Reduced Incidence of New-Onset Diabetes Mellitus after Renal Transplantation with 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitors (Statins)
Abstract
Statins have anti-inflammatory effects, modify endothelial function and improve peripheral insulin resistance. We hypothesized that statins influence the development of new-onset diabetes mellitus in renal transplant recipients. The records of all previously non-diabetic adults who received an allograft in Toronto between January 1, 1999 and December 31, 2001 were reviewed with follow-up through December 31, 2002. All patients receiving cyclosporine or tacrolimus, mycophenolate mofetil and prednisone were included. New-onset diabetes was diagnosed by the Canadian Diabetic Association criteria: fasting plasma glucose ≥7.0 mmol/L or 2-h postprandial glucose ≥11.1 mmol/L on more than two occasions. Statin use prior to diabetes development was recorded along with other variables. Cox proportional hazards models analyzing statin use as a time-dependent covariate were performed. Three hundred fourteen recipients met study criteria, of whom 129 received statins. New-onset diabetes incidence was 16% (n = 49). Statins (p = 0.0004, HR 0.238[0.109–0.524]) and ACE inhibitors/ARB (p = 0.01, HR 0.309[0.127–0.750]) were associated with decreased risk. Prednisone dose (p = 0.0001, HR 1.007[1.003–1.010] per 1 mg/d at 3 months), weight at transplant (p = 0.02, HR 1.022[1.003–1.042] per 1 kg), black ethnicity (p = 0.02, HR 1.230[1.023–1.480]) and age ≥45 years (p = 0.01, HR 2.226[1.162–4.261]) were associated with increased diabetes. Statin use is associated with reduced new-onset diabetes development after renal transplantation.
Introduction
New-onset diabetes after transplantation, previously called as post-transplant diabetes mellitus, is an important complication of renal transplantation (1,2) that leads to reduced graft function (3) and increased patient morbidity and mortality (4,5). Important risk factors include age, ethnic background, obesity, tacrolimus, prednisone and hepatitis C viral infection (2,6–10). Because of its immense impact on clinical outcomes, prevention of new-onset diabetes is highly desirable. However, new-onset diabetes remains quite prevalent (1) and may actually be increasing in incidence (11), with the current preventative strategies seemingly inadequate.
Therapy with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) is common after renal transplantation due to the favorable effect of statins on lipid levels (12) and cardiovascular outcome (13). In addition, statins have several pleiotropic effects unrelated to lipid levels including reduced inflammation, improved endothelial function (14) and improved insulin sensitivity (15). Statins may improve insulin resistance even in non-diabetic patients (16), and may actually have a protective effect against the development of diabetes in the general population (17). We therefore hypothesized that statins would demonstrate a protective effect against new-onset diabetes in renal transplant recipients (RTR), whose risk factors for diabetes are well defined (2,10) and somewhat different (6–9).
Methods
The nephrology clinics at the two institutions in Toronto, Canada performing adult renal transplant procedures (St. Michael's Hospital and Toronto General Hospital) together provide post-transplant care to approximately 2300 prevalent adult single-organ RTR. The records of the sub-population who received a renal allograft between January 1, 1999 and December 31, 2001 were reviewed for this study. Criteria for inclusion included the administration of a calcineurin-inhibitor (CNI) agent (cyclosporine or tacrolimus), mycophenolate mofetil (MMF) and prednisone as primary immunosuppressive therapy. Patients were excluded if there was a history of diabetic nephropathy, diabetes mellitus or glucose intolerance; if they were in pharmacotherapeutic studies involving other agents, had primary graft non-function, or received a multi-organ transplant including a double-kidney transplant. Employing a retrospective cohort study design, patients were classified into two groups on the basis of whether they had received statins or not. Additional information collected included recipient variables such as age, gender, ethnic origin, hepatitis C infection status, cause for ESRD and transplant centre; donor variables such as age and source; and peri-transplant variables including the cold ischemia time (CIT), HLA mismatch and most recent panel reactive antibody titre. Post-transplant factors included the occurrence of delayed graft function (DGF, defined as the requirement for dialysis in the first post-transplant week), biopsy-proven acute rejection based on Banff 97 criteria, and graft loss; the starting and cessation dates and dose for a statin, ACE inhibitor and ARB if any, the use of β-blockers and thiazides, renal function estimated by a serum creatinine (SCr) measurement at discharge and 1, 3, 6, 12 and 36 months post-transplant, as well as fasting lipid levels (including total cholesterol, HDL and LDL cholesterol and triglycerides) every 6 months, and dose (mg/d) of CNI, and prednisone at each point in time. If a CNI was switched or stopped post-transplant, the type of CNI at diabetes development, or in the case of those that did not develop diabetes, the last CNI used, was utilized in the CNI classification.
The primary outcome was the development of new-onset diabetes post-transplant in individuals previously without diabetes. New-onset diabetes was diagnosed by applying the current Canadian Diabetes Association (CDA) guidelines: a fasting plasma glucose (FPG) level ≥7.0 mmol/L and/or casual value (CPG) ≥11.1 mmol/L on at least two occasions, and in the absence of acute illness. Blood glucose levels are typically obtained with each blood work; weekly twice for the first 3 months, weekly thereafter to 9 months, once every 2 weeks to 12 months and monthly thereafter. The date of diabetes development was taken as the date of the first value consistent with the diagnosis. Resolution of new-onset diabetes was defined as the demonstration of normal FPG and/or CPG on more than two occasions in the absence of hypoglycemic therapy. Data were censored upon the development of new-onset diabetes or December 31, 2002, whichever was earlier.
Univariate comparisons were made by an unpaired Student's t-test, repeated-measures ANOVA, or chi-square analysis as appropriate. Since statins were introduced at varying points in time post-transplantation, their use was coded as a time-dependent covariate. Differences identified between the statin and non-statin groups were accounted for in the multivariate model. Furthermore, to explore and account for other potential confounding influences on the development of diabetes in this population, differences between the group that developed diabetes and the group that did not, in addition to statin use, were sought. Variables were then entered into a crude multivariate Cox proportional hazards model and a stepwise backward elimination method used to attain the final model. Goodness-of-fit was verified for each model. New-onset diabetes was the dependent variable for all analyses. All data are reported as mean ± SD unless otherwise specified, and for all associations a two-tailed p-value <0.05 was considered significant. SAS® software (Cary, NC) was used for all the analyses. The Research Ethics Boards at St. Michael's Hospital and Toronto General Hospital approved the protocol for this study.
Results
During the study period, 498 single-kidney allograft implantations were performed on 498 patients at the two institutions, of whom 346 received a CNI, MMF and prednisone as their primary immunosuppressive therapy. Of this subgroup, 314 (91%) had no prior history of diabetes and formed the group for this study. Seven patients had hepatitis C viral infection. The excluded patients included those on a CNI, MMF and prednisone (n = 32) who had pre-transplant diabetes, as well as those with primary graft non-function (n = 3) and those (n = 149) who participated in clinical trials involving the administration of other agents. The demographic characteristics of the latter (age 46.3 ± 13 years, 65% male, 74% Caucasian, weight at transplant 71.5 ± 15 kg, diabetes rate 21%) were not significantly different from the overall (n = 314) study group (p = NS for each), except that there were more cadaveric donors in the non-study group (56 vs. 43%, p = 0.003).
A total of 129 of 314 (41%) patients were given statins during the period of follow-up and prior to the development of diabetes. The time of introduction or re-introduction of a statin post-transplant is provided in Figure 1. Statin therapy was resumed in all patients on a statin prior to transplantation. Patient characteristics of the statin and non-statin groups are provided in Table 1. The statin group was older, more likely to be on cyclosporine and a β-blocker, and had a shorter CIT. The statin group also had higher fasting baseline total cholesterol, LDL cholesterol and triglyceride levels, which resolved by 12 months post-transplant, and a lower weight throughout the follow-up period (Table 2). However, there were no differences in cmulative steroid exposure.
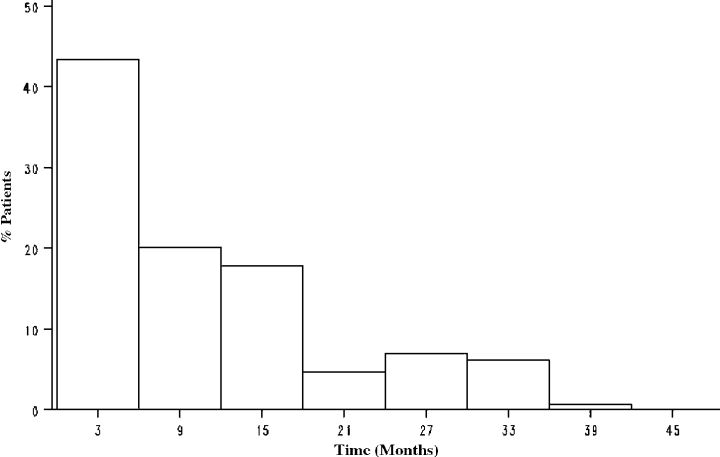
Frequency distri- bution of the time-to-introduction of a statin in the statin group.
| Characteristic | Statin group (n = 129) | Non-statin group (n = 185) | p-Value |
|---|---|---|---|
| Recipient age (years) | 46.7 ± 12 | 43.1 ± 13 | 0.02 |
| Recipient gender (% male) | 57.3 | 56.5 | 0.88 |
| Recipient ethnicity (%) | 0.97 | ||
| White | 64.3 | 67.8 | |
| Black | 9.3 | 8.9 | |
| Asian | 19.4 | 19.1 | |
| Other | 7.0 | 4.2 | |
| Cause of ESRD | 0.32 | ||
| Glomerulonephritis | 51.1 | 47.6 | |
| Hypertension | 6.2 | 9.5 | |
| Polycystic kidney disease | 20.9 | 16.1 | |
| Congenital anomalies | 15.5 | 14.9 | |
| Other/unknown | 6.3 | 11.9 | |
| Recipient weight at transplant (kg) | 69.9 ± 15 | 73.8 ± 16 | 0.04 |
| Recipient hepatitis C viral infection (%) | 0.8 | 2.9 | 0.18 |
| Pre-transplant panel reactive antibody titre (%) | 2.7 ± 9 | 5.3 ± 16 | 0.11 |
| HLA (A, B, DR) mismatch | 3.7 ± 1.7 | 3.6 ± 1.7 | 0.77 |
| Donor age (years) | 42.3 ± 11 | 40.0 ± 14 | 0.14 |
| Donor type (% cadaveric) | 41.1 | 44.1 | 0.61 |
| Cold ischemia time (h) | 8.7 ± 10 | 11.5 ± 10 | 0.04 |
| DGF (%) | 10.2 | 14.8 | 0.23 |
| Acute rejection (%) | 10.9 | 11.9 | 0.78 |
| Calcineurin-inhibitor type (%) | 0.0004 | ||
| Cyclosporine | 70.8 | 50.3 | |
| Tacrolimus | 29.2 | 49.7 | |
| Beta-blocker use (%) | 62.7 | 34.5 | <0.0001 |
| Thiazide use (%) | 14.7 | 18.3 | 0.08 |
| ACE inhibitor/ARB use (%) | 32.5 | 24.4 | 0.12 |
| Variable | Group (n) | Baselinea | Three months | Six months | Twelve months | Thirty-six months |
|---|---|---|---|---|---|---|
| Serum creatinine (μmol/L) | Statin (n) | 144.5 ± 81 (129) | 138.5 ± 46 (128) | 139.7 ± 48 (127) | 133.3 ± 44 (126) | 141.4 ± 41 (43) |
| Non-statin (n) | 152.2 ± 92 (185) | 137.4 ± 48 (160) | 137.4 ± 69 (158) | 128.4 ± 39 (152) | 143.9 ± 54 (43) | |
| Weight (kg) | Statin (n) | 68.6 ± 15 (129) | 71.0 ± 15 (117) | 73.0 ± 16 (124) | 73.7 ± 17 (124) | 72.7 ± 20 (42) |
| Non-statin (n) | 73.8 ± 17** (185) | 75.9 ± 17** (137) | 77.2 ± 17** (145) | 77.6 ± 18* (143) | 82.8 ± 18** (37) | |
| Serum total chol (mmol/l) | Statin (n) | 6.13 ± 1.3 (129) | NA | 5.94 ± 3.6 (112) | 5.15 ± 1.3 (107) | 4.97 ± 1.0 (44) |
| Non-statin | 5.34 ± 1.1*** (185) | NA | 5.04 ± 0.7** (111) | 4.98 ± 0.9 (95) | 5.01 ± 0.9 (44) | |
| Serum HDL chol (mmol/l) | Statin (n) | 1.40 ± 0.4 (129) | NA | 1.41 ± 0.4 (100) | 1.41 ± 0.4 (103) | 1.43 ± 0.4 (36) |
| Non-statin (n) | 1.33 ± 0.3 (185) | NA | 1.32 ± 0.3 (91) | 1.31 ± 0.3 (89) | 1.19 ± 0.4* (36) | |
| Serum LDL cholesterol (mmol/l) | Statin (n) | 3.55 ± 1.2 (129) | NA | 3.22 ± 1.2 (97) | 2.80 ± 1.0 (99) | 2.64 ± 0.9 (36) |
| Non-statin (n) | 3.07 ± 0.7** (185) | NA | 2.90 ± 0.7* (89) | 2.89 ± 0.7 (89) | 2.89 ± 0.8 (36) | |
| Serum TG (mmol/l) | Statin (n) | 2.58 ± 1.4 (129) | NA | 2.27 ± 1.7 (112) | 2.01 ± 1.2 (105) | 2.01 ± 1.0 (44) |
| Non-statin (n) | 2.04 ± 2.5** (185) | NA | 1.84 ± 0.7* (111) | 1.80 ± 0.7 (95) | 1.95 ± 0.9 (44) | |
| CsA dose (mg/d) | Statin (n) | 487 ± 356 (85) | 384 ± 162 (92) | 307 ± 185 (70) | 254 ± 149 (66) | 134 ± 126 (49) |
| Non-statin (n) | 509 ± 465 (95) | 369 ± 139 (79) | 269 ± 168 (88) | 225 ± 150 (84) | 107 ± 127 (42) | |
| Tac dose (mg/d) | Statin (n) | 5.1 ± 4.0 (44) | 6.4 ± 3.9 (33) | 4.3 ± 3.9 (44) | 3.7 ± 3.6 (44) | 1.4 ± 2.6 (24) |
| Non-statin (n) | 6.3 ± 5.5 (90) | 6.0 ± 3.4 (74) | 4.3 ± 3.2 (86) | 4.0 ± 2.9 (86) | 2.5 ± 3.7 (27) | |
| Cmulative steroid dose (mg) | Statin (n) | 1636 ± 811 (129) | 2788 ± 726 (122) | 3448 ± 1185 (113) | 4793 ± 1657 (112) | 8145 ± 3038 (60) |
| Non-statin (n) | 1711 ± 1144 (185) | 2964 ± 1094 (179) | 3828 ± 4505 (145) | 5416 ± 6972 (141) | 8595 ± 12089 (59) |
- aRefers to data collected at hospital discharge (renal function, weight and calcineurin-inhibitor data) or within/at the first post-transplant month (lipid profiles and cmulative steroids). *p < 0.05, **p < 0.01 and ***p < 0.001.
- Abbreviations: Chol—cholesterol, CsA—cyclosporine A, NA—not available/performed, tac—tacrolimus and TG—triglycerides.
There were 26 conversions from cyclosporine to tacrolimus (median: 165 days) and five conversions from tacrolimus to cyclosporine (median: 180 days) during the period of follow-up. Ten conversions from cyclosporine to tacrolimus were for acute rejection and the remainder for cyclosporine-related side effects other than new-onset diabetes. All conversions from tacrolimus to cyclosporine patients were for side effects other than new-onset diabetes.
The average number of blood glucose measurements was 66 ± 17 in the statin and 65 ± 16 in the non-statin group (p = 0.32). There were 49 new-onset diabetes events during follow-up (overall incidence rate: 16%). All diagnoses of diabetes were made on the basis of two consecutive elevated CPG; this was subsequently confirmed by FPG testing. There were nine overall events in the statin group for an incidence rate of 7%. In the non-statin group, there were 40 overall events for an incidence rate of 22%. During the first 90 days, there were two events in the statin group and 18 events in the non-statin group. Figure 2A,B demonstrates the time distribution of diabetes occurrence in the statin and non-statin groups. The median time to the development of diabetes post-transplant was 301 days in the statin group and 69 days in the non-statin group (p = NS). In the statin group, the median time-to-diabetes post-statin introduction was 190 days. Diabetes resolved in one patient; 15 patients required oral agents and six required insulin, while the remainder were managed through dietary modification alone. Discontinuation of statin therapy occurred in four patients for unknown reasons. The statin preparations used were atorvastatin (85%), pravastatin (7%), simvastatin (4%) and fluvastatin (4%). Five patients were prescribed ACE inhibitors prior to developing diabetes; one was also on a statin.
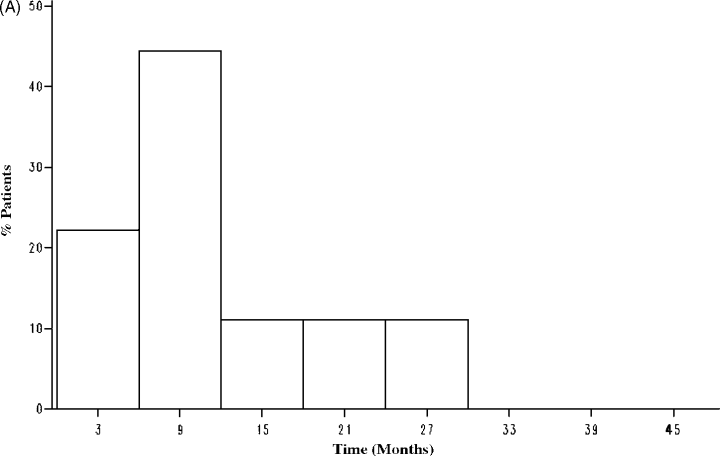
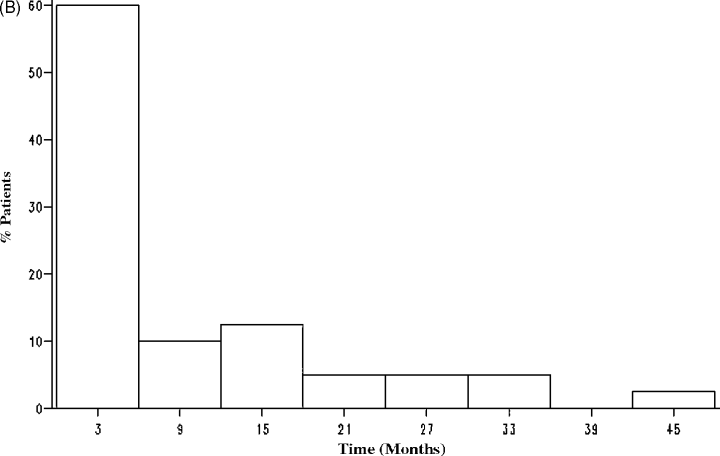
Frequency distribution of the time-to-new-onset diabetes in the statin group (A) and non-statin group (B).
The patients that developed new-onset diabetes were heavier at transplant compared to those that did not develop diabetes (78.5 ± 16 vs. 70.7 ± 15 kg, p = 0.0001) had a higher cmulative prednisone dose (37.34 ± 64.78 vs. 7.86 ± 14.04 g, p = 0.0001) and acute rejection rate (21 vs. 10%, p = 0.05), were more likely to be black (20 vs. 6.9%, p = 0.03), and less likely to be on a statin (18 vs. 45%, p = 0.0001) or ACE inhibitor (14 vs. 30%, p = 0.02). There was no difference in hepatitis C infection rate (2 vs. 2%, p = 1.0) or tacrolimus use (40 vs. 42%, p = 0.80).
The univariate analysis of risk factors utilizing diabetes as the outcome is provided in Table 3. Statin use was coded as a time-dependent covariate. Weight at transplant, black ethnicity, prednisone dose at 3 months, statin use and ACE inhibitor use were significant in this analysis. In the final multivariate Cox model (Table 4), statin use, along with prednisone dose, weight at transplant, age ≥45 years, and black ethnicity were the significant variables. Type of CNI used was not significant. To distinguish the effects of acute rejection and steroid use, the effect of steroids on diabetes development was analyzed separately in those that did not develop acute rejection. Steroid dose remained related to diabetes development (p = 0.03). Finally, to determine whether the statin effect was dependent upon lipid lowering, a Cox model including fasting serum total cholesterol, HDL, LDL and triglycerides was studied. The statin effect was found to be independent of lipid lowering.
| Variable | p-Value | Hazard ratio | 95% Hazard ratio limits | |
|---|---|---|---|---|
| Lower | Upper | |||
| Prednisone dose (per 1 mg/d above 0, at 3 months) | <0.0001 | 1.008 | 1.005 | 1.010 |
| Statin usea | 0.0001 | 0.244 | 0.118 | 0.506 |
| Black ethnicity (vs. non-black) | 0.003 | 2.882 | 1.432 | 5.802 |
| Weight at transplant (per 1 kg above 45 kg) | 0.004 | 1.025 | 1.008 | 1.043 |
| ACE inhibitor/ARB use | 0.02 | 0.383 | 0.172 | 0.854 |
| Acute rejection | 0.11 | 1.751 | 0.870 | 3.527 |
| Baseline fasting TG level (per mmol/l) | 0.11 | 1.196 | 0.958 | 1.491 |
| Age ≥45 years (vs. <45 years) | 0.21 | 1.436 | 0.809 | 2.551 |
| Cold ischemia time (per hour) | 0.22 | 1.017 | 0.989 | 1.046 |
| Cause of ESRD (GN vs. other) | 0.40 | 1.086 | 0.896 | 1.317 |
| Transplant centre (1 vs. 2) | 0.45 | 0.800 | 0.445 | 1.439 |
| Fasting total chol level (per mmol/l) | 0.55 | 0.924 | 0.709 | 1.203 |
| Cyclosporine use (vs. tacrolimus) | 0.93 | 0.977 | 0.538 | 1.774 |
| Beta-blocker use | 0.98 | 1.007 | 0.571 | 1.775 |
- aStatin use was coded as a time-dependent covariate.
- Abbreviations: chol—cholesterol, GN—glomerulonephritis, TG—triglycerides.
| Variable | Parameter estimate | Standard error | Chi-square | p-Value | Hazard ratio | 95% hazard ratio limits | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Statin usea | −1.433 | 0.401 | 12.758 | 0.0004 | 0.238 | 0.109 | 0.524 |
| Prednisone dose (per 1 mg/d above 0 at 3 months) | 0.006 | 0.001 | 15.800 | 0.0001 | 1.007 | 1.003 | 1.010 |
| Weight at transplant (per 1 kg above 45 kg) | 0.021 | 0.009 | 5.038 | 0.02 | 1.022 | 1.003 | 1.042 |
| Age ≥45 years (vs. <45 years) | 0.800 | 0.331 | 5.829 | 0.01 | 2.226 | 1.162 | 4.261 |
| Black ethnicity (vs. non-black) | 0.207 | 0.094 | 4.857 | 0.02 | 1.230 | 1.023 | 1.480 |
| ACE inhibitor/ARB use | −1.174 | 0.452 | 6.736 | 0.01 | 0.309 | 0.127 | 0.750 |
- aCoded as a time-dependent covariate.
Discussion
In this study, we have demonstrated that statin administration is associated with a reduction in the subsequent development of diabetes mellitus in RTR. This difference in diabetes incidence appears early and is sustained for up to 3 years post-transplant. The effect appears to be independent of lipid lowering, and expands the list of possible indications for statin use in the RTR population.
An understanding of the effects of statins at the cellular level may provide an explanation for their favorable influence on the development of diabetes. Insulin resistance is the cardinal feature associated with the type II diabetes. This may be due to impaired insulin signaling at the cellular level that results from mutations or post-translation modification of insulin itself or its downstream effector molecules (18). Statins may have an effect either on the delivery of substrate to insulin-sensitive tissues, or increase the activity of signaling cascades that mediate glucose uptake (18). Statins are known to increase the expression of endothelial nitric oxide synthase, which in turn may result in increased recruitment of capillaries in peripheral tissues leading to increased glucose metabolism (19). Another effect of statins that might mediate glucose uptake is the activation of PI3-K and Akt, which are molecules that regulate the translocation of the insulin-sensitive glucose transporter GLUT-4 to the cell surface, similar to insulin. Statins also decrease the levels of cytokines such as TNF-α and IL-6 that inhibit GLUT-4 translocation. Finally, statins inhibit cellular cascades like Rho-kinase that inactivate the insulin receptor (18). Whether any of the aforementioned effects of statins are operational in RTR, in whom the environment also includes chronic immunosuppressive therapy, however, remains speculative at this point.
Two major trials evaluating statins in the general population have addressed the issue of preventing new-onset diabetes. In the West of Scotland Coronary Prevention Study (WOSCOPS) (17), using the definition of two FPG values ≥7.0 mmol/L and at least one value ≥2.0 mmol/L above baseline, pravastatin significantly and favorably influenced the development of diabetes in the multivariate model, reducing the hazard by 30% (p = 0.042). The authors could not rule out the possibility, however, that this beneficial effect was mediated through triglyceride lowering. In the Heart Protection Study (HPS), in which simvastatin was used and the definition of new-onset diabetes was oral hypoglycemic or insulin therapy initiation, there was no difference in new-onset diabetes incidence between the statin (4.6%) and the placebo (4.0%) groups (p = 0.10) (20,21). These differing results may be related to population or drug differences, level of cardiovascular prevention (primary vs. secondary), method of reporting and definitions for diabetes used. Impaired glucose tolerance, however, is a macrovascular disease risk factor in itself (22) even without progression to overt diabetes. In this context, the prospectively recorded occurrence of new-onset diabetes in the RTR population, in whom there is one major trial to-date (13), will be an outcome of great interest.
The weakness of this study relates to its retrospective nature. We have shown that statin use is associated with a reduced incidence of new-onset diabetes, but the mechanism(s) behind this association remain to be elucidated. In some instances, statins were introduced several weeks or months after transplantation, even when patients had been on statins prior to transplantation. One might assume that the clinical effects of statins may persist for a while after discontinuation. However, a significant number of new-onset diabetes events occurred beyond the first 3 months of transplantation. This may indicate that the protective effect may be more important in the long term rather than in the early post-transplant period, when other interventions, such as immunosuppressive medication dose adjustment and the prevention of acute rejection may have a greater protective impact. The optimum timeframe for the introduction of a statin after transplantation remains to be defined, but it seems reasonable to commence therapy as soon as feasible. While other well-described risk factors, such as advanced age, steroid dose and black ethnicity were also significantly associated with diabetes in this study, the choice of CNI was not. The reason for lack of CNI-type effect remains speculative, but may relate to sample size, undefined population characteristics, choice of definition used in the study, and less likely perhaps, unrecognized confounding by statins in previous studies. The protective association seen with the ACE inhibitors is intriguing, and similar to the case of statins, warrants prospective investigation. In all, however, the retrospective nature of the study, which cannot account for unmeasured variables, as well as the small patient numbers limits the firmness of any conclusions that can be made.
We have used a rigorous definition for diabetes in this study, in an attempt to arrive at a more precise and clinically relevant estimation of new-onset diabetes incidence in this population. The wide disparity in reported diabetes incidence in the published literature is likely the result of utilizing varied definitions for the disease, such as the need for different forms of pharmacotherapy. We urge that for future studies of diabetes after transplantation definitions widely used and accepted in the general population be applied.
Beneficial effects of statins specific to the transplant population that have been demonstrated previously include favorable effects on cardiac events (13), chronic and acute rejection (23,24), and associations with improved patient survival (25), lower blood pressure (26) and improved bone mineral density (27). In this study it has been demonstrated that statins have a favorable influence on the development of new-onset diabetes after transplantation as well. This effect appears to be independent of their lipid lowering effect. While it remains to be seen if this can be shown prospectively, given the negative impact of new-onset diabetes on health-care expenditure (28), quality of life, graft (3) and patient (5) survival, we urge that appropriate preemptive measures against diabetes in this population be given strong consideration.
Acknowledgments
This study was supported by unrestricted educational grants from Novartis Canada and Hoffman La Roche Canada.




