Genomic structure, developmental distribution and functional properties of the chicken P2X5 receptor
Abstract
We report here the cloning of a chicken cDNA (402 aa) showing high sequence similarity to the previously cloned rat and human P2X5 receptors (67 and 69%, respectively). The chicken P2X5 subunit is encoded by a gene composed of 12 translated exons, which shows conserved genomic structure with mammalian P2X genes. In HEK-293 cells heterologously expressing chicken P2X5 receptors, ATP activates a current that desensitizes in a way that is dependent on the presence of extracellular divalent cations. ATP and 2-methylthio ATP are equipotent agonists (EC50 ∼ 2 µm) and suramin and pyridoxal 5-phosphate-6-azophenyl-2′,4′-disulfonic acid are potent antagonists. Additionally, reversal potential measurements indicate that chicken P2X5 is permeable not only to cations but also to chloride (PCs+/PCl- ∼ 1.9), as has been described for native P2X receptor mediated responses in embryonic chicken skeletal muscle. mRNA distribution of chicken P2X5 was determined by in situ hybridization analysis in both whole embryos and on tissue slices of heart and skeletal muscle. Our results suggest that chicken P2X5 receptors are expressed in developing muscle and might play a role in early muscle differentiation.
Abbreviations used
-
- ACh
-
- acetylcholine
-
- αβmeATP
-
- α,β-methylene-ATP
-
- 2MeSATP
-
- 2-methylthio-ATP
-
- PPADS
-
- pyridoxal 5-phosphate-6-azophenyl-2′,4′-disulfonic acid
-
- RACE
-
- rapid amplification of cDNA ends.
ATP acts as a neurotransmitter in the central and in peripheral nervous system (Burnstock 1999). Additionally, responses to extracellular ATP have been found in a number of isolated cells and tissues including smooth muscle, and skeletal and heart myocytes (Kolb and Wakelam 1983; Hume and Honig 1986; Benham and Tsien 1987; Friel 1988).
ATP is released from nerve terminals together with other neurotransmitters, including acetylcholine (ACh), noradrenaline and GABA (Sneddon and Westfall 1984; Zimmermann 1994; Jo and Schlichter 1999). Under metabolic stress (e.g. hypoxia), a non-synaptic mechanism of ATP release has been described for muscle cells (Williams and Forrester 1983), and almost every cell can release ATP in a nonlytic way after mechanical stimulation (Silinsky et al. 1999). At the mature neuromuscular junction synaptically released ATP is converted to adenosine, which in turn inhibits the release of ACh via presynaptic adenosine receptors (Sebastião et al. 1999; Silinsky et al. 1999). However, at the developing neuromuscular junction, ATP enhances the spontaneous release of ACh (Fu 1995) and produces strong depolarizations at embryonic skeletal muscle preparations by itself (Hume and Honig 1986). The distinct effects of extracellular ATP in developing and in adult animals correlate well with the disappearance of ATP-mediated contractility of chicken embryo skeletal muscle upon innervation (Wells et al. 1995). The effect of ATP on embryonic chicken myoblasts and myotubes is mediated by P2X receptors that desensitize and show little recovery from desensitization (Hume and Honig 1986; Thomas and Hume 1990a). Moreover, they are the only P2X receptors described so far to be permeable to both cations and chloride (Thomas and Hume 1990b).
Seven rat P2X subunits (P2X1–P2X7) have been cloned so far (Soto et al. 1997; North and Surprenant 2000). Additionally, a chicken P2X receptor cDNA (cP2X8) homologous to mammalian P2X5 subunits but showing sequence divergence at the C-terminal end has recently been reported (Bo et al. 2000). We have isolated the same cDNA from chicken heart. The amino acid sequence similarity level between cP2X8 and rP2X5 (67%) is comparable to what has been described for rat and chicken P2X4 subunits (75%, Ruppelt et al. 1999) and higher than the sequence similarity between different P2X subunits from the same species (maximally 50%, Soto et al. 1997). For these reasons we consider cP2X8 the chicken orthologue of rP2X5 and therefore refer to it as cP2X5. We have analyzed the gene structure of cP2X5 and determined that the last coding exon created the differences between rP2X5 and cP2X5. Additionally, we further characterized the functional properties of homomeric cP2X5 receptors heterologously expressed in HEK-293 cells and studied the mRNA transcript distribution at different stages of chicken development.
Materials and methods
Materials
Solutions for electrophysiology were made with highly purified water (NANOpure, Barnstead, Debuque, IA, USA) using chemicals of analytical grade. ATP (potassium salt); α,β-methylene-ATP (αβmeATP; lithium salt) and 2-methylthio ATP (2meSATP; sodium salt) were purchased from Sigma (St Louis, MO, USA). Suramin was purchased from RBI (Nactick, MA, USA) and pyridoxal 5-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) was obtained from Tocris Cookson (Bristol, UK). Solutions containing ATP or other nucleotides were prepared freshly each day and the pH of the solution was readjusted.
cDNA cloning and genomic screening
A partial cDNA for the cP2X5 receptor (∼400 bp) was obtained using degenerate PCR as described (Soto et al. 1996) and used to screen a 17-day-old chicken embryo brain cDNA library (Stratagene, La Jolla, CA, USA) (Ruppelt et al. 1999). One positive phage was isolated and cloned. Protein-sequence analysis revealed a cDNA encompassing the 3′-coding sequence of cP2X5 up to amino acid 257. To isolate the unknown 5′-coding sequence of the cP2X5 mRNA, we used RACE techniques (Marathon cDNA amplification kit, Clontech, Palo Alto, CA, USA). Specific antisense oligonucleotide (5′-ATCCAGACGGCTAAAAGAAT-3′) targeted to cP2X5 was used for PCR on a library of adaptor-ligated 14-day-old embryo heart cDNA following manufacturer's recommendations. A clone that contained an inframe stop codon in the 5′-untranslated sequence was joined to the previously isolated partial cDNA using an internal EcoRI site to form the full-length cP2X5 cDNA. In order to determine if additional C-terminal domains with higher homology to rP2X5 were present in chicken embryos, 3′-RACE was performed on adaptor-ligated cDNA libraries synthesized by using mRNA isolated from 14-day-old embryo brain and skeletal muscle and adult brain as described above. The specific sense oligonucleotides used for nested PCR were: 5′-CGAAACCTCCCACCACACTATCCT-3′ and 5′-CCGTTCCGTCCCTTTAAATTGTAGT-3′.
To screen a commercially available chicken genomic library (Clontech, Palo Alto, CA, USA) a 1042-bp probe (nt 248–1289) was used. Fourteen positive phages were isolated. One 17-kb long phage (λ71) that hybridized with probes containing N- and C-terminal sequences of cP2X5 cDNA was used for further analysis. The isolated λDNA was digested with XhoI/Asp718I (containing exon 1–2), Asp718I/HindIII (exon 3–5), HindIII (exon 6–12), cloned in pBluescript II KS(+) and sequenced. Comparison of the genomic sequence with the cDNA sequence was used to determine the exon–intron junctions.
All cDNA clones were sequenced in both strands using a Dye Terminator Cycle Sequencing Kit and an ABI377 DNA sequencer (Applied Biosystems, Foster City, CA, USA).
RNAse protection analysis
In the RNAse protection assay, a 575-bp fragment containing the last 439 bp of the cP2X5 coding sequence plus 23 bp of 3′-untranslated sequence (nt 715–1289) was used to generate a [32P]UTP-labeled antisense RNA probe using T7-RNA polymerase. Twenty micrograms of total RNA obtained as previously described (Chomczynski and Sacchi 1987) were hybridized with the probe and unprotected RNA was digested with RNAse A and RNAse T1 following the manufacturer's instructions (Ribonuclease protection assay RPA III, Ambion, Austin, TX, USA). DNA ladder (100 bp, Gibco BRL, Karlsruhe, Germany) was [32P]CTP-labeled using Terminal transferase (Boehringer Mannheim, Mannheim, Germany) following the protocol described by the manufacturer.
In situ hybridization
For whole mount in situ hybridization, 450 bp of cP2X5 C-terminal sequence (nt 825–1274) or 506 bp of extracellular domain sequence (nt 370–875) were used to generate antisense digoxigenin-labeled RNA probes using the Boehringer Mannheim kit (Boehringer Mannheim, Mannheim, Germany). The hybridization pattern obtained with both antisense probes was essentially identical. The corresponding sense probes were used as negative control. Embryos corresponding to Hamburger and Hamilton developmental stages 11 (HH11), 15 (HH15) and 18 (HH18) (Hamburger and Hamilton 1951) were fixed overnight in 4% paraformaldehyde. Whole mount in situ hybridization was performed as described (Pera and Kessel 1997).
In situ hybridization was performed on tissue slices of embryonic skeletal muscle and on heart cryostat sections using synthetic antisense and sense oligonucleotide probes. The oligonucleotides used were: antisense 5′-AACCCTTCCAGGACACCTGCCCCAT TCTCCCAGCTCTCTTTA-3′ or 5′-TCTTTCAAACAACGGCCA GTCTTAACCCCATTACCAGCCACCAC-3′; sense: 5′-GTGGTG GCTGGTAATGGGGTTAAGACTGGCCGTTGTTTGAAAGA-3′. Tissue preparation, hybridization, dipping and counterstaining were performed as previously described (Stocker and Pedarzani 2000). The following controls were performed: (i) adjacent sections were hybridized with sense oligonucleotide, or (ii) hybridized with a mixed oligonucleotide probe containing a 500-fold excess of cold oligonucleotide. The silver grain densities observed in control sections were considered as background.
Stable transfection of HEK-293 cells and electrophysiological measurements
The full-length cP2X5 cDNA was subcloned into the mammalian expression vector pcDNA3 (Invitrogen, Groningen, the Netherlands). Human embryonic kidney (HEK-293) cells were stably transfected using a modification of the calcium phosphate precipitation method (Chen and Okoyama 1987). Independent foci were selected in the presence of 1 mg/mL and expanded in the presence of 0.5 mg/mL geneticin (Sigma, St Louis, MO, USA). The cells were grown as monolayer cultures in 1 : 1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 nutrient mix (GibcoBRL, Rockville, MD, USA) supplemented with 10% fetal bovine serum and 0.5 mg/mL geneticin at 37°C, 5% CO2. For electrophysiological measurements, cells from subconfluent cultures were plated onto glass coverslips 24–48 h before use and maintained in culture medium containing 3% fetal bovine serum.
Membrane currents were recorded using the standard whole-cell configuration of the patch-clamp technique (Sakmann and Neher 1995) as previously described (Korngreen et al. 1998). Once the whole-cell configuration was established, the cell was continuously superfused with the desired extracellular solutions via a gravity-fed perfusion system. Experiments were initiated at least 2 min after establishing the whole-cell configuration to allow equilibration of the cytosol with the pipette solution. Membrane currents were recorded under voltage-clamp using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA) and stored on VCR tape for subsequent analysis (VR-10B, Instrutech, Port Washington, NY, USA). The sampling frequency was set to at least two times the corner frequency of the low-pass filter. The standard extracellular solution contained (mm): 150 CsCl, 1.5 CaCl2, 5 TES, 10 d-glucose, adjusted to pH 7.4 with CsOH. The standard pipette solution contained (mm): 155 CsCl, 5 TES, 0.1 EGTA, 0.096 CaCl2 (0.1 µm Ca2+, estimated by Max Chelator 6.0), adjusted to pH 7.2 with CsOH. Deviations from these solutions are noted in the figure legends.
Sigma Plot™ 2.0 scientific software (Jandel Scientific, San Rafael, CA, USA) was used for non-linear curve fitting.
Results
Isolation and analysis of cP2X5 cDNA and genomic sequences
A cDNA fragment with 62% sequence identity to rat P2X5 was amplified by degenerated PCR on 14-day-old chicken embryo cardiomyocyte mRNA as previously described (Soto et al. 1996; Ruppelt et al. 1999). The full-length cDNA was then isolated by combining the screening of a chick brain cDNA library with PCR amplification of the 5′ end by RACE techniques. The predicted amino acid sequence (402 aa) shows 67% and 69% similarity to rat (Collo et al. 1996; Garcia-Guzman et al. 1996) and human P2X5 (Lêet al. 1997) with a significantly lower sequence identity to other P2X subunits, suggesting that at least structurally this protein represents the chicken ortologue of P2X5. The identity between rP2X5 and cP2X5 subunits is well distributed along the sequence with the exception of the C-terminal domain (Fig. 1). cP2X5 is 54 aa shorter than rP2X5 and the similarity to the rat orthologue disappears shortly after the second transmembrane domain. We performed additional 3′-RACE reactions using mRNA isolated from embryonic skeletal muscle, heart and brain, and adult chicken brain, and while the C-terminal domain described here could always be amplified, a C-terminal domain with higher similarity to the rat P2X5 sequence was not found by this approach.
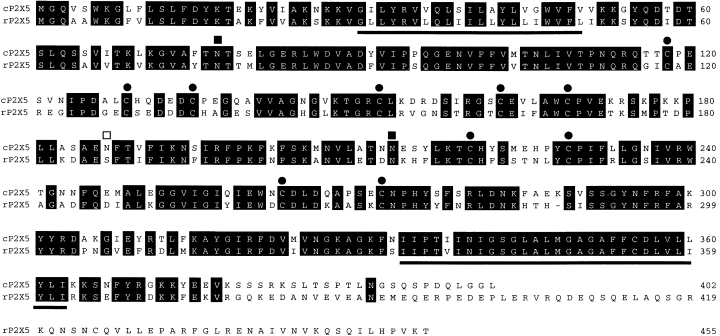
Amino acid sequence alignment for chicken and rat P2X5 receptors. Residues identical in both sequences are denoted by shaded boxes. Squares, putative N-glycosylation sites [N-X-(S/T)], open square denote the non-conserved N-glycosylation site between rat and chicken P2X5. Circles, conserved cystein residues. Underlined, proposed transmembrane segments (M1 and M2).
Screening of a chicken genomic library allowed us to determine the gene structure of cP2X5 including the exon–intron borders by comparison with the cDNA sequence (Figs 2a and b). Chicken P2X5 gene consists of 12 translated exons spanning a stretch of genome of approximately 10 kb (Fig. 2a). The gene structure is strongly conserved between cP2X5 and the mammalian P2X genes described so far (Brandle et al. 1997; Souslova et al. 1997; Buell et al. 1998; Nawa et al. 1998; Lynch et al. 1999). The last coding exon of cP2X5 (exon 12) starts at the point where the divergence between the rat and the chicken P2X5 protein sequence is apparent (Fig. 2b) indicating that an exchange or modification of that exon during evolution gave rise to two different C-terminal domains.
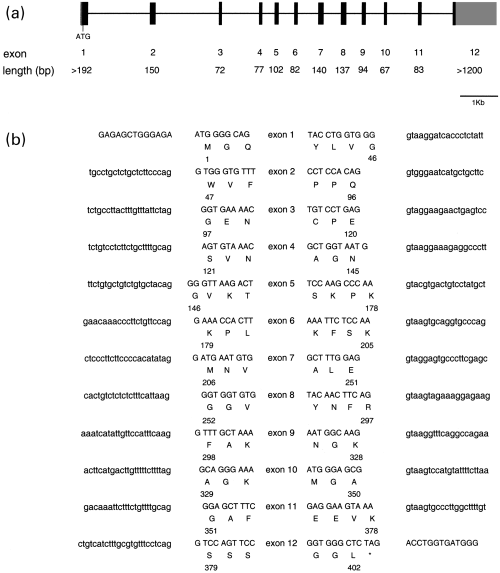
Exon–intron boundaries of the chicken P2X5 receptor gene. (a) Schematic representation of the cP2X5 gene. The exon–intron structure is drawn to scale. Exons are represented by boxes: gray boxes indicates untranslated sequence and black boxes protein coding sequence. Introns are represented by solid lines. The length of each exon is shown (bp). (b) The putative donor and acceptor splicing sites are based on the comparison of the genomic and cDNA sequences and consideration of the consensus sequence for splice junctions. Capital letters represent presumed exonic DNA, small letter intronic DNA. The sequence of the cP2X5 gene has been submitted to the EMBL nucleotide sequence database (accession number AJ308518).
Additionally, we performed RNAse protection analysis (RPA) on RNA isolated from heart and skeletal muscle of a 14-day-old chicken embryo in order to confirm the existence of chicken exon 12 and to investigate the possible presence of mRNA containing an alternative C-terminal exon. For this purpose, a 32P-labeled 575 bp long antisense cRNA probe that overlaps the last 439 bp of cP2X5 coding sequence plus 23 bp of 3′ untranslated region was synthesized (exon 8–12) (Fig. 3, undigested probe). The main band detected in both tissues corresponds to a protected RNA containing exon 8–12 (463 bp) (Fig. 3), strongly supporting the idea that the cP2X5 predominantly expressed in chicken heart and skeletal muscle contains exon 12 as last coding exon. Additionally, two faint bands of approximately 375 and 325 bp were detected (Fig. 3). The 375 bp band corresponds to the length of an additional C-terminal domain found by 3′-RACE containing exon 8–11 and going into intron 11–12 where an immediate stop codon in frame and polyadenylation signal are present (not shown).
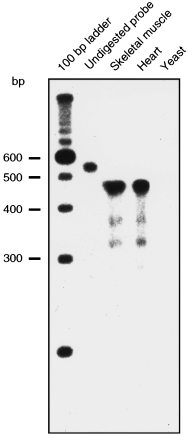
RNAse protection analysis of total RNA isolated from skeletal muscle and heart of a 14-day-old embryo. Lane 1, 100 bp DNA ladder (Gibco); lane 2, undigested probe; lane 3, 20 µg of digested skeletal muscle total RNA; lane 4, 20 µg of digested heart total RNA; lane 5, 10 µg of digested yeast RNA. A main protected band of 463 bp corresponding to the expression of RNA containing exon 8–12 is detected.
Tissue distribution of cP2X5 subunits
Since the highest expression level of cP2X5 mRNA was found by northern blot analysis in skeletal and heart muscle tissue of chicken embryos (not shown), we used radioactively labeled oligonucleotides to determine the transcript distribution in slices from both tissues. Figure 4(a and e) show an overview of the labeling from an X-ray film image, showing a homogeneous distribution of cP2X5 transcripts in cardiac (heart; frontal section) and skeletal muscle (lower limb; transversal section), respectively. Figure 4(b and f) present the corresponding sense controls. Both dark-field images and bright-field images showing cellular resolution of emulsion coated sections of the ventricular myocardium and skeletal muscle are presented in Fig. 4(c, d, g and h). In heart, no preferential localization of P2X5 transcripts to atria vs. ventricle could be detected.
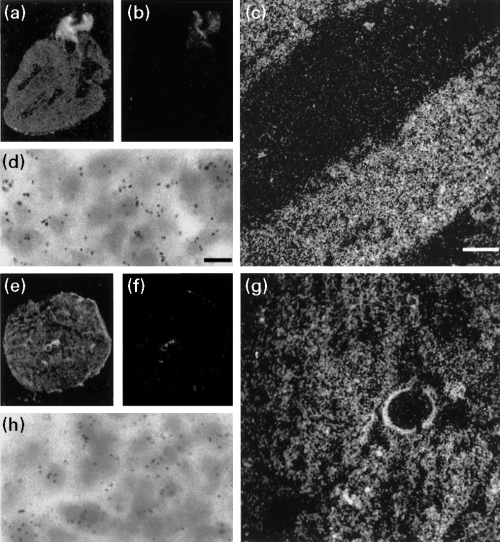
In situ hybridization on cardiac and skeletal muscle sections of 14-day-old embryos using radioactive oligonucleotides for cP2X5. (a) and (b) Autoradiograms of frontal sections of 14-day embryonic heart and corresponding negative control (sense oligonucleotide). No differences in labeling could be detected between atrium and ventricle. (c) Dark-field photomicrograph showing the distribution of cP2X5 transcripts at a cellular resolution in ventricular myocardium. (d) The cardiac muscle cells are depicted to a higher magnification. (e) and (f) Autoradiograms of transversal sections of 14-days embryonic lower leg and corresponding negative control (sense oligonucleotide). (g) Dark-field photomicrograph showing the distribution of cP2X5 transcripts at a cellular resolution in chicken skeletal muscle. (h) The muscle cells are depicted at a higher magnification in bright field optics. Sense oligonucleotides showed a low level of non-specific labeling. Scale bars: 250 µm for (c) and (g); 25 µm for (d) and (h).
cP2X5 transcripts were also detected in 3–4-day-old (HH18–HH22) embryos by northern blot analysis (not shown). Therefore, we used digoxigenin-labeled probes to investigate the distribution of P2X5 mRNA in whole-mount embryos (Fig. 5b; HH18). Chicken P2X5 mRNA was found in somites, otic vesicle, heart, brachial arches and embryonic brain (Fig. 5b). Expression in somites was also present in HH15 embryos (not shown) while no transcripts at all were detected in younger animals (HH11) (Fig. 5a). Transversal (Fig. 5c) and sagittal (Fig. 5d) sections of somites in a late stage of maturation (stage XI–XX; Christ and Ordahl 1995) showed a strong staining in the myotome, the first differentiated skeletal myocytes to appear and source of skeletal muscle tissue of trunk and limbs (Ordahl et al. 2000). No labeling could be detected in early stage somites (Figs 5a and b).
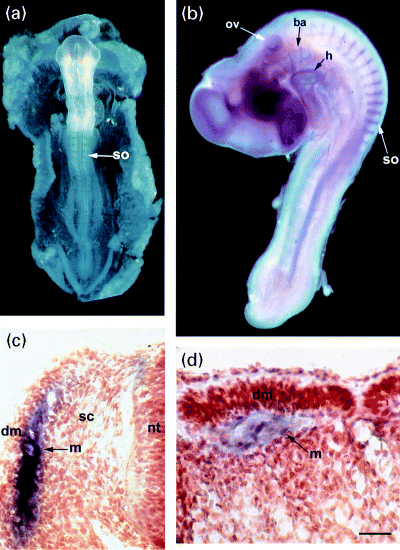
Whole-mount in situ hybridization of chicken embryos with digoxigenin-labeled cP2X5 probes. (a) Whole-mount embryo stage HH11. cP2X5 transcripts were not detected. (b) Whole-mount embryo stage HH18. Labeling can be observed in heart, otic vesicle, brachial arches, brain and somites. (c) Transversal and (d) saggital section of a late stage somite (XI–XX; Christ and Ordahl 1995). cP2X5 transcripts are confined to the myotome of the somite. ba: brachial arches; dm: dermomyotome; h: heart; m: myotome; nt: neural tube; ov: otic vesicle; so: somite; st: sclerotome. Scale Bar: 25 µm for (c) and (d).
Functional characterization of cP2X5 receptors
To characterize the functional properties of cP2X5 receptors, the full-length cP2X5 clone was stably transfected in HEK-293 cells. The currents elicited by ATP in the transfected cells were then investigated using the whole-cell configuration of the patch-clamp technique. In extracellular solutions containing 1.5 mm CaCl2, at a holding potential of − 60 mV, application of ATP (10 µm) induced a rapid inward current (100–700 pA) that desensitized with a time constant of 4.4 ± 1.3 s (n = 7) (Fig. 6a, lower trace). Only 30% of the initial current recovered after washing for 5 min (n = 5). The observed desensitization properties of the cP2X5 receptor are unlikely to arise from the activation of endogenous P2Y receptors (Schachter et al. 1997), since including a non-hydrolysable analogue of GTP (GDP-βS; 200 µm) in the pipette solution did not significantly affect the rate of desensitization at either negative or positive potentials (n = 8).
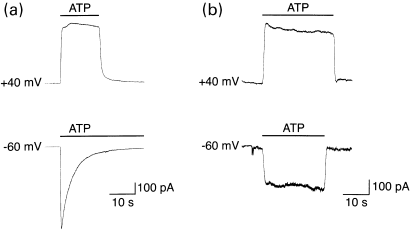
Extracellular divalent cations facilitate desensitization of cP2X5 receptors at negative membrane potentials. (a) and (b) Whole-cell membrane currents activated by 10 µm ATP (indicated by horizontal bars) in extracellular solutions containing either 1.5 mm CaCl2 (a) or 0.1 mm CaCl2 (b). In the presence of 1.5 mm CaCl2 in the extracellular solution, the current activated by ATP remained relatively constant for several seconds at + 40 mV (top trace), while at −60 mV the current desensitized exponentially with a time constant of 4.6 s (bottom trace). Rapid desensitization at −60 mV was absent in extracellular solutions containing 0.1 mm CaCl2. The currents in A and in B are from two different cells. Standard CsCl solutions.
When the membrane potential was held at + 40 mV, the rate of desensitization of the ATP-activated current was dramatically reduced (Fig. 6a, upper trace). The rate of desensitization at −60 mV was also reduced by lowering the concentration of extracellular Ca2+(Fig. 6b). The effect of extracellular Ca2+ on desensitization was mimicked by both extracellular Ba2+ and Mg2+ (1.5 mm) (data not shown). Thus, divalent cations appear to facilitate the rate of desensitization of cP2X5 receptors by interacting with a site within the voltage field.
Due to the desensitization and lack of recovery from desensitization that cP2X5 receptors exhibited at negative holding potentials with 1.5 mm Ca2+ in the extracellular solution, most experiments were carried out at + 20 mV with 0.5 mm CaCl2 in the extracellular solution. Under these experimental conditions, the decline in the ATP response was substantially slower, and thus reliable responses to multiple applications of agonists could be obtained (Fig. 7a). Dose–response relationships for ATP and 2MeSATP were constructed by bracketing each tested concentration of agonist by a measurement with 3 µm of the agonist, which served as a reference. The currents were then corrected for rundown by normalizing the current activated by a given concentration of agonist to the preceding response to 3 µm. The resulting dose–response relationships for added ATP and 2meSATP, shown in Fig. 7(b) (n = 5–15), gave an apparent concentration for half-maximal response (EC50) to ATP and 2MeSATP of 2.0 µm and 1.8 µm, respectively. Application of 100 µmαβmeATP elicited currents that were 80 ± 3% (n = 3) of the maximal current evoked by 10 µm ATP (Fig. 7a). Figure 7(c) shows that the currents elicited through cP2X5 receptors by 10 µm ATP were fully inhibited either by 1 µm suramin or by 1 µm PPADS. Dose-inhibition curves were not constructed since it was not possible to reliably determine the contribution of current rundown to the observed reduction in current amplitude due to partial irreversibility of the antagonist effect.
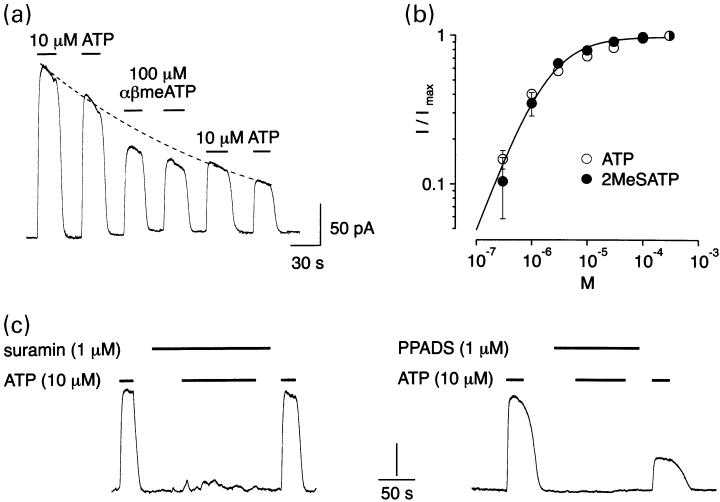
Pharmacological properties of cP2X5 receptors. (a) αβmeATP is less effective in activating cP2X5 channels in comparison to ATP. Continuous whole-cell current record measured at + 20 mV. The response to ATP declined exponentially with a time constant of 3.5 min (dashed line). The response to two consecutive applications of 100 µmαβmeATP (indicated by horizontal bars) are bracketed by the responses to 10 µm ATP. (b) Normalized dose–response curves for ATP (empty symbols) and for 2MeSATP (full symbols). Each tested concentration of agonist was bracketed by a measurement with 3 µm of the agonist. The currents were then corrected for rundown by normalizing the response to a given concentration of agonist to the preceding response to 3 µm of the agonist. Each point represents the mean (± SEM) of 5–15 experiments. The data were fitted with the equation I/Imax=a{[ATPo]n/([ATPo]n + 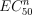 )}, where I is the current activated by a given concentration of ATPo, Imax is the maximal current activated by ATPo, EC50 is the concentration of ATPo ([ATPo]) yielding a current half the maximum, a is a normalization factor and n is the Hill coefficient. EC50, n, and a are 2.0 µm, 0.96, and 0.98 for ATP and 1.8 µm, 1.01, and 0.98 for 2MeSATP, respectively. The solid line is the least squares fit to the ATP data. C. Continous whole-cell current records measured at + 20 mV showing that both suramin (1 µm) and PPADS (1 µm) fully inhibited the current activated by 10 µm ATP. Calibration bar: 100 pA.
)}, where I is the current activated by a given concentration of ATPo, Imax is the maximal current activated by ATPo, EC50 is the concentration of ATPo ([ATPo]) yielding a current half the maximum, a is a normalization factor and n is the Hill coefficient. EC50, n, and a are 2.0 µm, 0.96, and 0.98 for ATP and 1.8 µm, 1.01, and 0.98 for 2MeSATP, respectively. The solid line is the least squares fit to the ATP data. C. Continous whole-cell current records measured at + 20 mV showing that both suramin (1 µm) and PPADS (1 µm) fully inhibited the current activated by 10 µm ATP. Calibration bar: 100 pA.
P2X receptors in chicken skeletal muscle have been reported to be permeable to Cl− (Thomas and Hume 1990b). Hence, we estimated the relative permeability of cP2X5 receptors channels to Cs+ and Cl− with the Goldman–Hodkin–Katz (GHK) equation by measuring the zero-current (reversal) potential of the net current activated by ATP. The reversal potential was measured using an intracellular solution, which contained 155 mm CsCl, and an extracellular solution, which contained either 150 mm CsCl or 30 mm CsCl. The osmolarity of the solution was maintained constant with sucrose. The net current activated by ATP was assessed by subtracting from the current activated by 10 µm ATP either the current recorded in the absence of ATP (ATP minus control) or the current remaining after inhibition by 10 µm suramin (ATP minus-ATP + suramin). Examples of net currents activated by ATP in either extracellular solution in response to voltage ramps are presented in Fig. 8(a). In 150 mm CsCl, the net current activated by ATP reversed at + 0.5 mV, and exhibited little rectification between −40 and + 40 mV (Fig. 8a) as described for the rat orthologue (Garcia-Guzman et al. 1996). The reversal potential shifted to −8.5 mV when the extracellular solution was changed to 30 mm CsCl. The average reversal potentials obtained using the two different estimates of the net current activated by ATP are presented in Fig. 8(b). The average reversal potential in 30 mm CsCl was −10.7 ± 1.3 mV (n = 5) and −9.5 ± 1.0 mV (n = 12) and in 150 mm CsCl was + 0.8 ± 0.8 mV and 0.0 ± 0.6 mV in the suramin and control subtracted currents, respectively. Assuming activity coefficients of 0.847, and 0.738 for 30 and 155 mm CsCl, respectively, a relative permeability ratio (PCs+/PCl-) of 1.98 and 1.82 was obtained using the GHK equation for control and suramin experiments, respectively. These results show that cP2X5 receptors are highly permeable to Cl−, as has been reported for native P2X receptors in chicken skeletal muscle (Thomas and Hume 1990b).
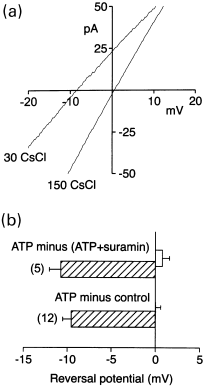
cP2X5 receptors are permeable to chloride ions. (a) Average whole-cell currents activated by ATP in response to voltage ramps in extracellular solutions containing either 150 mm CsCl or 30 mm CsCl. The osmolarity of the solution was maintained the same by substituting 120 mm CsCl with 210 mm sucrose. The concentrations of CsCl in the extracellular solution are indicated by each current trace. In each extracellular solution, the net current activated by ATP was assessed by subtracting the current recorded in extracellular solutions containing both ATP (10 µm) and suramin (10 µm) from the current activated by ATP (10 µm). Five consecutive current traces were averaged in each experimental condition. The extracellular solution also contained 5 mm TES and was adjusted to pH 7.4. Standard intracellular solution. Digitized at 1 kHz, filtered at 0.5 kHz. (b) Mean (± SEM) reversal potentials of the current activated by ATP in extracellular solutions containing either 150 mm CsCl (open bars) or 30 mm CsCl (hatched bars). Both extracellular solutions were tested in each cell. The ATP-activated current was assessed either by subtracting the current remaining in ATP (10 µm) and suramin (10 µm) (ATP minus-ATP + suramin) from the current activated by ATP (10 µm) (top bars) or by subtracting the current recorded under control conditions (ATP minus control) from the current recorded in the presence of ATP (bottom bars). The relative permeability of Cs+ and Cl− (PCs/PCl) was estimated to be 1.9 using the GHK equation.
Discussion
We present here for the first time the genomic structure of a non-mammalian P2X subunit. The cP2X5 gene consists of 12 translated exons that spread over 10 kb of genomic sequence. Exon–intron borders and exon lengths are conserved between the cP2X5 gene and the mammalian P2X subunits with known genomic organization (Brandle et al. 1997; Souslova et al. 1997; Buell et al. 1998; Nawa et al. 1998; Lynch et al. 1999). While most of the amino acid sequence is highly identical (67%) between the rat and chicken P2X5, the last coding exon of cP2X5 shows no similarity with the corresponding rat amino acid sequence. To investigate the possibility of an alternative last coding exon for cP2X5, 3′-RACE was performed on RNA isolated from different embryonic tissues and from adult chicken brain. The C-terminal domain coded by exon 12 could be always amplified, discarding possible recombination mistakes during the construction of the cDNA library. However, no additional exon showing more similarity to the rat P2X5 sequence could be detected. The relative abundance of the exon combination 8–12 in relation to putative alternative coding exons was investigated by RNAse protection. The result obtained clearly points to a majority expression in embryonic heart and skeletal muscle of cP2X5 mRNA containing exons 8–12.
cP2X5 transcripts were previously described to be present in somites of 4-day-old chicken embryos by whole mount in situ hybridization (Bo et al. 2000) but no labeling could be observed in somites of 3-day-old embryos. We have found cP2X5 transcripts in somites at earlier developmental stages (HH15 embryos; approximately 2 days old). Transversal and sagittal sections of HH18 (approximately 3 days old) embryos showed that the labeling in the somites was confined to the myotome. This finding together with the lack of cP2X5 transcripts in early stage somites and in younger embryos indicate a close correlation between the formation of myogenic precursor cells from the dermomyotome (Ordahl et al. 2000) and the appearance of cP2X5 expression. We also analyzed the expression of P2X5 transcripts in older embryos by in situ hybridization. Both, in lower limb skeletal muscle and in heart of a 14-day-old embryo, transcripts for cP2X5 were detected. To our knowledge, the effect of extracellular ATP on chicken heart has not been studied. However, ATP-elicited contraction in most skeletal muscles of 14-day-old chicken embryos and these responses to ATP disappear around the time of hatching (Wells et al. 1995). It will be interesting to determine whether lack of expression of cP2X5 mRNA in skeletal muscle correlates with the described disappearance of ATP-elicited contraction.
Heterologous expression of cP2X5 in HEK-293 cells leads to the formation of functional homomeric receptors that desensitize with a time constant of 4 s and do not recover from desensitization. Bo et al. (2000) observed the same phenomena in Xenopus oocytes heterologously expressing cP2X5 receptors. We found that desensitization required both negative membrane potentials and the presence of extracellular divalent cations suggesting that desensitization probably depends on permeation of divalent cations through the receptors. In this context it will be interesting to investigate whether the mechanism of desensitization of cP2X5 receptors is similar to the Ca2+-dependent desensitization described for NMDA receptors (Legendre et al. 1993). Desensitization of P2X2 receptors has been shown to depend on phosphorylation at a conserved threonine residue in the N-terminal domain (Boué-Grabot et al. 2000). The fact that (i) the putative phosphorylation site (T18 in rat P2X2) is conserved in both rat and chicken P2X5 subunits and (ii) rP2X5 receptors do not desensitize (Collo et al. 1996; Garcia-Guzman et al. 1996) argues against a role of PKC in cP2X5 desensitization. Mechanisms of desensitization of P2X receptors are more diverse, since desensitization of homomeric P2X1 receptors has been shown to be independent of current flow and Ca2+ entry (Werner et al. 1996).
ATP and 2MeSATP are equipotent agonists and suramin and PPADS are potent antagonists at cP2X5 receptors. This pharmacological profile is similar to that of the rat P2X5 receptor (Collo et al. 1996; Garcia-Guzman et al. 1996). However, αβmeATP-activated cP2X5 receptors while it did not evoke any response at rP2X5 receptors (at 500 µm; Garcia-Guzman et al. 1996). Sensitivity to αβmeATP can be exchanged between P2X1 and P2X2 by swapping the extracellular loop connecting the two transmembrane domains (Werner et al. 1996). Hence, amino acid differences between the extracellular domain of rat and chicken P2X5 receptors are likely to account for the different sensitivity to the ATP analogue.
Permeation studies on P2X receptors have focused mainly on monovalent and divalent cations (Evans et al. 1995; Virginio et al. 1998). Reversal potential measurements after substitution of Cl− by gluconate (Brake et al. 1994; Soto et al. 1996) indicated that P2X2 and P2X4 receptors heterologously expressed in Xenopus oocytes are not permeable to Cl−. In contrast, the present study shows that cP2X5 is permeable not only to cations but also to Cl− as has been described for the native ATP-mediated responses in embryonic chicken skeletal muscle (Thomas and Hume 1990b).
Thus, functional properties of heterologously expressed cP2X5 receptors are similar to the ATP-activated current in chicken embryo myotubes in that native receptors do not recover from desensitization (Thomas and Hume 1990a) and showed permeability not only to cations but also to Cl− (Thomas and Hume 1990b). However, in contrast to cP2X5, native receptors are not sensitive to αβmeATP (Thomas et al. 1991), pointing to cP2X5 as a constituent subunit of the native skeletal muscle receptor though additional P2X subunits might be present to confer insensitivity to αβmeATP.
Acknowledgements
We acknowledge Professor Walter Stühmer for support, lab space and equipment and for critically reading the manuscript. We are grateful to Dr Michael Kessel, Hendrik Knötgen, Dr Martin Stocker and Marco Gymnopolous for technical advice concerning the in situ hybridization experiments. We thank the Department of Immunocytochemistry, particularly Ms. M. Praetor for performing the sequencing. We are grateful to Daniel Kerschensteiner for help with the RPA experiments, for invaluable discussion and for critically reading the manuscript.




