Calcium Channels in the GABAergic Presynaptic Nerve Terminals Projecting to Meynert Neurons of the Rat
Abbreviations used : ACSF, artificial CSF ; AP-5, 2-amino-5-phosphonovaleric acid ; ω-Cg-GVIA, ω-conotoxin-GVIA ; ω-Cm-MVIIC, ω-conotoxin-MVIIC ; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione ; HVA, high-voltage-activated ; IPSC, inhibitory postsynaptic current ; LVA, low-voltage-activated ; nBM, nucleus basalis of Meynert ; TTX, tetrodotoxin ; VH, holding potential.
Abstract
Abstract : Effects of selective Ca2+ channel blockers on GABAergic inhibitory postsynaptic currents (IPSCs) were studied in the acutely dissociated rat nucleus basalis of Meynert (nBM) neurons attached with nerve endings, namely, the “synaptic bouton” preparation, and in the thin slices of nBM, using nystatin perforated and conventional whole-cell patch recording modes, respectively. In the synaptic bouton preparation, nicardipine (3 × 10-6M) and ω-conotoxin-MVIIC (3 × 10-6M) reduced the frequency of spontaneous postsynaptic currents by 37 and 22%, respectively, whereas ω-conotoxin-GVIA had no effect. After blockade of L- and P/Q-type Ca2+ channels, successive removal of Ca2+ from external solution had no significant effect on the residual spontaneous activities, indicating that N-, R-, and T-type Ca2+ channels are not involved in the spontaneous GABA release. Thapsigargin, but not ryanodine, increased the frequency of spontaneous IPSCs in both the synaptic bouton and slice preparations, suggesting the partial contribution of the intracellular Ca2+ storage site to the spontaneous GABA release. In contrast, ω-conotoxin-GVIA (3 × 10-6M) and ω-conotoxin-MVIIC (3 × 10-6M) suppressed the evoked IPSCs by 31 and 37%, respectively, but nicardipine produced no significant effect. The residual evoked currents were abolished in Ca2+-free external solution but not in the external solution containing 10-5M Ni2+, suggesting the involvement of N-, P/Q-, and R-type Ca2+ channels but not L- and T-type ones in the evoked IPSCs. Neither thapsigargin nor ryanodine had any significant effects on the evoked IPSCs. It was concluded that Ca2+ channel subtypes responsible for spontaneous transmitter release are different from those mediating the transmitter release evoked by nerve stimulation.
Neurotransmitter release is known to be triggered by Ca2+ influxes into the presynaptic nerve terminal through voltage-dependent Ca2+ channels (Miller, 1987 ; Tareilus and Breer, 1995). Mammalian neurons express multiple types of voltage-dependent Ca2+ channels. Biophysical criteria effectively isolate low-voltage-activated (LVA) from high-voltage-activated (HVA) Ca2+ channels : The LVA Ca2+ channel is activated at hyperpolarized potentials relative to HVA Ca2+ channels and exhibits rapid inactivation (Carbone and Lux, 1984 ; Akaike et al., 1989 ; Tarasenko et al., 1997). So far, at least one LVA (T-type) and five HVA (L-, N-, P-, Q-, and R-type) Ca2+ channels have been found in mammalian neurons (Birnbaumer et al., 1994). Molecular biological studies have also identified several types of the α1 subunit (Birnbaumer et al., 1994). The α1A channel is sensitive to ω-agatoxin-IVA and has similarities to both P- and Q-type Ca2+ channels. The α1B channel shows properties similar to those of the N-type Ca2+ channel, which is inhibited by ω-conotoxin-GVIA (ω-Cg-GVIA). The α1C and α1D channels produce the L-type Ca2+ channels, which are sensitive to dihydropyridines. The recently cloned α1G channel shows almost the same electrophysiological properties as the T-type Ca2+ channel, although the α1E channel does not fit exactly to any type of the Ca2+ channels reported in native neurons (Birnbaumer et al., 1994 ; Perez-Reyes et al., 1998). This diversity of Ca2+ channel types generates the hypothesis that different types of Ca2+ channels contribute to different functions. These various types of Ca2+ channels can differ in their distribution among and within neurons (Bean, 1989 ; Mintz et al., 1992 ; Ishibashi et al., 1995 ; Kavalali et al., 1997). Accordingly, it is also important to identify which Ca2+ channel types are crucial for both presynaptic Ca2+ entry and transmitter release.
The nucleus basalis of Meynert (nBM) is populated by large cholinergic neurons and is the major source of cholinergic input to the cerebral cortex in mammals (Johnston et al., 1979 ; Wenk et al., 1980 ; Bigl et al., 1982). The nBM plays an important role in higher brain function by modulating cortical activity (McCormick, 1992 ; Jones, 1993). The activity of nBM itself is also modulated by GABAergic, noradrenargic, serotonergic, and dopaminergic afferent fibers from the nucleus accumbens, locus coeruleus, dorsal raphe nucleus, and ventral tegmental area, respectively. We previously reported that the spontaneous synaptic currents in acutely dissociated rat nBM neurons with adherent functional synaptic boutons were mediated by GABAA receptors (Akaike et al., 1992). However, the properties of the presynaptic Ca2+ channels regulating the inhibitory postsynaptic currents (IPSCs) in this “synaptic bouton” preparation have yet to be elucidated. We therefore investigated the effects of selective Ca2+ antagonists on both the spontaneous GABAergic IPSCs in the synaptic bouton preparation and the evoked IPSCs in the slice preparation and report here that the HVA Ca2+ channels responsible for the spontaneous IPSCs are different from those mediating the evoked IPSCs.
MATERIALS AND METHODS
Acutely dissociated neurons with and without synaptic boutons
The nBM neurons with synaptic boutons were acutely dissociated from 2-week-old Wistar rats that were decapitated under pentobarbital sodium anesthesia (50 mg/kg, i.p.). The brains were quickly removed and immersed in an ice-cold artificial CSF (ACSF) solution containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.5 mM NaH2PO4, 26 mM NaHCO3, and 20 mM glucose (pH 7.4), well oxygenated with 95% O2 and 5% CO2. Coronal slices (400 μm thick) including the nBM were prepared with the use of a Vibratome tissue slicer (model DTK-1000 ; Dosaka, Kyoto, Japan). Thereafter, the slices were transferred into the dish, and a firepolished glass pipette was touched lightly onto the surface of the nBM. The pipette was vibrated horizontally at ~5-10 Hz for ~1 min, and then the slices were removed. The neurons mechanically dissociated without using any enzymes adhered to the bottom of the dish within 20 min, thus enabling the electrophysiological studies to be conducted. These neurons retained their original morphological features such as proximal dendritic processes.
The dissociation technique of preparing the nBM neurons without synaptic boutons was used as described previously (Ishibashi et al., 1995). The brain slices (400 μm thick) containing the nBM were enzymatically treated with 0.2 mg/ml Pronase for 40 min at 31°C and subsequently with 0.2 mg/ml thermolysin for 20 min at 31°C. After the enzyme treatment, the nBM region was identified under a binocular microscope (SMZ-1 ; Nikon, Tokyo, Japan) and micropunched out with an electrolytically polished injection needle. The micropunchedout pieces were mechanically triturated in a 35-mm-diameter culture dish (Primaria 3801 ; Becton Dickinson, Rutherford, NJ, U.S.A.) with a fire-polished Pasteur pipette under a phase-contrast microscope (TMS-1 ; Nikon). The dissociated neurons adhered to the bottom of the dish within 20-30 min.
The membrane currents were recorded from the somata of the nBM neurons using the nystatin perforated patch recording mode with some modifications under voltage-clamp conditions (Horn and Marty, 1988 ; Akaike and Harata, 1994). The standard external solution contained 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose (pH. 7.4). For the Ca2+-free external solution, CaCl2 in the standard external solution was replaced with equimolar MgCl2, and EGTA was added (2 mM). These external solutions routinely contained 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX ; 5 × 10-6M) and DL-2-amino-5-phosphonovaleric acid (DL-AP5 ; 10-5M) to block the glutamatergic synaptic currents. The pipette solution contained 70 mM CsCl, 80 mM cesium methanesulfonate, and 10 mM HEPES (pH 7.2, adjusted with Tris-OH). A stock solution containing 10 mg/ml nystatin was prepared and added at a final concentration of 200 μg/ml to the patch pipette solution. After the formation of a stable perforated patch, the series resistance ranged from 10 to 20 MΩ. The currents and voltage were measured with a patch-clamp amplifier (EPC-7 ; List Electronic, Darmstadt, Germany) and were monitored simultaneously on an oscilloscope (MS-5100A ; Iwatsu, Tokyo) and a pen recorder (RECTI-HORIT-8K ; San-ei, Tokyo). The electrical recordings were carried out at room temperature (21-24°C). All drugs were applied using the Y-tube method as described previously (Akaike et al., 1992). The analysis of spontaneous IPSCs was carried out using DETECTiVENT, a spontaneous event analysis software (Ankri et al., 1994).
Brain slice preparation
Both the brain slice preparation and the electrical recording method from the preparation were done the same as previously described (Edwards et al., 1989). In brief, coronal brain slices (200 μm thick) containing the nBM were made from 2-week-old Wistar rats using a Vibratome tissue slicer (DTK-1000 ; Dosaka) and kept in a well-oxygenated ACSF solution for at least 1 h at room temperature before performing the electrical measurements. A single slice was then transferred to a recording chamber, in which it was continuously perfused with the oxygenated ACSF solution. The nBM neurons were visually identified with the use of a water immersion objective attached to a Zeiss upright microscope (Axioscope), and the current recordings were made in the nBM neurons at a holding potential (VH) of -80 mV under voltage-clamp conditions. The pipette solution contained 43 mM CsCl, 92 mM cesium-methanesulfonate, 5 mM tetraethylammonium chloride, 2 mM EGTA, 1 mM MgCl2, 4 mM magnesium ATP, and 10 mM HEPES (pH 7.2, adjusted with Tris-OH). Ionic currents were recorded with an EPC-7 patch-clamp amplifier (List Electronic) and stored on magnetic tapes with the use of a digital audiotape recorder (RD-120TE ; TEAC, Tokyo). The pipette access resistance was compensated for in the same manner as that explained by Llano et al. (1991). The bathing solution was superfused at 2-3 ml/min. Stimulation and on-line data acquisition were performed using the PULSE program on a Macintosh computer (HEKA). The signals were filtered at 3 kHz and digitized at 10 kHz. Extracellular stimulation for eliciting the synaptic currents was performed by applying short (100-μs) voltage pulses between a platinum reference electrode and a glass pipette with a 5-10-μm tip diameter filled with the ACSF solution.
Drugs
The drugs used included DL-AP5, bicuculline, CNQX, EGTA, nicardipine, and nystatin from Sigma ; ω-Cg-GVIA and ω-conotoxin-MVIIC (ω-Cm-MVIIC) from the Peptide Institute (Osaka, Japan), and tetrodotoxin (TTX) from Sankyo (Tokyo). All drugs were dissolved just before use.
Statistics
The results are expressed as mean ± SEM values. Student's paired t test and the Kolmogorov—Smirnov test were used for statistical analysis. Values of p < 0.05 were considered significant.
RESULTS
Spontaneous postsynaptic currents
The spontaneous postsynaptic currents were recorded from acutely dissociated rat nBM neurons attached with nerve endings, the so-called synaptic bouton preparation, by the nystatin perforated patch recording mode at a VH of -50 mV, as reported previously (Akaike et al., 1992). In the presence of 5 × 10-6M CNQX and 10-5M DL-AP5, the residual spontaneous currents were completely abolished by adding 10-5M bicuculline in a reversible manner (Fig. 1A), indicating that they are GABAergic IPSCs. The frequency of the spontaneous IPSCs was further reduced by adding TTX (3 × 10-7M ; Fig. 1B), suggesting the partial contribution of action potentials to the appearance of IPSCs. To avoid the contribution of the Na+ channels to the spontaneous IPSCs, the following experiments using the synaptic bouton preparation were done in the presence of 3 × 10-7M TTX.
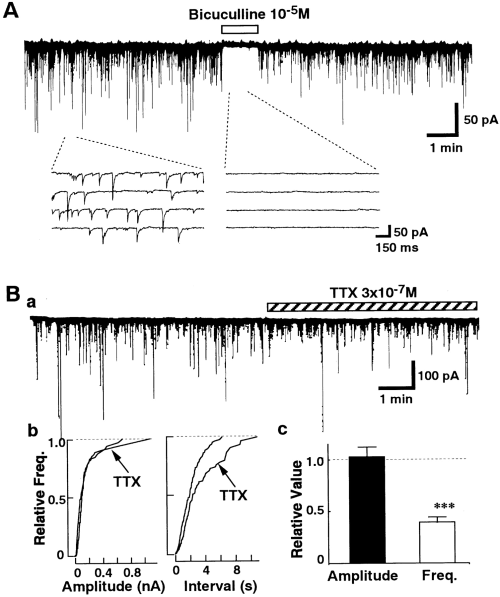
Spontaneous IPSCs in nBM neurons. Currents were recorded at a VH of -50 mV in the presence of both CNQX (5 × 10-6M) and DL-AP5 (10-5M). A : Effects of bicuculline on spontaneous synaptic currents. The figure is representative of six other neurons. B : Effects of TTX on spontaneous IPSCs. TTX did not affect the amplitude of spontaneous IPSCs but reduced their frequency. Ba : Representative current trace. Bb : Individual amplitude distributions of events in the left panel in the control and TTX treatment are not statistically significant (p = 0.32), as determined by Kolmogorov-Smirnov test. The number of events used for the cumulative plots was 425 for the control and 225 for TTX. Bc : Summary of the effects of TTX. Data were normalized to the values of the control period. The histograms show mean ± SEM (bars) values (n = 19). Asterisks indicate a significant difference from the control : ***p < 0.001 by paired t test.
The effects of Ca2+ channel blockers on the spontaneous IPSCs were examined. As shown in Fig. 2, ω-Cg-GVIA (3 × 10-6M), a blocker of N-type Ca2+ channels, had no significant effect on the spontaneous IPSCs (n = 6). In contrast, subsequent application of ω-Cm-MVIIC (3 × 10-6M), which inhibits not only P/Q-type but also N-type Ca2+ channels (Hillyard et al., 1992), decreased the frequency of the spontaneous IPSCs by 21.8 ± 4.2% (n = 6) without affecting the amplitude distribution. ω-Cm-MVIIC (3 × 10-6M) did not affect the GABA-induced current in acutely dissociated nBM neurons without synaptic boutons (n = 4 ; data not shown), indicating that ω-Cm-MVIIC itself does not directly act on the GABA-activated current. Figure 3 shows the effects of dihydropyridines on the spontaneous IPSCs. The L-type Ca2+ channel antagonist nicardipine (3 × 10-6M) reduced the frequency of spontaneous IPSCs by 37.1 ± 6.0% (n = 9 ; Fig. 3A), and the L-type Ca2+ channel agonist Bay K 8644 (10-6M) increased the frequency of the spontaneous IPSCs by 248.6 ± 28.5% (n = 7 ; Fig. 3B). Both dihydropyridines produced no significant effect on the amplitude distribution. Application of Ca2+-free external solution produced no further inhibition of the frequency of spontaneous IPSCs in the presence of both nicardipine and ω-Cm-MVIIC (Fig. 4). These results suggest that L- and P/Q-type HVA Ca2+ channels, but not T-, N-, and R-type Ca2+ channels, strongly contribute to the spontaneous GABA release from the presynaptic nerve terminal.

Effects of Ca2+ channel antagonists on spontaneous GABAergic IPSCs. A : Effects of ω-Cg-GVIA (3 × 10-6M ; n = 6) and ω-Cm-MVIIC (3 × 10-6M ; n = 6) on TTX-resistant spontaneous IPSCs. The cumulative amplitude (Ab) and cumulative frequency (Ac) distributions are plotted for IPSCs in the presence and absence of Ca2+ antagonists, which were applied cumulatively. Individual amplitude distributions of events in the control and ω-Cg-GVIA and ω-Cg-MVIIC treatment in Ab are not statistically significant, as determined by the Kolmogorov-Smirnov test. The number of events used for cumulative plots was 323 for the control, 169 for ω-Cg-GVIA, and 126 for ω-Cg-MVIIC. B : Summary of the effects of ω-Cg-GVIA and ω-Cm-MVIIC. Data were normalized to the values of the control. Asterisks indicate a significant difference from the control : **p < 0.01 by paired t test.
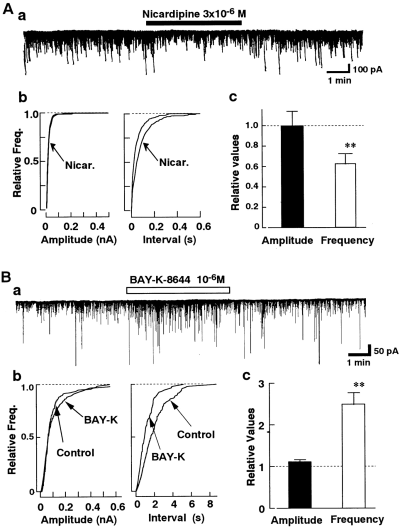
Effects of dihydropyridines on spontaneous IPSCs. A : Effect of nicardipine on spontaneous IPSCs. Nicardipine (Nicar. ; 3 × 10-6M) did not affect the amplitude of spontaneous IPSCs (p = 0.64 by Kolmogorov-Smirnov test) but reduced their frequency (n = 9). The number of events for the cumulative plots was 783 for the control and 413 for nicardipine. B : Effects of Bay K 8644 (10-6M ; n = 7) on the spontaneous IPSCs. The number of events for the cumulative plots was 704 for the control and 750 for Bay K 8644. **p < 0.01 by paired t test.
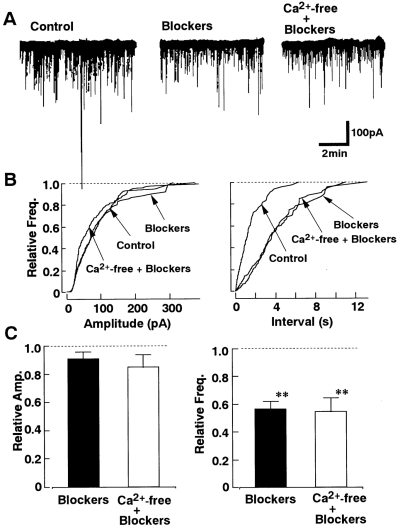
Effect of Ca2+-free external solution on spontaneous IPSCs after blockade of L- and P/Q-type Ca2+ channels. A : Comparison of spontaneous IPSCs recorded in control solution, test solution containing nicardipine (3 × 10-6M) and ω-Cm-MVIIC (3 × 10-6M), and finally in Ca2+-free solution with these blockers. B : Cumulative amplitude and frequency distributions are plotted for control, Ca2+ antagonists, and Ca2+-free solution with Ca2+ antagonists. Individual amplitude distributions of events in control, Ca2+-free solution, and blockers in the left panel are not statistically significant, as determined by the Kolmogorov-Smirnov test. The number of events used for cumulative distributions was 396 for control, 232 for Ca2+ antagonists, and 337 for Ca2+-free solution. C : Summary of the effects of Ca2+ antagonists and Ca2+-free solution containing these antagonists. Data were normalized to the values of the control (n = 8). **p < 0.01 by paired t test.
Because the spontaneous IPSCs were observed even in the Ca2+-free extracellular solution (Fig. 4), the effects of ryanodine and thapsigargin on the spontaneous IPSCs were investigated to assess the partial contribution of the Ca2+ release from intracellular Ca2+ storage sites. As shown in Fig. 5, thapsigargin (10-6M) increased the frequency of the spontaneous IPSCs by 256.0 ± 28.2% (n = 8). On the other hand, ryanodine (3 × 10-6M) had no significant effect. The results suggest the additional contribution of the inositol trisphosphate-sensitive Ca2+ release site to the spontaneous IPSCs.
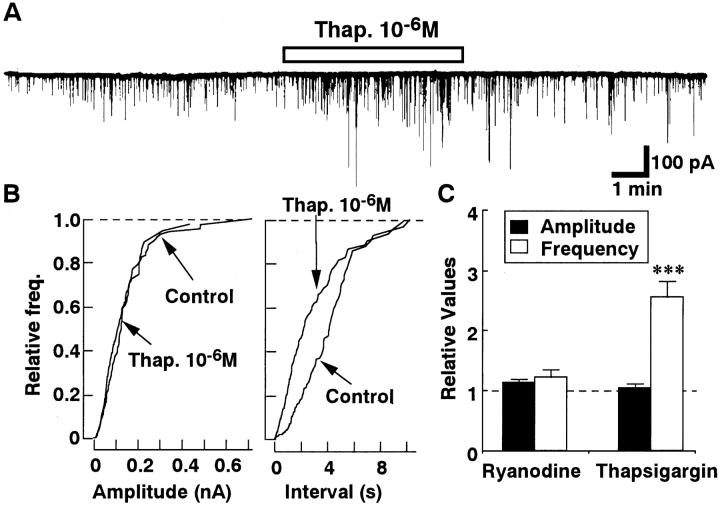
Effects of thapsigargin (Thap.) and ryanodine on spontaneous IPSCs. A : Representative current traces recorded before and during application of 10-6M Thap. B : Cumulative amplitude and frequency distribution. Thap. (10-6M) did not affect the amplitude of the spontaneous IPSCs (p = 0.24 by Kolmogorov-Smirnov test) but increased their frequency. The number of events used for cumulative distributions were 160 for control and 394 for Thap. C : Summary of the effects of Thap. (n = 8) and ryanodine (n = 5). Data were normalized to the values of the control period. Asterisks indicate a significant difference from the control : ***p < 0.001 by paired t test.
Evoked postsynaptic currents
The conventional whole-cell patch recordings of the evoked postsynaptic currents were made on the nBM neurons of each slice preparation. The VH was -80 mV. The postsynaptic currents recorded in the normal ACSF solution consisted of both the inhibitory and excitatory components. As shown in Fig. 6A, CNQX (3 × 10-6M) reduced a fraction of the evoked currents, indicating the existence of the glutamate-mediated excitatory component. The residual postsynaptic current was fully inhibited by 3 × 10-6M bicuculline, indicating that the evoked currents observed in the presence of CNQX are the GABA-mediated IPSCs. The evoked IPSCs were also fully abolished by application of Ca2+-free ACSF solution, suggesting that the evoked IPSCs could be elicited by Ca2+ influx into the presynaptic nerve terminals through the voltage-dependent Ca2+ channels.
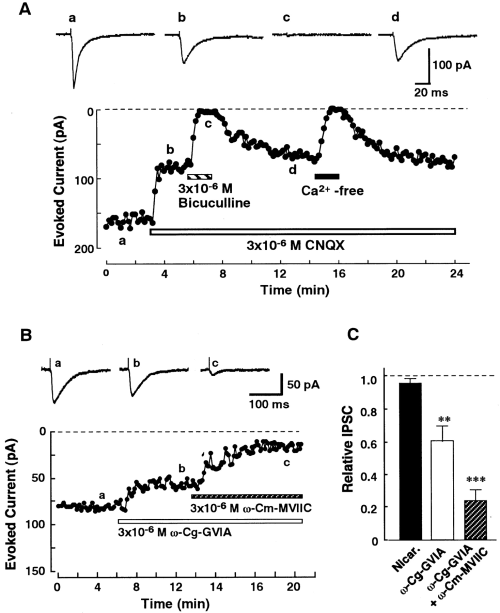
Effects of Ca2+ channel antagonists on evoked IPSCs in nBM neurons. A : Effect of Ca2+-free ACSF containing 200 μM EGTA on bicuculline-sensitive evoked IPSCs. The postsynaptic currents elicited by the electrical stimulation (10 V, 100 μs, 0.1 Hz) were recorded at a VH of -80 mV. The amplitude of each current was plotted as a function of time. The current traces in the upper panel (a-d) were recorded at the same time indicated in the lower panel. The same results were obtained from five other neurons. B : Representative records of the evoked IPSCs in control conditions (a) and with organic Ca2+ antagonists (b and c). The toxins were applied cumulatively. C : Effects of nicardipine (Nicar. ; 3 × 10-6M ; n = 7), ω-Cg-GVIA (3 × 10-6M ; n = 6), and ω-Cm-MVIIC (3 × 10-6M ; n = 6) on the evoked IPSCs. Asterisks indicate a significant difference from the control : **p < 0.001, ***p < 0.001 by paired t test.
To assess the Ca2+ channel types mediating the evoked IPSCs recorded in the nBM neurons, the effects of selective Ca2+ channel antagonists were examined in the continuous presence of 3 × 10-6M CNQX. Nicardipine (3 × 10-6M ; n = 7) had little effect on the evoked IPSCs (Fig. 6C), thus suggesting that the L-type Ca2+ channel was not involved in the evoked IPSCs. In contrast, ω-Cg-GVIA (3 × 10-6M) inhibited the evoked IPSCs by 30.8 ± 9.5% (n = 6 ; Fig. 6B). The dihydropyridine- and ω-Cg-GVIA-resistant evoked IPSCs were partially blocked by cumulative application of 3 × 10-6Mω-Cm-MVIIC (Fig. 6B and C). The average inhibition was 36.8 ± 7.2% (n = 6). Successive application of ω-Cm-MVIIC at 6 × 10-6M produced no further inhibition (data not shown), thus suggesting such blockage to be maximal. These residual IPSCs in the presence of these Ca2+ antagonists were not affected by application of 10-5M Ni2+ (n = 4 ; data not shown). This concentration of Ni2+ inhibited almost 50% of the LVA Ca2+ channel currents but had no effect on the HVA Ca2+ channel (authors' unpublished data). Therefore, the present data suggest that the T-type channel does not contribute to the evoked IPSCs. The residual IPSCs were completely eliminated by removal of extracellular Ca2+ (data not shown), suggesting the contribution of the R-type channel. Ryanodine (3 × 10-6M ; n = 5) and thapsigargin (10-6M ; n = 6) had no effect on the evoked IPSCs (data not shown), suggesting that the intracellular Ca2+ storage site does not contribute to the evoked IPSCs. Consequently, these results suggest that the N-, P/Q-, and R-type Ca2+ channels contribute to the evoked IPSCs in the presynaptic nerve terminals.
Spontaneous IPSCs in the slice preparation
Because the Ca2+ channel subtypes responsible for the evoked IPSCs were different from those mediating the spontaneous IPSCs, the effect of nicardipine on the spontaneous IPSCs in the slice preparation was also investigated. As shown in Fig. 7, nicardipine (3 × 10-6M) inhibited the spontaneous IPSCs recorded from the slice preparation by 32.2 ± 7.0% without changing the amplitude distribution (n = 8). We also tested the effects of ryanodine and thapsigargin on the spontaneous IPSCs in the slice preparation. As shown in Fig. 7C, ryanodine (3 × 10-6M, n = 6) had no significant effect on the spontaneous IPSCs recorded from the slice preparation. However, thapsigargin (10-6M) could increase the frequency of the spontaneous IPSCs by 154 ± 11.8% (Fig. 7C ; n = 7) even in the slice preparation, as observed in the synaptic bouton preparation (Fig. 5).
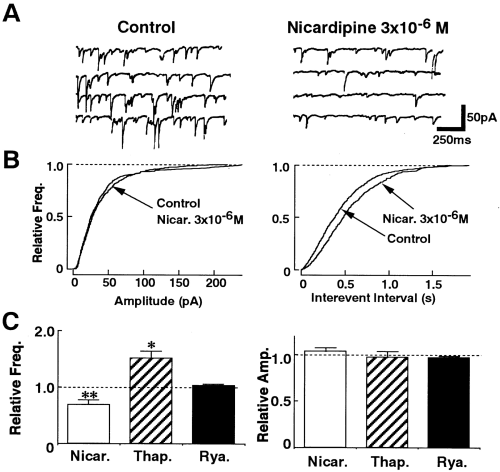
Spontaneous IPSCs in the slice preparation. A : Representative current traces recorded before and during application of 3 × 10-6M nicardipine. The VH was -80 mV. B : Cumulative amplitude and frequency distribution. Nicardipine (Nicar.) did not affect the amplitude of the spontaneous IPSCs (p = 0.33 by Kolmogorov-Smirnov test) but reduced their frequency. C : Effects of Nicar. (3 × 10-6M ; n = 8), thapsigargin (Thap. ; 10-6M ; n = 7), and ryanodine (Rya. ; 3 × 10-6M ; n = 6) on spontaneous IPSCs in the slice preparation. Data were normalized to the values of the control. Asterisks indicate a significant difference from the control : *p < 0.05, **p < 0.01 by paired t test.
DISCUSSION
The present study demonstrated that multiple subtypes of HVA Ca2+ channels are involved in GABA release at the inhibitory nerve terminals that project to the Meynert neurons of the rat. The spontaneous GABAergic IPSCs were not significantly affected by application of ω-Cg-GVIA but were inhibited by ω-Cm-MVIIC (Fig. 2), indicating the contribution of presynaptic P/Q-type Ca2+ channels to the spontaneous GABA release. The frequency of spontaneous IPSCs was also reduced by nicardipine and was increased by Bay K 8644 (Fig. 3), indicating the involvement of the L-type Ca2+ channel in the spontaneous GABA release. After blockade of the L- and P/Q-type Ca2+ channels, the residual spontaneous IPSCs were not affected by removal of Ca2+ from the external solution (Fig. 4). These pharmacological features indicated that no other types of Ca2+ channels, besides the L and P/Q type, were involved in the spontaneous GABA release from the presynaptic GABAergic nerve terminal, and the results were clearly different from those observed for evoked IPSCs. The evoked GABAergic IPSCs were inhibited by both ω-Cg-GVIA and ω-Cm-MVIIC (Fig. 6B and C), thus suggesting the existence of N- and P/Q-type Ca2+ channels in the GABAergic nerve terminal. Nicardipine had no significant effects on the evoked IPSCs (Fig. 6C). Moreover, the evoked IPSCs observed in the presence of ω-Cg-GVIA and ω-Cm-MVIIC were not affected by 10-5M Ni2+ but were completely abolished by removal of Ca2+ from the external solution, suggesting the additional involvement of R-type, but not T-type, Ca2+ channels in the evoked IPSCs. These data suggest a possible contribution of N-, P/Q-, and R-type Ca2+ channels to the evoked IPSCs.
In the present study, nicardipine reduced the frequency of spontaneous IPSCs recorded from both the synaptic bouton and slice preparations (Figs. 3 and 7), and the inhibition ratio of the spontaneous IPSC frequency by nicardipine was almost the same in both preparations. In addition, the frequency of spontaneous IPSCs was increased by thapsigargin, but not by ryanodine, in both preparations (Figs. 5 and 7). These results suggest that the contribution of Ca2+ channel subtypes and the intracellular calcium storage site to the spontaneous IPSCs does not change as a result of dissociation.
The presynaptic Ca2+ channels have been reported to be clustered within the active zone, the site of transmitter secretion (Heuser et al., 1979). In such active zones, it has been assumed that synaptic vesicles undergoing exocytosis must be present very close to the Ca2+ channels for release to be rapid (Burgoyne and Morgan, 1995). Accordingly, the distribution of Ca2+ channel subtypes within the nerve terminal is important to understand the significance of the particular types of Ca2+ channels for transmitter release. Miller (1987) postulated that L-type Ca2+ channels are present at the presynaptic nerve terminal but distant from the active zone and suggested that Ca2+ influx into the presynaptic nerve terminal through L-type Ca2+ channels induced by an action potential could not trigger the transmitter release. On the other hand, N-type channels are clustered near the transmitter release site and play a crucial role in the nerve-evoked transmitter release (Miller, 1987). Present results are consistent with the hypothesis of Miller (1987) and also suggest that the P/Q-type Ca2+ channels can mediate both spontaneous and evoked transmitter release. The contribution of P-type Ca2+ channels to both types of transmitter release has also been reported in rat cerebellum (Momiyama and Takahashi, 1994).
Recently, Reuter (1995) has shown that the distribution of the presynaptic N-type Ca2+ channel is nonuniform in the cultured hippocampal neurons using FM1-43. In his report, at some terminals, exocytosis was blocked completely by ω-Cg-GVIA, whereas at others, it was only partially blocked. In addition, at autaptic synapses in cultured hippocampal neurons, the mixture of N- and P/Q-type Ca2+ channel subtypes varies markedly from terminal to terminal (Reid et al., 1997). The Q-type Ca2+ channel has also been reported to be present on the neurohypophysial terminals, where they control vasopressin but not oxytocin secretion (Wang et al., 1997). In contrast, Poncer et al. (1997) reported that the inhibitory synapses originating from a single presynaptic neuron in the hippocampal slice cultures apparently express only one subtype of Ca2+ channel. According to their report, the inhibitory transmission between interneurons in the striatum radiatum and pyramidal neurons is selectively mediated by the N-type Ca2+ channel, whereas that between interneurons in the striatum lucidum and pyramidal neurons is mediated by the P/Q-type Ca2+ channels. The present study was made from a large number of synapses terminating at neurons freshly dissociated from the rat nBM region. The Ca2+ channel subtypes at the single presynaptic nerve terminals projecting to the rat nBM neurons should therefore be examined in a future study.
According to our previous observation (Akaike et al., 1992), the spontaneous IPSCs recorded in the Meynert neurons acutely dissociated from 8-week-old rats were regulated by the presynaptic intracellular free Ca2+ (a) accumulated via the voltage-dependent Ca2+ channels and (b) released from ryanodine-sensitive Ca2+ release sites. In the present study, performed with Meynert neurons dissociated from 2-week-old rats, the frequency of spontaneous IPSCs was increased by application of thapsigargin but not of ryanodine (Fig. 5). Therefore, the Ca2+ release from the intracellular Ca2+ store regulating the spontaneous GABA release might change during the postnatal development.
The present results clearly showed that the combination of Ca2+ channels mediating the nerve-evoked GABA release at the inhibitory nerve endings is different from that mediating the spontaneous GABA releases. The difference in the combination between the Ca2+ channels responsible for the evoked IPSCs and those mediating spontaneous IPSCs has been also reported at the cerebellar inhibitory synapses (Momiyama and Takahashi, 1994) and the hippocampal inhibitory synapses (Doze et al., 1995) of the rats. It has also been reported that the HVA Ca2+ channels are composed of different subunits : α1, α2/δ, and γ (Ahlijanian et al., 1990 ; Witcher et al., 1993). At least seven different genes encoding α1 subunits and three distinct genes encoding β subunits have been isolated from various types of tissues (Vardi et al., 1995 ; Perez-Reyes et al., 1998). The α1 subunit alone is able to direct the permeation of Ca2+ and also has specific binding sites for Ca2+ antagonists. The differences in presynaptic Ca2+ channels mediating the evoked and spontaneous IPSCs might result from the functionally and/or regionally different expression of the Ca2+ channel subunits in the presynaptic GABAergic nerve terminals.
Acknowledgements
This study was supported by Grants-in-Aid for Scientific Research 10770010 (to J.-S.R.), 0495 (to H.I.), and 10044301, 10044301, and 10470009 (to N.A.) from the Ministry of Education, Science and Culture, Japan.




