more conservative than the 10% threshold outlined for consideration as a conservation population under the Memorandum of Understanding and Conservation Agreement for Westslope Cutthroat Trout (Allendorf et al. 2001; MTFWP 2007).
At each site, we collected all Oncorhynchus species present by using a backpack electrofishing unit until (1) we obtained a sample size of 25 individuals, (2) sampling time exceeded 2.5 h, or (3) the sample reach exceeded 550 m. For each fish, we measured total length (mm), removed a tissue sample, and placed the sampled tissue in 95% ethanol for genetic analysis. For sites that were sampled in multiple years, we compared the genotypes of sampled individuals to ensure that the same individual was not represented more than once in our data set.
To assess habitat quality and other tributary characteristics known to influence hybridization at the site scale, we recorded elevation, distance from the confluence (Stream_km), stream slope, temperature, and bank stability at each site (Figure 2). Elevation and Stream_km were measured in ArcMap (ESRI 2010) using U.S. Geological Survey digital elevation map layers and the Montana Streams layer maintained by MFWP (//nris.mt.gov/gis/). We calculated stream slope as the change in elevation from the confluence to the site divided by Stream_km. We obtained mean August temperatures for each site from the NorWeST Stream Temp interactive map (www.sciencebase.gov/flexviewer/NorWeST/).
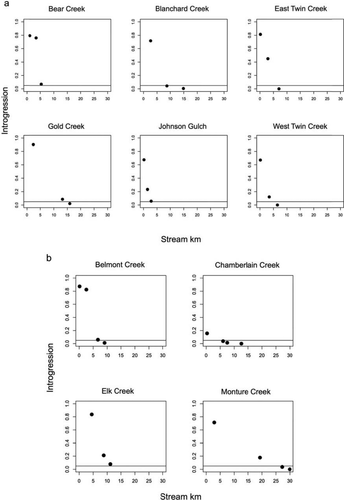
Level of introgression (proportion of Rainbow Trout alleles in a sample) versus distance upstream from the confluence (Stream_km) for all Blackfoot River basin sites sampled in (a) disease-negative streams and (b) disease-positive streams. The horizontal line represents 5% introgression of Westslope Cutthroat Trout with Rainbow Trout.
We measured bank stability at each sampling site to quantify measures of habitat quality, as grazing is the primary anthropogenic riparian disturbance in this watershed. Previous studies have found a positive association between introgression with Rainbow Trout alleles and either logging activity or road density (Heath et al. 2009; Muhlfeld et al. 2009b). Authors of those studies attributed the pattern to stream alterations resulting from human activities and infrastructure, including increases in fine-sediment deposition and changes in hydrologic regimes. Although logging and the presence of roads are common disturbances throughout the Blackfoot River basin, agriculture and grazing practices are the most widespread disturbances in the watershed, often causing riparian degradation and loss of bank stability (Pierce et al. 2013). To assess bank stability and animal impacts, we used rating systems for vegetation cover, bank stabilization by rock, and animal damage (wild or domestic) as outlined by Stevenson and Mills (1999) and summed the ratings across the three categories to obtain a single variable for bank stability at a site.
Genetic Analysis
To ensure that our data were representative of the population at a given site, we analyzed all fish between 70 and 230 mm TL. We did not sample fish smaller than 70 mm because these individuals are typically young of the year and it is difficult to obtain a sufficient tissue sample without lethal effects. Based on the expert opinion of local biologists, we did not analyze fish larger than 230 mm to minimize the likelihood of including migratory fish that were using the site as summer habitat.
All genetic analyses were conducted at the Conservation Genetics Laboratory, University of Montana, Missoula.
Stream-Scale Data Collection
To examine which variables best predicted the size of the hybrid zone (i.e., the distance from the confluence at which introgression declined to 5%), we first estimated the Stream_km where introgression would equal 5% by fitting a linear regression (introgression versus Stream_km) between the two sites where introgression was closest to 5%. When possible, we interpolated between two adjacent sampling sites that tested above and below this threshold. In a given stream, if we were unable to obtain a sample with introgression below 5%, we extrapolated and used the two adjacent sites with introgression levels that were closest to 5%. We did not fit a logistic or linear regression using all data points for a given stream because this would have provided a model that best fit all points rather than more accurately pinpointing the location where population-level introgression reached 5%.
To obtain a slope measure that was independent of the response variable (i.e., distance from the upper end of the hybrid zone to the confluence) and that represented the stream scale, we used the slope of the entire stream as a variable for predicting the size of the hybrid zone. We calculated whole-stream slope as the change in elevation over the distance from the headwaters of the main stem to the confluence using data layers in ArcMap as described above (ESRI 2010). We did not include elevation in this analysis because the change in elevation throughout the hybrid zone was incorporated into our slope parameter, and we had no prediction for how elevation at the end of the hybrid zone would influence the overall size of the zone.
To obtain a measure of temperature corresponding to the hybrid zone in each stream, we used the average of temperatures at the confluence and at the upper limit of the hybrid zone (Temp_zone), as obtained from the NorWeST Stream Temp interactive map for each stream. To quantify bank stability within the hybrid zone, we averaged the scores for this variable across all sampling sites within the hybrid zone to obtain a single estimate of bank stability (Bank_zone).
Statistical Analyses
Which variables are associated with introgression at a site?—To evaluate whether WD presence influenced the level of introgression at a given site, we standardized variables and used a generalized linear mixed regression model with a logit-link function. The WD, temperature (Temp), elevation (Elev), Stream_km, slope, and bank stability (Bank) variables were included as fixed effects. We evaluated a one-way interaction between WD and all other fixed effects because we hypothesized that the presence of WD would alter the influence of these variables on introgression. Because there were multiple sites within a stream, “Stream” was included as a random effect in every model.
We calculated the variance inflation factor (VIF) for each fixed effect to assess multicollinearity. The VIF quantifies the degree to which the variance increases as a result of multicollinearity with other variables in an ordinary least-squares regression model. For example, a VIF of 10 for a single variable would mean that the variance of the parameter estimate is 10 times larger than it would be if that variable was completely uncorrelated with all others in the model (Montgomery et al. 2012). This assessment of multicollinearity allows a model to include variables that might be correlated but that have differing relationships with the response variable. If the VIF was high (>5) for a given combination of variables, we created several global models so that variables with a high degree of multicollinearity could be included in separate models.
Elevation was used to predict temperature in the NorWeST Stream Temp models. As a result, these two variables were inherently confounded, so we created separate global models to avoid including these variables together in the same model. Preliminary analysis of models that included both WD and Slope or both Temp and Stream_km revealed that these two sets of variables were significantly correlated. Specifically, results from models that included these combinations of variables suggested associations between these variables and introgression that were not observed in the raw data, thus indicating that multicollinearity between predictor variables was affecting the model results (Montgomery et al. 2012). A Welch's t-test revealed that slope was significantly shallower in streams where WD was present (P < 0.001; Figure 2a). We also found that Temp was significantly correlated with Stream_km (r = −0.51, P < 0.01).
-
Model structure A: Introgression ∼ WD × (Elev + Stream_km + Bank) + (1|Stream),
-
Model structure B: Introgression ∼ WD × (Temp + Bank) + (1|Stream),
-
Model structure C: Introgression ∼ Slope + Elev + Stream_km + Bank + (1|Stream),
and
-
Model structure D: Introgression ∼ Slope + Temp + Bank + (1|Stream),
Model selection was based on Akaike's information criterion (AIC; Burnham and Anderson 2002) and error around parameter estimates. The top model from a given subset of models was the one with the lowest AIC value that also had significant parameter estimates (α = 0.05) for all interaction terms as well as for any base variables not included in the interaction terms (Arnold 2010). Error structure was calculated by using the same methods for all four model sets. As a result, we were able to select the best overall model as the model with the lowest AIC value (Burnham and Anderson 2002).
-
Model structure Zone_A: Hybrid Zone Size ∼ WD + Slope,
-
Model structure Zone_B: Hybrid Zone Size ∼ WD + Temp_zone,
and
-
Model structure Zone_C: Hybrid Zone Size ∼ WD + Bank_zone.
We compared all possible subsets of these global models. In each case, the top model was the model with the lowest value of AIC corrected for small sample size (AICc) and in which parameter estimates for all variables were significantly different from zero (α = 0.05). The overall best model describing hybrid zone size from these three global model structures was the top model that explained the highest proportion of variance as indicated by the R2 value.
All statistical analyses were conducted in R (R Development Core Team 2012), and the following R packages were used: lme4, Hmisc, HH, and MASS (Venables and Ripley 2002; Bates et al. 2014; Harrell 2014; Heiberger 2014).
RESULTS
Quantification of Introgressive Hybridization and the Size of the Hybrid Zone
We obtained a minimum of 25 tissue samples at all sites except for five sites in three different streams (West Twin, Monture, and Gold creeks); we were unable to achieve a sample size of 25 fish at those sites due to low densities of Westslope Cutthroat Trout (0.03–0.09 fish/m; Table S.1). At four of the five sites, our sample sizes ranged from 20 to 24 individuals, but in one case (West Twin Creek site WT3), we obtained only 13 individuals over three sampling years. However, all of the fish captured at that site were nonhybridized. Based on the 10 diagnostic markers, we still had a 92.6% probability of detecting as little as 1% population admixture (Kanda et al. 2002) with these 13 samples, so the WT3 site was included in the analyses as a nonhybridized site.
To estimate the size of the hybrid zone, we generally interpolated between the highest-elevation sites in each stream (Table 1). The exceptions were Johnson Gulch, Bear Creek, and Elk Creek, where we did not obtain a sample with population-level introgression less than 5% (Figure 3). In Elk Creek, we detected 8.6% admixture at the highest-elevation site (EK3), but Westslope Cutthroat Trout were not present at the next site upstream. The highest-elevation site sampled in Bear Creek (BR3) had 7.7% admixture, but we only obtained two fish at the next site upstream of BR3. In Johnson Gulch, the uppermost site (JG2) had 6.3% admixture, and we were not able to access higher sites. For these three streams, we estimated hybrid zone size by extrapolating from the two highest-elevation sampling sites.
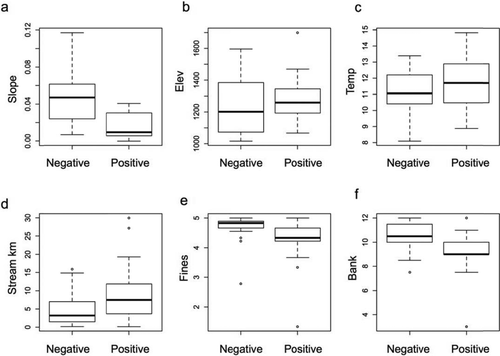
Box-and-whisker plots showing the range of site-scale variables between whirling disease-negative and disease-positive streams sampled in the Blackfoot River basin: (a) slope, (b) elevation (m), (c) temperature (°C), (d) distance upstream from the confluence (Stream_km), (e) fine-sediment deposition (1 = >75% of surface covered by fines; 2 = 50–75% coverage; 3 = 25–50% coverage; 4 = 5–25% coverage; 5 = 0–5% coverage), and (f) bank stability ranking (as summed across rankings of animal damage, bank stabilization by rock, and vegetation cover; 3 = low stability and 12 = high stability) across all sites. Whiskers represent 1.5 times the interquartile range.
Across all sites, individuals that tested positive for Rainbow Trout alleles appeared to be backcross hybrids. No F1 hybrid individuals (i.e., fish with 50% admixture that were heterozygous for Rainbow Trout and Westslope Cutthroat Trout alleles at each diagnostic locus) and no pure Rainbow Trout were observed in this study.
Which Site-Scale Variables Are Associated with Introgression?
In each evaluation of global model structures (A–D) for explaining the level of introgression, the top model reported had the lowest AIC value and met our criteria for significant parameter estimates (outlined in Methods); in each case, no other models had AIC values that were within 2 points of the top model's AIC value (Table 2).
| Model structure | Description | k | Log-likelihood | AIC | Uninformative parameters |
|---|---|---|---|---|---|
| A | WD × (Stream_km + Elev + Bank) + (1|Stream) | 9 | −292.6 | 603.1 | WD |
| B | WD × (Temp) + Bank + (1|Stream) | 6 | −1,100.0 | 2,212.1 | WD |
| C | Slope + Elev + Stream_km + Bank + (1|Stream) | 6 | −291.1 | 594.1 | None |
| D | Slope + Temp + Bank + (1|Stream) | 5 | −1,220.5 | 2,451.0 | None |
Results of top model from evaluation of global model A indicated that lower levels of introgression at a particular site were associated with increasing Stream_km, higher elevation, and higher-quality habitat as indicated by greater bank stability (Table 3). The presence of WD increased the effects of elevation and bank stability but decreased the effect of Stream_km. Although the association between introgression and Stream_km was still negative in the presence of WD, the effect of Stream_km was reduced.
| Top model A | Top model B | Top model C | Top model D | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | Estimate | SE | P-value | |||
| Fixed effects | |||||||||||||||
| Intercept | −2.13 | 0.35 | <0.0001 | −1.49 | 0.54 | <0.01 | −2.00 | 0.31 | <0.0001 | −1.58 | 0.73 | <0.05 | |||
| WD | −0.10 | 0.36 | 0.79 | −1.04 | 0.55 | 0.06 | |||||||||
| Slope | −0.35 | 0.05 | <0.0001 | −0.32 | 0.04 | <0.001 | |||||||||
| Elev | −2.39 | 0.09 | <0.0001 | −2.52 | 0.08 | <0.0001 | |||||||||
| Stream_km | −1.04 | 0.11 | <0.0001 | −0.73 | 0.07 | <0.0001 | |||||||||
| Temp | 2.48 | 0.05 | <0.0001 | 2.45 | 0.05 | <0.001 | |||||||||
| Bank | −0.45 | 0.04 | <0.0001 | −0.53 | 0.04 | −0.52 | 0.03 | <0.0001 | −0.40 | 0.04 | <0.001 | ||||
| WD × Elev | −0.40 | 0.09 | <0.0001 | ||||||||||||
| WD × Stream_km | 0.41 | 0.10 | <0.0001 | ||||||||||||
| WD × Temp | −0.75 | 0.04 | <0.0001 | ||||||||||||
| WD × Bank | −0.12 | 0.03619 | <0.01 | ||||||||||||
| Variance of random effects | |||||||||||||||
| Stream | 1.17 | 2.87 | 0.98 | 4.51 | |||||||||||
Results of the top model from evaluation of global model B suggested that introgression at a site declined with greater bank stability and increased with higher temperatures. In the presence of WD, the relationship between temperature and introgression was reduced.
Results of the top model from evaluation of global model C indicated that introgression declined with increases in stream slope, elevation, Stream_km, and bank stability. Similarly, the top model based on the evaluation of global model D indicated that introgression was lower at sites with steeper slopes, higher bank stability, and cooler temperatures.
All four top models from the evaluations of model structures A–D were consistent in their results, indicating lower introgression at sites that were cooler, were further from the confluence, were at a higher elevation, and had higher bank stability. The top model from the global model C evaluation had the lowest AIC value of all the top models, so it was considered the overall best model for explaining the level of introgression at a site.
What Influences the Spatial Extent of Introgression within a Stream?
For the Zone_A model structure, the top model for predicting the size of the hybrid zone contained only stream slope (Table 4) and explained nearly 40% of the variation observed in the response variable for this data set. No other Zone_A models had AICc values that were within 2 points of the top model's value. For the Zone_B and Zone_C model structures, two models’ AICc values were within 2 points of the top models’ AICc values. In each case, the model that contained WD alone had the lowest AICc value. Surprisingly, the presence of WD was positively associated with hybrid zone size, although the association was not significant. The second-best model (i.e., within 2 AICc points of the top model) for the Zone_B structure displayed a positive association between Temp_zone and hybrid zone size, whereas the second-best model for Zone_C had a negative association between Bank_zone and hybrid zone size. Across all three global model structures, the top model from the Zone_A evaluation explained the greatest proportion of variance in the size of the hybrid zone and was the only model in which parameter estimates were significantly different from zero. As a result, no other model was considered for selection as the overall best-fit model for predicting hybrid zone size.
| Model | Description | k | Multiple R2 | Estimate | SE | P-value | Negative log-likelihood |
|---|---|---|---|---|---|---|---|
| Zone_A1 | Slope | 2 | 0.39 | −4.35 | 1.93 | 0.05 | −30.63 |
| Zone_B1; Zone_C1 | WD | 2 | 0.16 | 2.80 | 2.26 | 0.26 | −32.23 |
| Zone_B2 | Temp_zone | 2 | 0.05 | 1.53 | 2.41 | 0.54 | −32.84 |
| Zone_C2 | Bank_zone | 2 | 0.06 | −1.77 | 2.39 | 0.48 | −32.75 |
DISCUSSION
In our study, WD was not included in the best overall model for examining associations with hybrid zone size at the whole-stream scale. In analyzing introgression at the site scale, the effects of distance from the confluence (Stream_km) and temperature were reduced in the presence of WD, contrary to our expectations. There are several possible reasons why we did not observe patterns that supported our hypotheses. First, studies on WD susceptibility have only been performed on fish of hatchery origin. It is possible that (1) the susceptibility of wild Rainbow Trout and hybrids does not differ from that of pure, wild Westslope Cutthroat Trout; or (2) the difference is so weak that there is not an observable effect on the overall levels of introgressive hybridization between the two species. Miller and Vincent (2008) found evidence that a wild strain of Rainbow Trout from Harrison Lake, Montana, underwent rapid natural selection for resistance to WD. It is also possible that the wild populations in this study experienced a similar process of selection after the disease initially emerged in the Blackfoot River basin. The presence of WD may have had an impact on hybridization between Rainbow Trout and Westslope Cutthroat Trout soon after M. cerebralis was introduced, but evolution of resistance to WD and subsequent recovery of Rainbow Trout and hybrid populations prior to our study could have obscured any apparent evidence of this interaction.
Stream slope repeatedly emerged as a strong predictor of hybridization between Rainbow Trout and Westslope Cutthroat Trout: introgression was lower at higher-gradient sites, and hybrid zones were smaller in shorter, more steeply sloping streams. In the Blackfoot River basin, landscape-level estimates of valley slope are correlated with stream slope at the site scale and serve as a good predictor of both fine-sediment loads and WD severity at a site (Pierce et al. 2008). Specifically, less-steep sites had higher disease severity in sentinel cage studies, presumably due to the higher loads of fine sediment, which provide habitat for the disease's alternative host, T. tubifex. In addition, less-steep, disease-positive streams registered some of highest instances of disease severity in sentinel cage studies conducted throughout the Blackfoot River basin. Monture Creek had the lowest stream slope and the largest hybrid zone in our data set. At our lowest-elevation sampling site in Monture Creek (2.9 km upstream from the confluence), we observed introgressive hybridization that was close to 80%. Over 90% of sentinel-caged fish at that location had mean grade infections exceeding 3 (MacConnell–Baldwin rating scale) in 2005, 2006, 2007, and 2009 (Pierce et al. 2008, 2012). If WD was impacting wild Rainbow Trout and hybrid populations in a manner that reduced introgressive hybridization with Westslope Cutthroat Trout, we would expect a stream like Monture Creek to have a much smaller hybrid zone and lower levels of introgressive hybridization at sites known to induce high-severity infection. These data highlight stream slope as a comprehensive variable influencing T. tubifex habitat and distribution and thus the presence of WD.
Slope may also influence habitat characteristics associated with the current distribution and spawning success of Rainbow Trout and Westslope Cutthroat Trout. The association of stream slope with introgression at the site scale may be explained by differences in life history between Rainbow Trout and Westslope Cutthroat Trout. Multiple studies comparing habitat and occupancy of Rainbow Trout, Cutthroat Trout, and hybrids have found that Rainbow Trout and hybrids occupy lower-gradient sections of stream in areas where Rainbow Trout have been introduced as well as in areas where the two species are naturally sympatric (Hitt et al. 2003; Weigel et al. 2003; Buehrens et al. 2013). Muhlfeld et al. (2014) found that the expansion of hybridization from 1978 to 2008 in the upper Flathead River basin of northwestern Montana was strongly correlated with decreases in May precipitation. Rainbow Trout and hybrids tend to spawn earlier in the spring as runoff associated with snowmelt increases and peaks, whereas Westslope Cutthroat Trout spawn later in the spring as high flows subside (Muhlfeld et al. 2009a; Corsi et al. 2013). Muhlfeld et al. (2014) attributed the expansion of hybridization in the Flathead River in part to lower spring runoff, which would result in reduced scouring of redds and disturbance of newly emerged juveniles. In this context, we would expect streams with steeper slopes to have faster, more turbulent flows during spring spates. Redds and juveniles in these more steeply sloping streams would likely experience more disturbance from spring flow events, and Rainbow Trout and hybrids would be particularly susceptible to these disturbances due to their timing of spawning and emergence. A similar association between stream slope and salmonid community composition has also been observed in mountain streams of the Pacific Northwest. Montgomery et al. (1999) found that on a basin scale, steeper streams favored salmonid species whose spawn timing resulted in egg incubation and juvenile emergence periods that were offset from the most severe flood events. Although fine-scale habitat characteristics certainly play a role, our results support landscape-level geomorphology as a factor determining salmonid community composition across both native and nonnative species.
As expected, introgression was lower at higher-elevation sites in this study. Elevation generally displayed a negative correlation with introgressive hybridization between our two focal species, as has been observed in other studies (Hitt et al. 2003; Bennett et al. 2010; Rasmussen et al. 2010; Yau and Taylor 2013). For example, in a study of the upper Oldman River (Alberta, Canada), Rasmussen et al. (2010) reported that the proportion of Rainbow Trout alleles present in a population decreased exponentially with increases in site elevation; introgression greater than 5% was only observed at 1 out of 16 sites with elevations of 1,471 m or higher (median introgression = 1%; maximum elevation = 1,722 m). Hitt et al. (2003) found a similar transition to nonhybridized Westslope Cutthroat Trout at roughly 1,450 m in the upper Flathead River basin. Among a total of 12 sites at elevations above 1,300 m, we observed only one site where introgression was greater than 5% (median introgression for those 12 sites = 1.4%; maximum elevation = 1,699 m). Results of these studies suggest that an elevational threshold exists for the persistence of Rainbow Trout and hybrids. Mechanistically, however, the associations are likely the result of changes in habitat and climate that follow an elevational gradient.
In previous studies, temperature was negatively associated with both the occurrence of hybridization and the degree of introgression at the site scale (Muhlfeld et al. 2009b; Yau and Taylor 2013). We identified similar associations in our data set, but it should be noted that temperature was not present in either the top model for explaining the level of introgression at a site or the top model for explaining the overall size of the hybrid zone. Elevation and distance from the confluence were significant predictors determining the level of introgression at a site, and temperature was associated with both of those predictor variables. Temperature did not emerge as a significant predictor of site-scale introgression or hybrid zone size at the whole-stream scale, suggesting that generalized summertime temperature metrics alone (e.g., mean August temperature from the NorWeST Stream Temp models) may not represent the key limiting climatic conditions that affect hybridization at a whole-stream scale. For example, Fausch et al. (2001) found that success of Rainbow Trout invasions in Colorado, the southern Appalachians, and Japan were strongly influenced by flow regime. Bennett et al. (2010) reported that tributaries to the upper Kootenay River (British Columbia) that were located in warmer and drier biogeoclimatic zones were associated with higher levels of introgression between Westslope Cutthroat Trout and introduced Rainbow Trout. In a study on physiological performance, Rasmussen et al. (2012) suggested that the metabolic needs of individuals with Rainbow Trout ancestry (both pure and introgressed) are not met in less-productive, high-elevation habitat, thereby allowing Westslope Cutthroat Trout to dominate those areas. These studies provide evidence that broader climatic variables incorporating aspects of temperature, precipitation, and flow regime serve as better predictors of hybridization between Rainbow Trout and Westslope Cutthroat Trout than temperature alone.
Distance from the confluence is associated with temperature and elevation, but it may also address variation in introgression associated with propagule pressure that is not represented in measures of temperature or elevation. Bennett et al. (2010) determined that introgression between Rainbow Trout and Westslope Cutthroat Trout at sites in the upper Kootenay River was strongly influenced by propagule pressure—a variable they defined as a combination of historical stocking intensity and distance from the stocking locations. Although the entire main stem of the Blackfoot River was heavily stocked with Rainbow Trout in the 20th century (1902–1974; Zachheim 2006), detailed stocking records are not available for this watershed (R. Pierce, personal observation). Consistent with other studies, we found that introgression decreased with increasing distance from the confluence, as main river sections are currently considered the putative source of Rainbow Trout alleles in the Blackfoot River basin and other river basins (Hitt et al. 2003; Weigel et al. 2003; Muhlfeld et al. 2009b; Rasmussen et al. 2010; Kovach et al. 2011).
Similar to the findings of Muhlfeld et al. (2009b), we observed that sites with higher habitat quality generally had lower levels of introgression. In our study streams, introgression tended to increase with disturbances that erode streambanks and increase rates of sedimentation, such as hoof shearing, lack of vigorous riparian vegetation, and bank stabilization by rocks. Such disturbances also tend to increase stream temperatures (which may favor Rainbow Trout and hybrids), an association that was observed in this data set as well as in other studies of the Blackfoot River basin (Pierce et al. 2013). An additional mechanism for this trend could be related to early development: embryos of Rainbow Trout and hybrids may have a higher tolerance for fine sediment than embryos of Westslope Cutthroat Trout. Sowden and Power (1985) did not find a negative association between nonnative Rainbow Trout survival and fine sediments (<2 mm in diameter) in a tributary to Lake Erie in Ontario, Canada. Conversely, fry emergence success declined significantly in redds with proportion of fine sediment less than 6.5 mm for Westslope Cutthroat Trout (Weaver and Fraley 1993) or less than 4 mm for Bonneville Cutthroat Trout O. clarkii utah (Budy et al. 2012). In short, habitat alterations resulting in an increased proportion of smaller substrate and fine sediment may inhibit the spawning success of Westslope Cutthroat Trout. However, more-direct studies of the effects of fine sediment and preferred spawning gravels for Rainbow Trout and hybrids are needed to better address this hypothesis.
In our study, hybridization between native Westslope Cutthroat Trout and invasive Rainbow Trout was not influenced by multispecies interactions that included an introduced parasite. Researchers have predicted that climate change will warm stream temperatures, resulting in reduced habitat for native trout and increased habitat for nonnative trout throughout the Rocky Mountains (Williams et al. 2009; Wenger et al. 2011). Similarly, human activities and climate change are expected to cause further expansion of wildlife diseases and to alter host–pathogen interactions (Daszak et al. 2001; Fuller et al. 2012; Gallana et al. 2013).
Cutthroat Trout inhabit some of the highest-gradient streams of all salmonids and often occupy reaches where no other fish are present (Bozek and Hubert 1992; Paul and Post 2001; Quist and Hubert 2004; Rasmussen et al. 2010; D'Angelo and Muhlfeld 2013). Geomorphic characteristics (e.g., stream slope) may limit species expansion in certain types of stream, such as high-gradient, high-elevation tributaries. Biologists should incorporate geomorphic variables in addition to variables like temperature and precipitation when outlining their expectations for community composition and native species conservation in the coming decades. Once hybridization has occurred, habitat restoration efforts alone cannot remove Rainbow Trout alleles from a population. Additionally, restoration cannot change the broad-scale geomorphic characteristics of habitat, such as stream slope. However, results from this study may help to prioritize areas where restoration aimed at maintaining temperatures, bank stability, and hydrologic regimes that favor Westslope Cutthroat Trout could reduce the likelihood of Rainbow Trout invasion.
As community assemblages continue to change, we must continually evaluate the effects of biotic interactions across communities and across landscapes. Interactions between various nonnative species could either control or facilitate their invasions. Knowledge of how nonnative species interact with each other and with native species in the communities and habitats they invade will assist managers in developing and prioritizing conservation action strategies for the long-term protection of native species in the wild.
ACKNOWLEDGMENTS
We thank W. Lowe, M. Schwartz, D. Patterson, and C. Muhlfeld for feedback on drafts of this work. We also acknowledge our funding sources for this study, including the National Science Foundation's Graduate Research Fellowship Program (DGE-0809127), the National Science Foundation's Integrative Graduate Education and Research Traineeship Program (DGE-0504628), the Westslope Chapter of Trout Unlimited, and the Winston Fly Rod Company. We are grateful to Craig Podner, Bradford Balis, and Julia Clymer for help in collecting samples and to Sally Painter, Angela Lodmell, and Taylor Wilcox for assistance with processing genetic samples in the laboratory. Finally, we thank the many private landowners of the Blackfoot Valley who allowed us to access many of our study sites through their properties.




