Significance of Anticardiolipin Antibodies on Short and Long Term Allograft Survival and Function following Kidney Transplantation
Abstract
The significance of anticardiolipin antibodies (ACAs) prior to renal transplantation is unclear. We studied a cohort of 337 patients who underwent renal transplantation from 1996 to 2001. Follow-up continued until allograft loss, patient death or 31 December 2002. The primary outcome was a composite endpoint of death-censored allograft loss or a 25% reduction in estimated glomerular filtration rate (GFR) from 1-month post-transplant. Secondary outcomes were allograft loss, a 25% reduction in GFR, acute rejection and creatinine at 1 year. IgG and IgM ACA titers were positive (≥15) in 18.1% of recipients. There were no significant differences at baseline between recipients, except coumadin therapy in those with positive ACA titers (20% vs. 7.4%). Post-transplant, there was no increase in the primary outcome in ACA-positive patients, even after adjustment for anticoagulation with coumadin (HR = 1.42 [0.68, 2.96]). There was no difference in secondary outcomes between those with or without positive titers. Two of five patients with very high titers (>50) who were not anticoagulated had early graft loss. A positive ACA titer prior to kidney transplantation was not associated with inferior renal outcomes after transplantation, although more research is required to address the prognostic significance of very high ACA titers.
Introduction
Inherited hypercoagulable states, such as those attributed to factor V Leiden, the prothrombin G20210A polymorphism and the thermolabile variant of methylene tetrahydrofolate reductase enzyme, have been associated with poor outcomes following kidney transplantation (1–3). This may be a consequence of enhanced microvascular and/or macrovascular thrombosis of graft vessels. Acquired thrombophilia, specifically the presence of anitphospholipid antibodies (APA), may also predispose to adverse outcomes after transplantation (4), but this association is less clear.
There are three major classes of APA, including anticardiolipin antibodies (ACA), anti-β2-glycoprotein-1 and the lupus anticoagulant (5). ACA are by far the most common, and their prevalence in the end stage renal disease (ESRD) population is considerably higher than in healthy controls. Various published series have reported ACA in 10–30% of dialysis patients, compared to 1–8% in the general population (4–7). It is unclear, however, whether these ACA are thrombogenic, or simply represent an epiphenomenon. For example, APA may frequently be detected in the setting of infection, but most studies have found no association with thrombophilia (8,9). In the dialysis population, the published data does not convincingly link APA with vascular access thrombosis (10,11).
Small retrospective studies have shown that APA are associated with a higher incidence of transplant artery and vein thrombosis early post-transplantation in the setting of systemic lupus erythematosis (SLE) or the primary antiphospholipid syndrome (APS) (12–15). The effect of APA on long term graft survival in these patients is unknown.
Patients with lupus or primary APS constitute a minority of all ESRD patients with a positive APA, and the significance of a positive pre-transplant APA in patients without SLE or primary APS is unclear. A single previously published report that attempted to address this question was a small retrospective case-control study (16). Furthermore, no studies to date have assessed long term graft survival and function in patients with positive APA measured prior to transplant. Because of the limited data concerning this potentially treatable condition, we investigated the impact of positive anticardiolipin antibody titers determined in ESRD patients prior to transplantation on the short and long term outcomes after kidney transplantation.
Methods
Study design
This retrospective observational cohort study consisted of 337 of the 361 patients who received a kidney transplant between 1 January 1996 and 31 December 2001 at Massachusetts General Hospital (MGH), in whom ACA was measured prior to transplant.
Data Collection
Demographic and clinical data was gathered from the medical records. Creatinine measures were taken at 1, 3, 6 and 12 months, and then yearly until the end of follow-up. Follow-up continued until allograft loss, patient death or end of follow-up on 31 December 2002.
Transplant operation and patient management
The individual surgeons and nephrologists caring for the patients dictated perioperative and long-term management. In general, beginning in 1997, the majority of patients (85%) were treated with triple immunosuppression consisting of a calcineurin inhibitor, steroids and mycophenolate moefetil. Recipients of cadaveric organs typically received antibody induction therapy (60%). Prior to 1997, azathioprine was used rather than mycophenolate moefetil, and induction therapy for recipients of cadaveric organs was not standard; 42 transplants (11%) were performed prior to 1997. Sirolimus therapy was used in only 3% of recipients.
In the majority of cases, when a patient was receiving pre-transplant anticoagulation, post-transplant anticoagulation was also given. However, in 10 recipients, anticoagulation was not given post-transplant. In some instances, patients not on pre-transplant anticoagulation were started post-transplant. No study protocol existed to guide these decisions, which were made by the treating physicians. Post-transplant anti-coagulation was performed more frequently in ACA positive recipients (21.8%) compared to ACA negative recipients (7.0%). In those patients who received coumadin after transplant, long-term anticoagulation was prescribed, except in five patients. These patients had elevated ACA titers but no history of the antiphospholipid syndrome, and anticoagulation was stopped within 1 month.
Definitions
The antiphospholipid syndrome was defined as previously reported, requiring the presence of at least one clinical criteria and at least one laboratory criteria as elaborated in the International Consensus Statement (at least one clinical episode of thrombosis combined with a positive APA) (17). Delayed graft function was defined as the transient requirement for dialysis after the transplant operation. Acute rejection was verified by biopsy in all cases, and classified as acute cellular rejection, or acute humoral rejection (18). Acute humoral rejection was defined histologically by the presence of neutrophils in the glomerular and peritubular capillaries, and by C4d deposition in the peritubular capillaries (19). Allograft loss was defined as the resumption of chronic dialysis before the end of the study period. Patients who died with a functioning allograft were censored. Glomerular filtration rate (GFR) was estimated by the Jelliffe-1 equation (CrCl =[98 - 16 ×[age - 20]/20]/SCr, then multiplied by 0.90 if female) for calculation of creatinine clearance. This equation has been validated for use in renal transplant recipients (20). Baseline renal function after transplant was defined by the creatinine at 1-month post-transplant.
Measurement of exposures
Since 1996, testing for ACA became a standard part of the pre-transplant work up at MGH. A single value for each test obtained prior to transplant was recorded to determine exposure status. If the test was done more than once prior to transplantation, the value obtained nearest to the transplant date was used. Measurement of anticardiolipin IgG and IgM antibodies was performed at MGH using a commercially available kit from INOVA Diagnostic Inc (San Diego, CA). The test employs an enzyme-linked immunosorbant assay (ELISA) performed in a 96 well plate, and an alkaline phosphatase colorimetric reaction for photometeric readout at OD405. The units GLA and MLA are generic IgG and IgM phospholipid units, respectively. A cutoff of ≥15 GLA or MLA was used to define a positive test. This cutoff represents >5 SD above the mean of normal healthy controls.
Outcomes
The primary endpoint was a composite of death-censored allograft loss and 25% reduction in estimated GFR. The secondary endpoints were (1) death-censored allograft loss, (2) a 25% reduction in estimated GFR, (3) acute rejection and (4) creatinine at 1 year.
Statistical analysis
The primary outcome was analyzed by Kaplan-Meyer survival curve with the log-rank test, and adjusted analysis was performed using Cox proportional hazards regression. The hazards ratio and 95% confidence limits was used as the primary measure of association. The Chi-square and Wilcoxon Rank Sum tests were used to compare binomial and continuous data, respectively. A p-value ≤0.05 was considered significant. Given 337 eligible subjects, the total number of primary endpoints (n = 59), and the prevalence of positive ACA titers in our cohort, we had 80% power to detect a two-fold increased risk of the primary endpoint among ACA positive recipients, comparable to the 2.5-fold higher odds of early graft loss in the earlier study (16).
Institutional Review
The MGH Institutional Review Board approved this study.
Results
Three hundred and sixty-one kidney transplants were performed from January 1996 through December 2001. Glomerulonephritis was the major cause of ESRD, reported in 131 patients (35.2%). ESRD was also attributed to diabetes (21.3%), polycystic disease (10.5%), obstruction or reflux (7.8%), hypertension (5.8%), hereditary nephritis (4.4%) and other (8.0%). The etiology of ESRD was unknown in 21 subjects (5.8%). Pre-transplant ACA IgG and IgM were measured in 337 patients. Sixty-one (18.1%) had a positive ACA titer (25 were positive for IgG, 27 were positive for IgM and 9 were positive for both). Of the patients with a positive ACA, five had a prior clotting history and thus met the criteria for APS prior to transplantation. These subjects were included in the analysis. Three patients had clotting histories, negative ACA, but a positive lupus anticoagulant, and thus were classified as having the APS.
Pre-transplant characteristics, stratified by ACA status, are shown in Table 1. Patients with negative and positive ACA did not differ with respect to age, sex, diabetes, hypertension, transplant type, transplant number, time on dialysis, degree of HLA mismatch, PRA and HCV antibody status. They did differ with respect to coumadin therapy; 11/55 (20%) of patients in the ACA positive group were on coumadin, compared to 18/243 (7.4%) in the ACA negative group.
| Covariate | ACA negative n = 276 (81.8%) | ACA positive n = 61 (18.2%) | p-value1 |
|---|---|---|---|
| Age (years) | 45.0 ± 13.2 | 44.4 ± 15.3 | 0.75 |
| Male sex | 169/276 (61.2) | 36/61 (59.0) | 0.77 |
| Hypertension | 238/275 (86.6) | 47/61 (77.1) | 0.08 |
| Diabetes | 74/274 (27.0) | 19/61 (31.2) | 0.53 |
| Type of transplant | 0.682 | ||
| Cadaveric donor | 139/275 (50.5) | 32/61 (52.5) | |
| Living related donor | 104/275 (37.8) | 20/61 (32.8) | |
| Living unrelated donor | 32/275 (11.6) | 9/61 (14.8) | |
| Re-transplant | 28/276 (10.1) | 11/61 (18.0) | 0.12 |
| Time on dialysis (months) | 25.5 ± 34.7 | 25.6 ± 24.2 | 0.99 |
| # HLA mismatches | 2.98 ± 2.03 | 3.1 ± 2.03 | 0.71 |
| Mean PRA | 8.9 ± 22.7 | 7.6 ± 21.0 | 0.67 |
| Coumadin therapy | 18/243 (7.4) | 11/55 (20.0) | 0.01 |
| HCV Ab positive | 21/272 (7.7) | 5/59 (8.5) | 0.20 |
- Results expressed as no./no. (%) or mean ± SD.
- 1p-values calculated using the Chi-Square test for categorical variables and the Wilcoxon rank sum test for continuous variables. Alpha set at 0.05.
- 2p-value for group comparison.
Patient characteristics after transplant are shown in Table 2. Patients with and without positive ACA titers were similar in terms of delayed graft function, 1-month serum creatinine and creatinine at the end of follow-up. Acute rejection rates were similar between the groups. Mean follow up time was shorter in patients with a positive titer (27.4 vs. 34.6 months, p = 0.02), and these patients were also more likely to be on coumadin (21.8% vs. 7.0%, p = 0.02).
| Covariate | ACA negative n = 276 (81.8%) | ACA positive n = 61 (18.2%) | p-value1 |
|---|---|---|---|
| Delayed graft function | 38/274 (13.9) | 6/60 (10.0) | 0.53 |
| Acute rejection | 0.562 | ||
| None | 148/276 (71.7) | 41/61 (67.2) | |
| Acute cellular rejection | 61/276 (22.1) | 14/61 (23.0) | |
| Acute humoral rejection | 17/276 (6.2) | 6/61 (9.8) | |
| 1-month serum creatinine | 1.6 ± 0.6 | 1.6 ± 0.9 | 0.32 |
| Mean creatinine at 1 year | 1.5 ± 1.0 | 1.5 ± 0.4 | 0.70 |
| Last creatinine | 1.4 ± 0.6 | 1.5 ± 0.4 | 0.95 |
| Mean follow up (months) | 34.6 ± 22.3 | 27.4 ± 16.7 | 0.02 |
| Coumadin therapy | 17/243 (7.0) | 12/55 (21.8) | 0.02 |
- Results expressed as no./no. (%) or mean ± SD.
- 1p-values calculated using the Chi-Square test for categorical variables and the Wilcoxon Rank Sum Test for continuous variables. Alpha set at 0.05.
- 2p-value for group comparison.
Occurrence of the primary outcome of combined death-censored allograft loss or 25% reduction in GFR was similar in the two groups (Figure 1, p = 0.27 by log-rank test). The unadjusted hazard ratio for the primary outcome was 1.43 (0.73, 2.80). There were no significant differences in secondary outcomes between the two groups. Mean creatinine at 1 year was 1.5 mg/dL in the ACA positive group and 1.5 mg/dL in the ACA negative group (p = 0.70). The HR for death-censored allograft loss alone, and 25% reduction in GFR alone were 1.62 (0.69, 3.80) and 1.09 (0.42, 2.87), respectively.
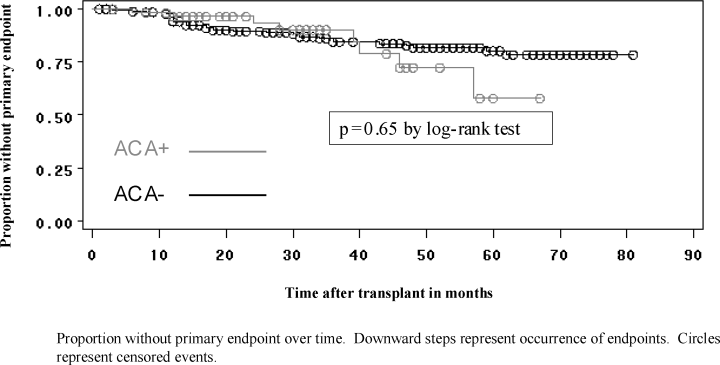
Kaplan–Meyer plot of primary endpoint of combined allograft loss or 25% reduction in estimated GFR. Proportion without primary endpoint over time. Downward steps represent occurrence of endpoints. Circles represent censored events.
Because anticoagulation with coumadin occurred more commonly in patients with positive ACA titers, we controlled for anticoagulation status using Cox proportional hazard regression (Table 3). The adjusted HR for the primary endpoint was 1.19 (0.55, 2.58) if coumadin was administered pre-transplant, and 1.42 (0.68, 2.96) if coumadin was given post-transplant.
| Unadjusted HR | Adjusted for coumadin (post-transplant) | |
|---|---|---|
| Allograft loss or 25% reduction in GFR1 | 1.43 (0.73, 2.80) | 1.42 (0.68, 2.96) |
| Allograft loss | 1.62 (0.69, 3.80) | 1.65 (0.69, 3.97) |
| 25% reduction in GFR | 1.09 (0.42, 2.87) | 1.01 (0.33, 3.11) |
- 1GFR, glomerular filtration rate.
To determine whether the type of ACA impacted the results, we constructed additional Cox proportional hazard models using IgG and IgM as separate exposures. No difference in the primary outcome was found between patients with a positive or negative IgG ACA (HR 1.20 [0.51, 2.82]), or between those with a positive or negative IgM ACA (HR 1.26 [0.50, 3.20]). To exclude the effect of absolute titer of ACA on the primary outcome, the analysis was repeated using ≥50 GLA or ≥50 MLA to define ACA positivity. The number of patients with titers above 50 was small (n = 9), and no significant difference in the primary outcome was found in multivariable modeling.
It is notable, however, that of the nine patients with ACA titers greater than 50 but no history of previous clotting episodes, four received some form of perioperative anticoagulation, whereas five did not. Two of the five patients (40%) who were not anticoagulated suffered graft loss from vascular thrombosis within the first month. One of these individuals had an IgM titer of 62.7 MLA and a normal IgG titer; the second individual had an IgG of 79.1 GLA and an IgM titer of 31.0 MLA. Six patients had less severe elevations of ACA, but that were still twice the upper limit of normal (titers 30–50). There were no graft losses, although four of the six did not receive anticoagulation. Among 46 recipients who had lower positive titers of ACA (15–30), only six received anticoagulation, yet there were no graft losses secondary to vascular thrombosis. Therefore, of the 61 ACA positive recipients, graft loss due to thrombosis occurred only among those with very high titer (i.e. ≥50) who were not anticoagulated.
Of the eight patients with the antiphospholipid syndrome, seven received perioperative anticoagulation; none of these patients lost their grafts in the first month post-transplant. The one patient with APS who did not receive perioperative anticoagulation lost the graft four days post-transplant due to transplant renal artery thrombosis.
Discussion
We conducted an observational cohort study to determine the effect of anticardiolipin antibodies on the outcome of kidney transplantation. The frequency of positive ACA titers in our cohort was similar to that in the general ESRD population (6,7), and rates of rejection and allograft survival were also similar to other reports (21). We found no difference in allograft survival or renal function during the study period between patients with or without positive ACA titers. Furthermore, rates of acute rejection and creatinine concentration at 1-year post-transplant, markers which are highly predictive of allograft outcome (22), were similar between the groups. The results were unchanged after adjusting for potential confounding by coumadin therapy. Although they represented a small fraction of the overall population of recipients with positive ACA titers, 40% of patients with very high titers (>50) who received no anticoagulation suffered early graft loss. We propose that low ACA titers in the ESRD population may represent an epiphenomenon without significant clinical ramifications, but that high titers of these antibodies may be associated with an increased risk of early graft loss.
The findings of this study contrast with the one other investigation designed to analyze the impact of ACAs in kidney transplant recipients in general, rather than focusing on the particular subgroups with SLE or primary APS (16). Wagenknecht et al. performed a hospital based case-control study of 110 subjects, and found that 57% of cases with early allograft loss (within 16 days of the transplant operation) had a positive APA titer, compared to 35% of controls. Several limitations temper the interpretation of that study. First, multivariable modeling to control for confounding factors was not performed. This is especially troublesome given that in their study, patients with graft loss compared to controls had higher PRA (22.8% vs. 11.8%) and a higher frequency of re-transplantation (30% vs. 13%). These factors are known to be associated with poor allograft outcomes (23,24), and their inclusion in a multivariable logistic regression model could potentially have nullified their findings with respect to APA status. Second, the blood from each case and control was subjected to 18 different tests for APA. This multiple testing presents a risk for increased false positive results and misclassification; although it is likely that this misclassification would be non-differential, it is impossible to say this with confidence given the differences in baseline characteristics between cases and controls. The multiple testing in that study probably also explains the prevalence of APA found (46%), which is approximately twice the prevalence typically described in the ESRD population. Third, it was unclear if any or all of the cases with allograft loss had the primary APS diagnosed pre-transplant, and which, if any, patients were anticoagulated. Finally, because only early graft loss was investigated (within 16 days of the transplant operation), no conclusions about the impact of APA on later outcomes could be elucidated. In our study, we found no such association with allograft loss, whether early or over longer periods of follow-up. The frequency of positive ACA titers in our study matches that which is expected from published data. It should be noted that the method of testing ACA in our study differed from the method used by Wagenknecht et al. Aside from coumadin therapy, we found no potential confounding factors that might have nullified an effect if one existed. Regression analysis was performed to adjust for coumadin therapy, and no effect of positive ACA titers was found.
To our knowledge, this is the first study to examine the impact of anticardiolipin antibodies on short- and long-term outcomes following kidney transplantation, and is the largest study of any kind to address this question. Our results imply that, in the absence of lupus, the antiphospholipid syndrome or very high titers of ACA, there may not be a deleterious association between anticardiolipin antibodies and renal allograft outcome. Rather, the presence of an elevated anticardiolipin antibody titer may simply reflect the high prevalence in the general ESRD population. The implication that in most ESRD patients, a positive APA represents an epiphenomenon, is comparable to data describing APA in patients with infections in which no convincing association with thrombophilia has been observed (8,9).
These data must be interpreted in the context of the study design. Because of excellent transplant outcomes, the number of patients reaching the primary (n = 59) and secondary (n = 33) endpoints was relatively small. It is possible, therefore, that in a much larger cohort a significant difference may have been found. This is especially true with respect to extremely high ACA titers (≥50), and class of ACA (IgG vs. IgM). Indeed, two of five patients with titers ≥50 who were not anticoagulated lost their allografts; therefore, from our data, it is not possible to conclude that high titers of ACA at the time of transplant have no prognostic implication. Clearly, more work is needed to resolve this important issue. Furthermore, treatment with anticoagulation was not standardized, allowing introduction of confounding by indication. Some patients with positive titers were anticoagulated, thus potentially biasing the results toward the null. The great majority of subjects that were anticoagulated, however, were known to have the antiphospholipid syndrome, and adjustment for coumadin use did not alter the results. Using medical records as the primary source for data collection introduces the potential for misclassification. Another source of misclassification was the use of a single pre-transplant ACA test to categorize patients as ACA positive and negative. Because of natural variation in ACA titers over time, it is possible that some ACA positive recipients were classified as ACA negative if their pre-transplant test was negative; this potential misclassification could have biased our study toward finding no association. However, our intention was to examine a single ACA measurement as part of a standard pre-transplant work-up, and thus we feel our results are valid, despite some misclassification. Only ACA were considered in defining exposure status, as complete testing for lupus anticoagulant and β2-glycoprotein-1 was not performed; however, ACA are the most common form of APA, and we do not feel that testing for these other two forms of APA would significantly alter the results. Finally, there is no single test for anticardiolipin antibody that is used uniformly in all centers across the country; thus, it is not possible to say with certainty that classification of subjects by an alternate assay would mirror the classification in this study. Despite these limitations, to our knowledge this is the largest study to date that analyzes the impact of positive anticardiolipin antibody titers in the general ESRD population on the outcome following kidney transplantation.
As a result of these findings and prior studies, we currently measure ACA titers in all renal transplant recipients before the transplant operation. If there is a history of lupus or prior thrombotic events (the APS), or if either the IgG or IgM ACA titer is greater than or equal to 50 GLA or MLA, then we anticoagulate with heparin in the perioperative period and convert patients to coumadin. If the patient does not have lupus or the APS, and the ACA titers are less than 50, we do not anticoagulate unless there is some other indication.
In conclusion, we found no significant differences overall in short- and long-term kidney transplant outcomes between patients with or without a positive ACA titer, although the significance of very high ACA titers in a subgroup of patients remains to be determined. These results do not contradict previous findings in patients with lupus or the antiphospholipid syndrome. Further prospective analyses of large cohorts of renal allograft recipients with ACA assays performed prior to transplantation are needed to confirm these results.




