Quantification of Axonal Damage in Traumatic Brain Injury
Affinity Purification and Characterization of Cerebrospinal Fluid Tau Proteins
Abbreviations used : BAP, bacterial alkaline phosphatase ; CNBr, cyanogen bromide ; CT, computed tomography ; cTau, cleaved tau ; DAI, diffuse axonal injury ; GCS, Glasgow Coma Scale ; Mab, monoclonal antibody ; MAP, microtubule-associated protein ; PAGE, polyacrylamide gel electrophoresis ; SDS, sodium dodecyl sulfate ; TBI, traumatic brain injury ; TBST, Tris-buffered saline containing Tween.
Abstract
Abstract : Diffuse axonal injury is a primary feature of head trauma and is one of the most frequent causes of mortality and morbidity. Diffuse axonal injury is microscopic in nature and difficult or impossible to detect with imaging techniques. The objective of the present study was to determine whether axonal injury in head trauma patients could be quantified by measuring levels of CSF tau proteins. Tau proteins are structural microtubule binding proteins primarily localized in the axonal compartment of neurons. Monoclonal antibodies recognizing the form of tau found in the CSF of head trauma patients were developed by differential CSF hybridoma screening using CSF from head trauma and control patients. Clones positive for head trauma CSF tau proteins were used to characterize this form of tau and for ELISA development. Using the developed ELISA, CSF tau levels were elevated >1,000-fold in head trauma patients (mean, 1,519 ng/ml of CSF) when compared with patients with multiple sclerosis (mean, 0.014 ng/ml of CSF ; p < 0.001), normal pressure hydrocephalus (nondetectable CSF tau), neurologic controls (mean, 0.031 ng/ml of CSF ; p < 0.001), or nonneurologic controls (nondetectable CSF tau ; p < 0.001). In head trauma, a relationship between clinical improvement and decreased CSF tau levels was observed. These data suggest that CSF tau levels may prove a clinically useful assay for quantifying the axonal injury associated with head trauma and monitoring efficacy of neuroprotective agents. Affinity purification of CSF tau from head trauma patients indicated a uniform cleavage of ~ 18 kDa from all six tau isoforms, reducing their apparent molecular sizes to 30-50 kDa. These cleaved forms of CSF tau consisted of the interior portion of the tau sequence, including the microtubule binding domain, as judged by cyanogen bromide digestion. Consistent with these data, CSF cleaved tau bound taxolpolymerized microtubules, indicating a functionally intact microtubule binding domain. Furthermore, epitope mapping studies suggested that CSF cleaved tau proteins consist of the interior portion of the tau sequence with cleavage at both N and C terminals.
Traumatic injury is the most common cause of death and permanent disability in the early decades of life (Frankowski et al., 1985). Traumatic brain injury (TBI) is responsible for the majority of these deaths and disabilities. The incidence of TBI peaks at 550 per 100,000 people 15-24 years of age and characteristically transforms young, productive individuals into dependent ones that often require decades of costly institutionalized care.
Diffuse axonal injury (DAI) is one of the most common forms of injury in TBI patients making up ~48% of all primary lesions (Gentry, 1990). DAI results from shear strain forces caused by marked rotational acceleration of the head during impact (Adams et al., 1989). DAI occurs primarily in the lobar white matter, particularly at the corticomedullary junction of the frontal and temporal lobes. In more severe cases, DAI also occurs in the corpus callosum and brainstem. Clinically, severe DAI is usually associated with impairment of consciousness, which begins at the moment of impact.
Despite the introduction of magnetic resonance imaging, computed tomography (CT) is the imaging modality of choice for evaluating TBI patients (Gentry, 1994). The advantages of CT include widespread availability, rapid imaging time, low cost, and safety. Unfortunately, most DAI lesions are not radiographically visible by CT (Gentry, 1990). Because most of the damage in DAI is microscopic, only 10% of patients with DAI demonstrate abnormalities on CT. That is, DAI lesions cannot be detected on CT unless they are large enough to demonstrate a focus of subtle hypodensity, possibly representing edema. These difficulties indicate the clinical need for an improved method for assessing DAI in head injury.
Several lines of evidence suggest that measuring levels of the microtubule-associated protein (MAP) tau in CSF may provide an alternative method of assessing axonal injury in TBI. First, tau is a protein localized primarily in neurons (Binder et al., 1985 ; Kosik and Finch, 1987). Second, tau is localized principally in the axonal compartment. The axonal localization of tau results from the targeted transport of its mRNA to the proximal portion of axons (Litman et al., 1993). Furthermore, tau demonstrates selective axonal stabilization, resulting in tau sequestration in the axonal compartment (Kanai and Hirokawa, 1995). Tau is expressed from a single gene that undergoes alternate splicing resulting in six tau isoforms with apparent molecular masses between 48 and 68 kDa (Goedert et al., 1989). Functionally, tau binds to axonal microtubules, resulting in the formation of axonal microtubule bundles. These bundles form important structural elements in the axonal cytoskeleton and are critical elements in the axoplasmic flow of proteins between the nerve terminal and neuronal cell body.
In the present study, monoclonal antibodies (Mabs) were developed that recognize the posttranslationally modified form of tau found in TBI patient CSF. This was accomplished by differential hybridoma screening. Hybridoma supernatants were selected that differentially bound to TBI patient CSF but not to control CSF. The developed Mabs demonstrated a >1,000-fold higher affinity for the modified form of tau present in CSF from head-injured patients than for intact full-length tau. These newly developed CSF tau Mabs were used to affinity-purify and to characterize tau from the CSF and brain of TBI patients and to develop a sensitive sandwich ELISA for quantifying tau in patient CSF.
MATERIALS AND METHODS
CSF samples
CSF samples were collected under an approved protocol from the University of Cincinnati Institutional Review Board. CSF was collected from hospitalized patients with severe closed head injury [Glasgow Coma Scale (GCS), [8] and four control groups (normal pressure hydrocephalus, multiple sclerosis, neurologic, and nonneurologic controls). For patients with head injury or normal pressure hydrocephalus, intraventricular catheters were surgically placed and continuously monitored. CSF was collected directly from transduction tubing at least every 24 h, centrifuged at 13,000 g for 15 min, and stored at -70°C until use. The multiple sclerosis group (n = 15) consisted of patients with either a progressive (n = 7) or relapsing (n = 8) course. The neurologic control group consisted of patients with migraine (n = 5), wrist pain (n = 2), seizure disorder (n = 3), Guillain-Barré syndrome (n = 2), transverse myelitis (n = 2), or optic neuritis (n = 1). The nonneurologic control group consisted of patients with a psychiatric disorder (n = 15). CSF was centrifuged at 13,000 g for 15 min and stored at -70°C. The GCS was used to quantify the patient's clinical condition during hospitalization (Jennett et al., 1977). The GCS measures verbal performance, motor response, and eye opening on a 3-15 point scale, with the highest score reflecting normal performance.
Antibodies
Mabs PHF-1 (IgG ; diluted 1:500) and Alz50 (IgM ; diluted 1:10), both raised against a paired helical filament preparation (Wolozin et al., 1986 ; Greenberg et al., 1992), were the generous gift of Dr. Peter Davies. Mab SMI33 (IgM ; diluted 1:750), which recognizes nonphosphorylated Ser235 of tau (Lichtenberg-Kraag et al., 1992), was purchased from Sternberger Monoclonals (Baltimore, MD, U.S.A.). Polyclonal antibody BYA-1074 (diluted 1:1,000), raised against bovine tau (Kosik et al., 1989), and tau Mab Tau-1 (IgG ; diluted 1:500), which recognizes nonphosphorylated Ser199 of tau (Liu et al., 1993), were purchased from Accurate Chemical & Scientific Corp. (Westbury, NY, U.S.A.). Polyclonal antibody Alz5 (diluted 1:300) was raised against a synthetic peptide corresponding to the C-terminal 13 amino acids of tau (Caputo et al., 1992).
Protein purification and expression
Tau purification, dephosphorylation, and digestion.
Tau was purified using a procedure modified from that of Nukina et al. (1987). In brief, postmortem human brains were homogenized in a volume 2.5 times their weight in 50 mM Tris-HCl (pH 6.8), 0.3 M NaCl, 1% β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and 5 μM leupeptin. The homogenate was centrifuged at 30,000 g for 5 min at 4°C. The supernatant was incubated on ice, and the pellet was homogenized a second time in a volume 2.5 times its weight with 50 mM Tris-HCl (pH 9.2), 0.3 M NaCl, and 1% β-mercaptoethanol. The homogenate was then centrifuged at 4°C for 5 min at 30,000 g. The supernatants were combined and boiled for 10 min. The samples were then centrifuged for 30 min at 30,000 g at 4°C. The samples were dialyzed overnight against 50 mM Tris-HCl before examination by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Tau antigen was prepared by electrophoresing the tau preparation on 10% SDS-polyacrylamide curtain gels. Proteins with molecular masses of 30-80 kDa were excised, electroeluted in a Schleicher & Schuell (Keene, NH, U.S.A.) Elutrap device containing 40 mM Tris-borate buffer (pH 8.64) and 0.8% SDS, and then dialyzed against 50 mM Tris-HCl before injection. Brain and CSF tau proteins were dephosphorylated overnight at 37°C in 50 mM Tris-HCl (pH 8.0) with or without 1 U/ml bacterial alkaline phosphatase (BAP ; Sigma Chemical Co., St. Louis, MO, U.S.A.). The effect of BAP treatment on Mab binding was assessed by solid-phase ELISA. Wells were coated with 300 ng of tau, blocked, and washed, and primary antibody was added for 1 h and assayed as described below. For epitope mapping studies, tau samples were digested in 200 μl of 70% formic acid containing 50 mg/ml cyanogen bromide (CNBr) solution and incubated over-night at room temperature. Samples were washed twice with 1 ml of double distilled water and evaluated on 15% SDS-PAGE gels.
Purification of microtubules.
Tubulin was purified from rat brain, and microtubules were assembled in the presence of taxol (Schiff et al., 1979 ; Vallee, 1982). MAPs were dissociated from the microtubules by suspending the microtubule pellet (600 μg) in assembly buffer (40 μM taxol in 0.1 M PIPES, 1.0 mM EGTA, 1.0 M MgSO4, and 1.0 mM GTP), and NaCl was added to a final concentration of 0.35 M. After repeated salt extractions (three times), all MAPs had been removed from the microtubule samples as judged by western blots of the third supernatant probed with antibody BYA-1074.
MAP purification.
NaCl was removed from the above microtubule pellet (600 μg) by washing in 1.3 ml of assembly buffer. Microtubules were pelleted at 30,000 g for 25 min ; CSF or human heat-stable proteins containing tau and 1.3 ml of assembly buffer were added to the pellet, incubated for 10 min at 37°C, and centrifuged at 30,000 g for 25 min. Proteins not bound to microtubules were removed by two cycles of washing (1.3 ml of assembly buffer, incubation for 10 min at 37°C, and centrifugation at 30,000 g for 25 min). Microtubule-bound proteins were dissociated by addition of 0.5 ml of assembly buffer with 0.35 M NaCl to the washed microtubule pellet. Following incubation for 10 min at 37°C, the sample was dialyzed against 50 mM Tris-HCl and analyzed by immunoblotting.
Recombinant tau.
Recombinant human tau was produced using the previously described pET-n123c and pET-n1234c plasmids expressed in the BL21(DE3) expression vector (Lee and Rook, 1992). The plasmids were the generous gift of Dr. Gloria Lee. Plasmid pET-n123c codes for the 352-amino acid three-repeat form of tau, whereas plasmid pET-n1234c codes for the 383-amino acid four-repeat form of tau containing exon 10. Following expression, tau was purified as above and was estimated to be ~90% pure as judged by Coomassie Blue-stained SDS-PAGE.
Mab production
Human tau Mabs.
Female BALB/c mice were injected intraperitoneally with 100 μg of the antigen preparation suspended in an equal volume of Freund's complete adjuvant (Sigma). Boostering was performed at 2-week intervals with 100 μg of the antigen suspended in incomplete Freund's adjuvant (Sigma). Mice were bled before each boostering and sera were titrated by ELISA. Mabs were produced as previously described (Kohler and Milstein, 1975). In brief, 1.8 × 108 spleen cells were mixed with 3.6 × 107 NS1/1-Ag-4 mouse myeloma cells. Fusion was induced by addition of 38% polyethylene glycol 1550. Cells were washed with Dulbecco's modified Eagle's medium (GibcoBRL, Gaithersburg, MD, U.S.A.) and resuspended in Super Dulbecco's modified Eagle's medium (GibcoBRL) containing 14% fetal calf serum and a hypoxanthine, aminopterin, and thymidine combination (Sigma). Cells were dispersed in 96-well microtiter plates coated with a mouse splenocyte feeder layer. Supernatants were screened after plating against CSF from CNS trauma patients or controls by ELISA and western blot. Colonies that produced supernatant found to react with CSF from CNS trauma patients but not control CSF were expanded and cloned by limiting dilution. To ensure isolation of monoclonal hybridomas, cloning was repeated until 100% of the wells showed specific Mab production. At the end of this process, three Mabs, designated cTau7, cTau8, and cTau12 (where cTau represents cleaved tau), were recovered.
Ascites production.
Male BALB/c mice were primed by intraperitoneal injection of 0.5 ml of 2, 6, 10, 14-tetramethylpentadecane (Pristane ; Sigma), followed 14 days later by injection of 106 hybridoma cells. After 7 days, the peritoneal cavities were tapped. Ascites fluids were titrated, pooled by hybridoma, and stored at -20°C. To ensure establishment of stable cell lines, hybridomas were passed twice through Pristane-primed mice. Following each passage, hybridomas were recloned. Stability was defined as 100% of hybridoma supernatants exhibiting immunoreactivity against antigen by ELISA. Stable hybridomas were then injected into mice, and ascites was collected for Mab purification (below).
Mab purification and tau affinity purification.
One milliliter of ascites was diluted 1:1 with 50 mM sodium acetate (pH 5.0) and applied to 2 ml of equilibrated protein G-Sepharose. After washing, Mabs were eluted with Gentle Ag/Ab Elution Buffer (Pierce, Rockford, IL, U.S.A.) and desalted over Sepharose G-25 (2SQ-B ; Isolab, Akron, OH, U.S.A.). Mab purity was confirmed by isoelectric focusing using Resolve agarose isoelectric focusing gels (Isolab) and SDS-PAGE. Purified Mabs 7A5, 8A12, and 12B2 were used to affinity-purify human tau using protein G-agarose (Boehringer Mannheim, Germany) as specified by the manufacturer. Purified Mabs were conjugated to horseradish peroxidase (Finnsugar) as described by Boorsma and Kalsbeek (1975). A checkerboard titration was performed to determine the optimal conjugate dilution for ELISA and immunoblot studies.
Immunoblotting
CSF or nondigested brain proteins were electrophoresed on 10% SDS-polyacrylamide gels (Laemmli, 1970) and transferred electrophoretically to nitrocellulose (BA-S 85 ; Schleicher & Schuell) as described by Towbin et al. (1979). Nonspecific binding was blocked with 5% bovine serum albumin in Tris-buffered saline containing Tween [TBST ; 0.1 M Tris-HCl and 0.9% NaCl with 0.1% (vol/vol) Tween 20] for 1 h. The membrane was washed for 30 min in TBST, incubated with primary antibody for 1 h, and blocked in 5% nonfat dry milk in TBST for 15 min. After washing (three times in TBST), a 1:200 dilution of biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, U.S.A.) in TBST was added for 30 min, and the membrane was washed and then transferred to Vectastain ABC-peroxidase (Vector Laboratories) in TBST for 30 min. The blot was washed and transferred to a substrate solution of diaminobenzidine tetrahydrochloride dihydrate in phosphate-buffered saline with 1% NiCl2 and 1% CoCl2. Mabs cTau7, cTau8, and cTau12 were diluted 1:1,000 for immunoblots.
ELISA
Tau sandwich ELISA. Immulon 2 plates were coated with affinity-purified Mab cTau12 (100 μl per well, 5 μg/ml) for 1 h and overcoated overnight with 5% nonfat dry milk and 0.5% gelatin in Tris-buffered saline. Plates were washed with TBST, 100 μl of affinity-purified tau was added per well (27-1,720 pg), and the plates were incubated for 1 h and then washed with TBST. A 1:1 mixture of horseradish peroxidase-conjugated Mabs cTau7 and cTau8 (100 μl per well ; diluted 1:2,000) was added, and the plates were incubated for 1 h and washed with TBST. Biotin-tyramine (100 μl, 3 μg/ml in 50 mM Tris-HCl and 0.001% H2O2, pH 8.0) was added for 15 min, and plates were washed (Bobrow et al., 1989). Color was developed with Vector Laboratories ABC-AP using nitrophenyl phosphate as substrate and assayed at 405 nm. Negative controls included exclusion of cTau12 or cTau7 and cTau8 or deletion of tau from the assay.
RESULTS
CSF tau Mabs
Mabs were developed that recognize the posttranslationally modified form of tau found in TBI patient CSF. Hybridoma supernatants were subjected to a differential solid-phase ELISA screen using CSF samples from TBI and control patients. Three monoclonal-producing hybridomas, cTau7, cTau8, and cTau12, were selected that maximally discriminated head injury from control CSF. For example, cTau7 CSF immunoblot data from four head injury patients are shown in Fig. 1. The CSF sample in lane a was collected on day 1 of hospitalization from a patient with DAI. This patient was comatose (GCS =4) on admission and at the time of CSF collection. CT revealed limited CNS pathology that was insufficient to explain the patient's clinical symptoms. The diagnosis of DAI was made based on clinical data and serial CT scans showing no disturbance of the mesencephalic cisterns or evidence of any midline shift, with CNS pathology limited to a small hemorrhagic focus in the right temporal region. Immunoblot analysis of unconcentrated CSF revealed labeling of 30-50-kDa proteins with all three Mabs developed (only cTau7 data are shown). Because normal tau demonstrates an apparent molecular size of 48-68 kDa, the decreased molecular size of labeled CSF tau suggests that the CSF form of tau is cleaved. CSF cTau levels for this patient were 1,202 ng/ml using the cTau ELISA described below. Western blot analysis of CSF collected the following day revealed appreciably less cTau Mab labeling, which was consistent with a decrease in CSF cTau levels to 413 ng/ml as measured by ELISA (data not shown).
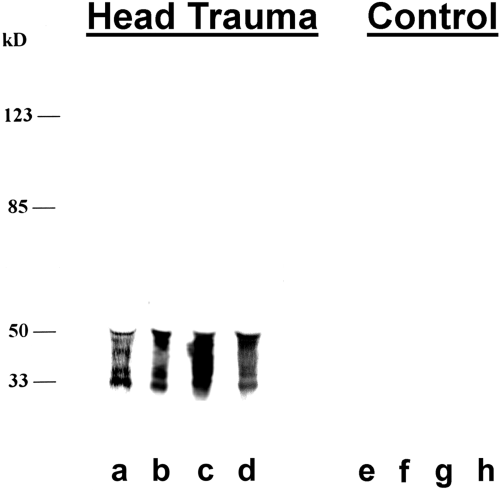
. Mabs recognizing CSF cTau proteins were developed by differential CSF screening. Mabs were developed that specifically recognized 30-50-kDa CSF cTau proteins using a differential CSF screen. Hybridomas were selected that labeled CSF proteins from patients with severe closed head injury (Head Trauma, lanes a-d) but not control patients (lanes e-h). Western blot of unconcentrated CSF samples was performed with Mab cTau7 (1:1,000). Lane a is 50 μl of CSF (60 ng of cTau by ELISA), lane b is 3 μl of CSF (35 ng of cTau), lane c is 50 μl of CSF (45 ng of cTau), lane d is 50 μl of CSF (41 ng of cTau), and lanes e-h are all 50 μl of CSF from control patients (nondetectable levels of cTau by ELISA). Molecular mass markers are shown at the left.
Similar 30-50-kDa labeling was observed in all other CSF samples from head-injured patients with the cTau Mabs developed (Fig. 1). The patient in lane b was admitted with severe head injury (GCS = 4) and demonstrated significant cTAu Mab labeling of 30-50-kDa proteins in CSF collected on day 1 of hospitalization. Day 1 CSF cTau levels were 11,810 ng/ml, decreasing to 4,754 ng/ml on day 2 and 397 ng/ml on day 3. The patient in lane c was also admitted with severe head injury and demonstrated significant labeling of 30-50-kDa CSF proteins on day 1 of hospitalization. Clinical data and CSF cTau levels were obtained for this patient during a 10-day course of hospitalization (Fig. 2). On admission, this patient was comatose (GCS = 6) and demonstrated an elevated CSF cTau level (904 ng/ml). CSF cTau levels rapidly decreased during the first 4 days of hospitalization, and this improvement in CSF cTau levels was associated with a significant clinical improvement as indicated by GCS ratings in the normal range by day 8 (Fig. 2). The patient in lane d was admitted with severe head injury (GCS = 6) and demonstrated similar labeling with cTau Mabs on western blots and decreasing CSF cTau levels during hospitalization : day 1 = 828 ng/ml, day 2 = 313 ng/ml, day 3 = 145 ng/ml, and day 4 = 15 ng/ml. In comparison, control patients demonstrated no CSF cTau Mab immunoreactivity on western blots and no detectable cTau levels by ELISA (Fig. 1 and Table 1).

. Levels of CSF cTau are correlated with patient clinical improvement. CSF cTau levels were measured during a 10-day course of hospitalization for a patient with closed head injury (see Results), and the patient's clinical condition was quantified with the GCS. A time-dependent improvement in patient CSF cTau levels was observed that was related to the patient's clinical improvement (GCS scores). The western blot of patient day 1 CSF with cTau Mab is shown in Fig. 1, lane c. GCS scores range from 3 to 15, with the highest score representing normal consciousness.
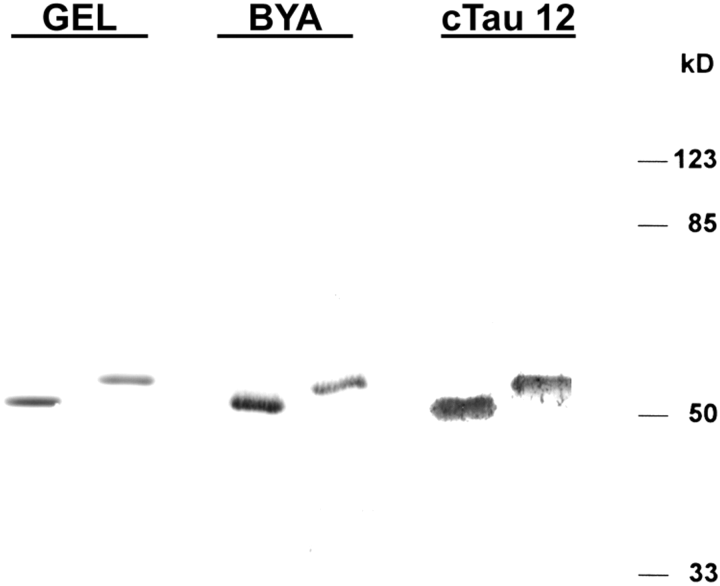
. CSF cTau Mabs label recombinant tau. A 10% gel with recombinant three-repeat tau (GEL, left lane, 1 μg) and four-repeat tau (GEL, right lane, 1 μg) was stained with Coomassie Blue. Recombinant tau blotted with antibody BYA-1074 (0.07 μg per lane) and cTau12 (2.5 μg per lane) is also shown. Molecular mass markers are shown at the right.
| CSF cTau (ng/ml) | |||
|---|---|---|---|
| Disease (no. of patients) | Mean ± SD | Range | Age (mean ± SD years) |
| Head injury (15) | 1,519.6 ± 3,019 a | 7-11,810 | 32.4 ± 14.1 |
| Hydrocephalus (5) | 0 ± 0 | 0-0 | 54.0 ± 22.5 |
| Multiple sclerosis (15) | 0.014 ± 0.05 | 0-0.21 | 36.4 ± 9.5 |
| Neurologic controls (15) | 0.031 ± 0.11 | 0-0.46 | 40.5 ± 12.0 |
| Nonneurologic controls (15) | 0 ± 0 | 0-0 | 49.8 ± 15.3 |
- a p < 0.001 when compared with control patients.
Characterization of cTau7-, cTau8-, and cTau12-labeled proteins
The experiments described below indicate that the 30-50-kDa proteins recognized by antibodies cTau7, cTau8, and cTau 12 are a cleaved form of the microtubule binding protein tau. These data demonstrate that (a) antibodies cTau7, cTau8, and cTau12 label recombinantly expressed tau, (b) the 30-50-kDa proteins recognized by cTau7, cTau8, and cTau12, similar to tau, contain a functionally intact microtubule binding domain, (c) antibodies cTau7, cTau8, and cTau12 label a 14.7-kDa CNBr digestion fragment comprising Pro215-Met419 of the tau primary sequence, (d) 30-50-kDa cTau proteins occur in both CSF from CNS trauma patients and postmortem brain, and (e) CSF 30-50-kDa proteins consist of the interior portion of the tau sequence from which the N- and C-terminal amino acids have been cleaved.
Recombinant tau immunoreactivity.
Mabs cTau7, cTau8, and cTau12 labeled recombinant tau expressed in Escherichia coli (Fig. 3). Recombinant tau containing either three or four repeats was expressed in BL21(DE3) cells and isolated. Recombinant tau was highly purified as judged by Coomassie Blue-stained SDS-PAGE (Fig. 3, GEL). Both tau isoforms demonstrated immunoreactivity with tau polyclonal antibody BYA-1074 and at higher protein loads Mabs cTau12 (Fig. 3), cTau7, and cTau8 (data not shown). These data suggest that Mabs cTau7, cTau8, and cTau12 demonstrate higher affinity for cTau than for intact full-length tau. This selectivity was examined by comparing serial dilutions of affinitypurified CSF tau with serial dilutions of recombinant three-repeat and four-repeat tau using the cTau sandwich ELISA described below. This study indicated that 2,752 times more three-repeat tau and 2,479 times more four-repeat tau were required to obtain comparable OD readings as those produced by affinity-purified CSF tau.
Affinity and microtubule purification of tau.
CSF proteins from head injury patients or a preparation of heat-stable postmortem brain proteins were affinity-purified with Mabs cTau7, cTau8, and cTau12 (Fig. 4). Affinity purification from either source revealed a band of 30-50-kDa tau proteins on immunoblot or on Coomassie Blue-stained SDS-PAGE (Fig. 4, AFFINITY PURIFIED). These data indicate that 30-50-kDa tau proteins are present in both brain and CSF.
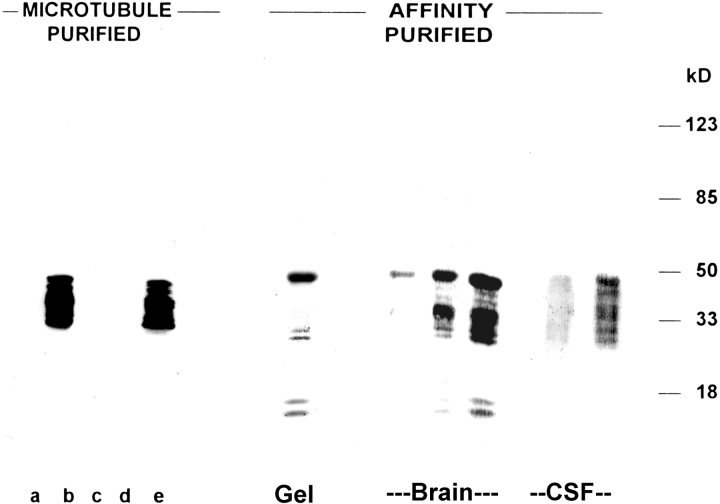
. CSF and brain cTau proteins bind microtubules. Microtubule purified : The presence of a functionally intact tau microtubule binding domain in 30-50-kDa cTau proteins was demonstrated by the purification of these cTau proteins from whole CSF using polymerized microtubules. Initially, taxol-polymerized microtubules were salt-extracted to insure that no cTau7-immunoreactive proteins remained present (lane a). Microtubules were then incubated with a preparation of CSF cTau proteins (lane b, 1 μg) and washed several times until the supernatant was free of cTau7 reactivity (lanes c and d). Microtubule-bound proteins were salt-extracted, yielding 30-50-kDa cTau7-reactive cTau proteins (lane e). Similar results were obtained with Mabs cTau8 and cTau12 (data not shown). Affinity purified : The cTau proteins were affinity-purified from either CSF or brain with Mab cTau7 coupled to protein G-agarose. Coomassie Blue-stained gels indicated that affinity-purified cTau consisted of a primary 50-kDa protein band (Gel, 1 μg). Immunoblots of affinity-purified CSF (100 and 500 ng) and brain (10, 30, and 100ng) with cTau7 revealed 30-50-kDa protein bands. Similar results were observed with Mabs cTau8 and cTau12 (data not shown). The position of molecular mass markers is shown at the right.
Similar to tau, CSF and brain 30-50-kDa proteins recognized by Mabs cTau7, cTau8, and cTau12 bind microtubules. Tubulin was purified from brain and polymerized into microtubules with taxol. Endogenous microtubule binding proteins were dissociated from the polymerized microtubules with all detectable endogenous MAPs removed by the third salt extraction (Fig. 4, lane a). Microtubules were incubated with CSF 30-50-kDa cTau proteins (Fig. 4, lane b) and microtubules washed several times to remove all proteins not tightly bound (Fig. 4, lanes c and d). Microtubule binding proteins were then salt-extracted from the washed microtubule preparation, revealing a cTau-7-labeled ladder of 30-50-kDa proteins (Fig. 4, lane e). Similar results were obtained with Mabs cTau8 and cTau12 (data not shown). No labeling of MAP-2 or neurofilaments with Mabs cTau7, cTau8, and cTau12 was observed in purified preparations of brain proteins (Vallee, 1982). The presence of 280-kDa MAP-2 in this preparation was confirmed by immunoblots with Mab AP-14 (Kalcheva et al., 1994), which labels both MAP-2a and MAP-2b, whereas the presence of 200-kDa neurofilament-H and 155-kDa neurofilament-M was confirmed with Mabs NE-14 (Gotow and Tanaka, 1994) and BF-10 (Anderton et al., 1982), respectively (data not shown).
Epitope mapping.
cTau7, cTau8, and cTau12 immunoblots of CNBr-digested CSF or brain 30-50-kDa tau proteins indicated that all three Mabs labeled the same CNBr-digested fragment (Fig. 5). This fragment could also be labeled with the Mab PHF-1, which recognizes phosphorylated Ser396 of tau (Otvos et al., 1994 ; Zemlan and Dean, 1996). The PHF-1 data identify the cTau7-, cTau8-, and cTau12-labeled fragment as the largest CNBr digestion product that comprises tau sequences Pro251-Met419. These data indicate that both CSF and brain cTau proteins demonstrate similar CNBr digestion products, suggesting that the labeled CSF and brain proteins are structurally similar.
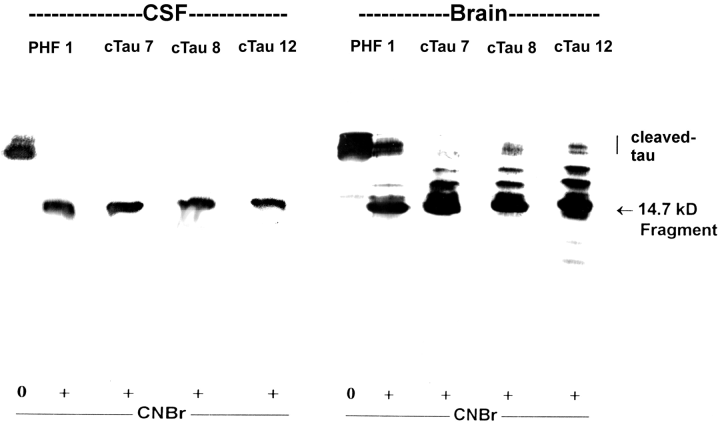
. Mabs cTau7, cTau8, and cTau12 recognize a 14.7-kDa CNBr digestion fragment occurring in patient CSF and brain. The cTau from CSF or brain (2 μg per lane) was treated either with (+) or without (0) CNBr and blotted with Mabs PHF-1, cTau7, cTau8, and cTau12. All four antibodies appeared to label predominantly the same CNBr digestion fragment consisting of tau amino acids Pro251-Met419. The PHF-1 fragment has a reported molecular size of 14.7 kDa (Zemlan and Dean, 1996). Blots are from a single 15% gel. The position at which non-CNBr-digested cTau migrated is shown (cleaved-tau).
Antibody BYA-1074 raised against full-length tau labeled a band of 48-68-kDa proteins in heat-stable extracts of postmortem brain (Fig. 6), whereas no BYA-1074 labeling of CSF proteins could be demonstrated under any conditions (data not shown). Antibodies cTau7, cTau8, and cTau12 labeled a band of 30-50-kDa proteins in postmortem brain, indicating that these cTau Mabs demonstrate a higher affinity for the cleaved form of tau than for the intact form of tau. These results are consistent with the above recombinant tau data that indicated that the cTau Mabs show a >1,000-fold higher affinity for the cleaved form of tau in comparison with either intact three-repeat or four-repeat tau. Mab PHF-1 produced a similar pattern of labeled postmortem proteins as the cTau Mabs, indicating that phosphorylation of Ser396 is more prevalent in the cleaved form of tau than in the intact form of this protein (Fig. 6, PHF 1). Mab Tau-1 labeled proteins spanning the 30-68-kDa region, indicating that Ser199 is poorly phosphorylated in both full-length and cleaved tau (Fig. 6, Tau 1). In comparison, the C-terminal antibody Alz5 and the N-terminal antibody Alz50 labeled only full-length tau, suggesting that these epitopes are not present in cTau.

. Postmortem brain contains both full-length 48-68-kDa tau proteins and 30-50-kDa cTau proteins. Intact tau proteins were selectively labeled with the tau antibody BYA-1074, the C-terminal tau antibody Alz5, and the N-terminal tau antibody Alz50 in heat-stable preparations of postmortem brain. The cTau proteins were selectively labeled with Mabs cTau7, cTau8, cTau12, and PHF-1 (which recognizes phospho-Ser396 of tau). Mab Tau-1, which recognizes nonphosphorylated Ser199 of tau, labeled both forms of tau. These data suggest that tau is cleaved at both the N and C terminals and phosphorylated at Ser396 in brain.
Experiments were performed to assess whether cleavage of full-length tau occurred in brain or CSF. One milliliter of heat-stable proteins purified from postmortem brain containing both cTau and full-length tau was incubated at 37°C with aliquots removed at 0, 1, 2, 3, 6, 7, and 20 days. Full-length tau was labeled with BYA-1074, and cTau was labeled with Mab cTau7 on western blots of the same samples (1 μg per lane). A steady decrease in content of BYA-1074-labeled proteins was observed with time, whereas a steady increase in content of cTau7-labeled proteins was observed (data not shown). To determine whether tau cleavage occurred in CSF, 5 μg of recombinant tau was added to 1 ml of CSF and incubated overnight at 37°C. CSF aliquots were electrophoresed on SDS-PAGE and blotted with BYA-1074, which indicated a decrease in neither BYA-1074-labeled full-length tau nor any faster-migrating BYA-1074-labeled species after incubation (data not shown).
ELISA development and patient CSF cTau levels
ELISA development.
Before sandwich ELISA development, it was important to determine whether cTau7, cTau8, and cTau12 epitopes were independent and whether the epitopes were phosphorylated. ELISA competition studies among cTau7, cTau8, and cTau12 resulted in noncompetitive binding to CSF cTau proteins (data not shown). Also, Mabs cTau7, cTau8, and cTau12 demonstrated binding to 30-50-kDa cTau proteins that was not affected by dephosphorylation with BAP. For example, BAP treatment produced a fivefold increase in the binding of Mab SMI33, which recognizes nonphosphorylated Ser235 of tau (BAP, 1.88 ; no BAP, 0.35 ; mean OD), whereas no effect of BAP treatment on cTau7 (BAP, 1.75 ; no BAP, 1.51), cTau8 (BAP, 1.99 ; no BAP, 1.61), or cTau12 (BAP, 1.72 ; no BAP, 1.53) binding was observed. A sensitive ELISA using catalyzed reporter deposition for signal enhancement was developed using Mab cTau12 for antigen capture, horseradish peroxidase-conjugated Mabs cTau7 and cTau8 for detection, and biotin-tyramine as the reporter. The sensitivity of this ELISA for affinity-purified CSF tau was 0.1 ng/ml.
Patient CSF cTau levels.
Patients with closed head injury demonstrated significantly elevated CSF cTau levels (p <0.001) with respect to all four control groups examined (Table 1). CSF cTau levels average 1,519 ng/ml in head injury patients, whereas levels were below the level of detection (0.1 ng/ml) in 48 of 50 control patients. Detectable levels of CSF cTau were measured in one patient with relapsing, severe late-stage multiple sclerosis (0.21 ng/ml) and one patient with Guillain-Barré syndrome (0.46 ng/ml). There was no overlap in CSF cTau levels between head injury patients (lowest value, 7.4 ng/ml) and control patients (highest value, 0.46 ng/ml). One possible interpretation of these data is that the elevated cTau levels in head injury patients were associated with the ventricular source of CSF used for assay in these patients as opposed to the lumbar source of CSF used for the multiple sclerosis, neurologic, and nonneurologic control patients. For this reason cTau levels were also determined in ventricular CSF collected from patients with normal pressure hydrocephalus (Table 1). CSF cTau levels were below the level of detection in all hydrocephalus patients examined. To determine whether blood cells sometimes occurring in CSF samples affected the performance of the cTau ELISA, CSF samples were rated on a 1-4 scale, and the relation to CSF tau levels was determined by regression analysis. No significant correlation was observed (>0.10). To determine whether blood cells were a source of antigen in the cTau ELISA, 100-μl samples of patient lysed blood cells were assayed, and no measurable immunoreactivity was found.
DISCUSSION
CSF proteins in TBI
CSF cTau proteins.
The present study suggests that CSF levels of a cleaved form of the microtubule binding protein tau reflect axonal damage after head injury. Highly elevated cTau levels were observed both by ELISA and on western blot in DAI, where axonal injury was identified as the primary diagnostic feature responsible for the clinically observed neurological deficits. Loss of axonal microtubules resulting from direct or indirect traumatic injury to CNS axons is a common feature of head trauma (Povlishock and Christman, 1995 ; Povlishock and Pettus, 1996). This loss of axonal microtubules following injury would be expected to release intracellular microtubule binding proteins, such as tau, into the extracellular space, where they would be transported by convective bulk flow to CSF (Segal, 1993). This interpretation is consistent with the present data where CSF cTau levels were elevated >1,000-fold in TBI patients when compared with controls (Table 1). Furthermore, there was no overlap in CSF cTau levels between TBI patients and control patients.
Tau is an intraneuronal nonreleased protein ; therefore, CSF tau levels in patients free of axonal injury would be expected to be low or nonexistent. A control group of patients with a demyelinating disease, multiple sclerosis, was included in the present study as there is a relative preservation of axons in this disorder. As expected, few (one of 15) demonstrated detectable levels of CSF tau. The one patient with detectable tau levels had severe, late-stage multiple sclerosis. In late-stage multiple sclerosis axons are injured and ultimately degenerate, probably accounting for the CSF tau detected in this patient (Shintaku et al., 1988 ; Sobel, 1995). Detectable CSF tau levels were only observed in one of 30 patients in the combined neurologic and nonneurologic control groups. This was a patient with Guillain-Barré syndrome, a polyneuropathy often associated with increased CSF protein levels. Increased CSF tau levels in Guillain-Barré syndrome have been previously reported and may reflect active neuronal degeneration in this disorder (Vandermeeren et al., 1993).
One potential problem with the present study is the different source of CSF collected from head injury patients (ventricular) compared with the multiple sclerosis, neurologic, and nonneurologic control patients (spinal). For this reason, ventricular CSF was collected from patients with normal pressure hydrocephalus. Nondetectable CSF tau levels were found in all hydrocephalus patients, indicating that the source and method of CSF collection were not responsible for the elevated tau levels observed in TBI patients (Table 1).
CSF studies of TBI :
enolase. Although the present study is the first to our knowledge to measure CSF tau levels in TBI patients, the relationship between other CSF proteins and head injury has been investigated. CSF enolase levels have been studied in patients with severe head injury (Scarna et al., 1982 ; Mabe et al., 1991). The γγ-enolase isoenzyme is found in high concentrations in neurons, platelets, and certain endocrine cells (Dauber-schmidt et al., 1983). Median CSF enolase levels have been reported (Ross et al., 1996) to be elevated ninefold in severe head injury patients compared with control patients with back pain. A significant correlation between initial CSF enolase levels and GCS scores was observed. As a measure of neuronal injury, CSF enolase shows promise for certain patients. The assay was first used to study head injury patients 15 years ago but has not been widely used, possibly because of assay limitations (Scarna et al., 1982). A primary problem is that platelets are a rich source of enolase, raising the concern that in CSF samples containing blood, one may be detecting enolase of platelet rather than neuronal origin (Marangos et al., 1979 ; Ross et al., 1996). In the present study, lysed blood cells demonstrated no immunoreactivity in the cTau ELISA. Therefore, blood contamination routinely found in some CSF samples should not be a source of tau proteins.
CSF studies of TBI : glutamate.
Changes in levels of CSF excitatory amino acids, particularly glutamate, following head injury have been recently studied (Baker et al., 1993). These studies were intended to address treatment issues. For example, several lines of evidence suggest that following CNS injury, extracellular levels of glutamate increase (Katayama et al., 1990 ; Nilsson et al., 1990). Glutamate is then thought to activate NMDA and α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA)/kainate receptors, resulting in cellular edema and the accumulation of intracellular calcium, causing cell death. As a measure of neuronal injury, CSF glutamate holds little promise. The increase in CSF glutamate levels after head injury is modest (Nilsson et al., 1990 ; Baker et al., 1993). Furthermore, serum levels of glutamate are 50-100 times higher than CSF glutamate levels, suggesting that glutamate detected in CSF may originate from both brain and peripheral sources (Faden and Salzman, 1992).
CSF cTau proteins
Developed Mab antigen.
In the present study, Mabs were developed that distinguished between head injury patient and control CSF. The developed Mabs labeled proteins present in head injury patients with an apparent molecular size of 30-50 kDa on western blots. The Mab antigen appeared to be a posttranslationally modified form of tau as (a) the 30—50-kDa Mab affinity-purified proteins, like tau, were microtubule binding proteins, (b) Mabs raised in other laboratories that possess well-defined tau epitopes labeled these 30—50-kDa proteins, and (c) the developed Mabs labeled recombinantly expressed tau.
The CSF tau proteins labeled with the developed Mabs demonstrated a faster gel mobility than that observed for full-length tau, whose six isoforms demonstrate an apparent molecular size of 48-68 kDa (Cleveland et al., 1977 ; Couchie and Nunez, 1985). This faster gel mobility appears to reflect the cleavage of CSF tau proteins at both the N and C terminals. Examination of 30—50-kDa CSF tau proteins in CNBr cleavage studies and antibody mapping studies suggests that these proteins consist of the interior portion of the tau primary sequence, including a functional microtubule binding domain.
Mab selectivity for cTau.
Mabs cTau7, cTau8, and cTau12 demonstrated selective labeling of cTau when equivalent protein loads of cTau and intact tau were examined by western blot. This suggests that cTau Mabs demonstrate a higher affinity for the cleaved form of tau than for full-length tau. Estimation of relative cTau Mab affinity by ELISA indicated that cTau Mabs possess a >1,000-fold higher affinity for cTau than for the full-length three-repeat or four-repeat forms of tau. This selectivity of Mabs cTau7, cTau8, and cTau12 is best explained by a conformational state assumed by tau following cleavage rather than differences in primary sequence. Previous research demonstrates that Mabs can specifically recognize tau secondary structure. Several tau Mabs, including Alz50, Tau-2, and MC1, recognize a specific tau conformation rather than tau primary structure (Kanemaru et al., 1992 ; Carmel et al., 1996 ; Vincent et al., 1996). For example, using tau deletion mutants Carmel et al. (1996) demonstrated that Alz50 recognized a conformation of tau where the N terminus is in close association with the microtubule binding domain. The KD of Alz50 for the native tau protein in this preferred conformation was >70-fold higher than for the protein without the preferred secondary structure. Similarly, Mabs cTau7, cTau8, and cTau12 appear to recognize a secondary structure that tau assumes after cleavage.
Tau cleavage.
The present study is the first detailed characterization study of tau proteins in head injury patient CSF. Previous studies have estimated the apparent molecular size of CSF tau primarily in patients with Alzheimer's disease. These studies have produced variable estimates of the molecular size of Alzheimer's disease CSF tau, including 68 kDa (Wolozin and Davies, 1987), 55 kDa (Vigo-Pelfrey et al., 1995), three bands ranging from 50 to 65 kDa (Arai et al., 1995), or a 26—28-kDa primary band (Johnson et al., 1997). Differences in molecular size estimates between the present study examining head injured patients and studies using Alzheimer's disease CSF may reflect exposure of tau to different proteases following head injury and Alzheimer's disease. For example, cell death in head injury is necrotic, whereas cell death in Alzheimer's disease is thought to be apoptotic (Li et al., 1997 ; Nishimoto et al., 1997).
Also, differences in apparent molecular size may reflect technical differences in quantifying tau. In the present study, the apparent molecular size of tau was determined directly in unconcentrated CSF samples. In comparison, tau is reportedly a low-abundance protein in Alzheimer's disease CSF. For example, Vigo-Pelfrey et al. (1995) purified 6,000 μl of Alzheimer's disease CSF to obtain sufficient tau for one lane on a western blot. In the present study, only 3-50 μl of unconcentrated CSF was required to demonstrate immunoreactivity on western blots.
In conclusion, the present study demonstrates that CSF cTau levels are elevated in patients with head injury. Furthermore, the elevation of CSF cTau levels in a comatose head-injured patient with an unremarkable CT scan indicates that CSF cTau levels are a more sensitive measure of axonal damage than CT. The correlation between the patient's clinical condition and CSF cTau levels suggests that CSF cTau levels may be a good predictor of severity of head injury and possibly patient outcome after discharge. Also, CSF cTau levels may prove valuable in assessing the efficacy of pharmacologic treatments of TBI.
Acknowledgements
This work was supported by grant AG 12572 from the National Institutes of Health.




