Role of Protein Kinase C (PKC) in Agonist-Induced α-Opioid Receptor Down-Regulation
II. Activation and Involvement of the α, ε, and ξ Isoforms of PKC
Abbreviations used : DAG, diacylglycerol ; DAMGO, [-d-Ala2,N-Me-Phe4,Gly-ol]enkephalin ; DIPR, diprenorphine ; DPDPE, [Tyr-d-penicillamine-Gly-Phe-d-penicillamine]enkephalin ; DSLET, Tyr-d-Ser-Gly-Phe-Leu-Thr ; IP3, inositol 1,4,5-trisphosphate ; PAGE, polyacrylamide gel electrophoresis ; PBS, phosphate-buffered saline ; PDBu, phorbol 12,13-dibutyrate ; PKC, protein kinase C ; PLC, phospholipase C ; PMA, phorbol 12-myristate 13-acetate ; PTX, pertussis toxin ; SDS, sodium dodecyl sulfate.
Abstract
Abstract : Phosphorylation of specific amino acid residues is believed to be crucial for the agonist-induced regulation of several G protein-coupled receptors. This is especially true for the three types of opioid receptors (μ, δ, and α), which contain consensus sites for phosphorylation by numerous protein kinases. Protein kinase C (PKC) has been shown to catalyze the in vitro phosphorylation of μ- and δ-opioid receptors and to potentiate agonist-induced receptor desensitization. In this series of experiments, we continue our investigation of how opioid-activated PKC contributes to homologous receptor down-regulation and then expand our focus to include the exploration of the mechanism(s) by which μ-opioids produce PKC translocation in SH-SY5Y neuroblastoma cells. [dAla2,N-Me-Phe4,Gly-ol]enkephalin (DAMGO)-induced PKC translocation follows a time-dependent and biphasic pattern beginning 2 h after opioid addition, when a pronounced translocation of PKC to the plasma membrane occurs. When opioid exposure is lengthened to >12 h, both cytosolic and particulate PKC levels drop significantly below those of control-treated cells in a process we termed “reverse translocation.” The opioid receptor antagonist naloxone, the PKC inhibitor chelerythrine, and the L-type calcium channel antagonist nimodipine attenuated opioid-mediated effects on PKC and μ-receptor down-regulation, suggesting that this is a process partially regulated by Ca2+-dependent PKC isoforms. However, chronic exposure to phorbol ester, which depletes the cells of diacylglycerol (DAG) and Ca2+-sensitive PKC isoforms, before DAMGO exposure, had no effect on opioid receptor down-regulation. In addition to expressing conventional (PKC-α) and novel (PKC-ε) isoforms, SH-SY5Y cells also contain a DAG-and Ca2+-independent, atypical PKC isozyme (PKC-ξ), which does not decrease in expression after prolonged DAMGO or phorbol ester treatment. This led us to investigate whether PKC-ξ is similarly sensitive to activation by μ-opioids. PKC-ξ translocates from the cytosol to the membrane with kinetics similar to those of PKC-α and ε in response to DAMGO but does not undergo reverse translocation after longer exposure times. Our evidence suggests that direct PKC activation by μ-opioid agonists is involved in the processes that result in μ-receptor down-regulation in human neuroblastoma cells and that conventional, novel, and atypical PKC isozymes are involved.
The recent cloning of the major opioid receptor types (μ, δ, and κ) has provided essential information about their plasticity (Evans et al., 1992 ; Kieffer et al., 1992 ; Chen et al., 1993 ; Wang et al., 1993 ; Yasuda et al., 1993). Repeated or prolonged exposure to opioid agonists reduces the responsiveness of opioid receptors to its agonists over time. This loss of receptor function is hypothesized to contribute to the cellular and biochemical changes that lead to opiate tolerance, dependence, and, possibly, addiction in humans (Nestler, 1992). These neuroadaptive processes also limit the use of narcotic analgesics in the treatment of chronic pain. The loss of receptor sensitivity that is observed in biochemical and behavioral assays appears to result from a sequence of events that begin shortly (within seconds) after addition of an agonist. In the absence of rapid agonist removal, a series of regulatory events occur that act to limit receptor activity and curtail the effect of opioid/opiate. Substantial experimental evidence has divided this loss of function into separate, but related, receptor events : desensitization, internalization, and down-regulation.
Rapid phosphorylation of the opioid receptor appears to be an absolute prelude to desensitization and down-regulation (Zhang et al., 1998). However, many protein kinases act on opioid receptors with high affinity and have been suggested to be important factors in agonist-mediated regulation. Of these, protein kinase C (PKC) remains a controversial contributor to receptor down-regulation. Several reports have demonstrated that prior activation of PKC by phorbol esters potentiates agonist-mediated functional desensitization and down-regulation of μ- and δ-opioid receptors (Gucker and Bidlack, 1992 ; Chen and Yu, 1994 ; Cai et al., 1997 ; Narita et al., 1997). Furthermore, intrathecal pretreatment of mice with the PKC inhibitor calphostin C prevents the development and expression of acute antinociceptive tolerance to repeated spinal applications of deltorphin-II (Narita et al., 1996). However, conflicting evidence has been presented regarding whether PKC is involved in the processes that underlie μ- and δ-opioid receptor regulation. Pei et al. (1995) concluded that PKC-dependent phosphorylation was not involved in desensitization of the δ-opioid receptor transfected into HEK-293 cells. In those experiments, total cellular PKC was believed to be down-regulated after prolonged exposure to the phorbol ester phorbol 12-myristate 13-acetate (PMA), which induces an enzymatic degradation of the PKC protein (Nakanishi and Exton, 1992 ; Hong et al., 1995 ; Keenan et al., 1995). However, in these “PKC-depleted” HEK-293 cells, the δ-opioid receptor was still capable of undergoing phosphorylation and desensitization in response to repeated exposure to the specific δ agonist [Tyr-d-penicillamine-Gly-Phe-d-penicillamine]enkephalin (DPDPE). Similar conclusions were drawn using a mutant form of the mouse δ-opioid receptor for studying down-regulation. Chronic exposure to opioids continued to produce significant δ-receptor down-regulation after Ser344, one putative site in the C terminus for phosphorylation by PKC, was replaced by Ala (Cvejic et al., 1996).
In our previous study (Kramer and Simon, 1999), we demonstrated that μ-selective opioids induce a translocation of PKC to the plasma membrane of SH-SY5Y neuroblastoma cells after relatively short periods of exposure (2-6 h). The onset of PKC translocation roughly parallels the down-regulation of [3H]diprenorphine ([3H]DIPR) binding sites, and the loss of opioid receptors continues despite the observed decrease in membrane PKC content that occurs over time. Overall, the pharmacokinetics of PKC translocation suggest that opioid-mediated PKC activation may be an important event during receptor down-regulation rather than the more rapid processes of desensitization/internalization. It is interesting that opioids have a bidirectional effect on PKC translocation depending on the duration of agonist exposure. Longer incubations (> 12 h) resulted in a loss of particulate PKC to levels well below control in a response that we have termed “reverse translocation.”
Our previous report (Kramer and Simon, 1999) used 3H-phorbol ester binding to quantify membrane PKC density. However, SH-SY5Y neuroblastoma cells express three of the 10 known PKC isoforms : the α [conventional group ; Ca2+ - and diacylglycerol (DAG)-regulated], the ε (novel group ; DAG-regulated), and the ξ (atypical group ; regulated by neither DAG nor Ca2+) (Turner et al., 1994) ; therefore, only the DAG-regulated isoforms will act as substrates for [3H]phorbol 12,13-dibutyrate ([3H]PDBu) (Nakanishi and Exton, 1992 ; Hong et al., 1995 ; Keenan et al., 1995). Consequently, changes in membrane [3H]PDBu binding represented the translocation (or reverse translocation) of the α and/or ε isoforms only and did not assess the sensitivity of non-DAG-regulated PKC isoforms to opioids. In the experiments described below, we examined whether individual, differentially regulated PKC isozymes are activated by opioids and describe how this may explain why opioid receptor down-regulation continued during the period of PKC-α and -ε reverse translocation. In addition, we investigated the mechanisms by which μ-selective opioids elicit physiological PKC translocation/activation in SH-SY5Y neuroblastoma cells and present evidence suggesting how PKC activation may contribute to opioid receptor down-regulation.
MATERIALS AND METHODS
Drugs and cell culture
Morphine sulfate [d-Ala2,N-ME-Phe4,Gly-ol]enkephalin (DAMGO), DPDPE, and Tyr-d-Ser-Gly-Phe-Leu-Thr (DS-LET) were received from the National Institute on Drug Abuse (Bethesda, MD, U.S.A.). Nimodipine and chelerythrine chloride were purchased from Research Biochemicals International (Natick, MA, U.S.A.), and pertussis toxin (PTX) was from GibcoBRL (Grand Island, NY, U.S.A.). PMA and 4α-PMA were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Undifferentiated SH-SY5Y neuroblastoma cells were a generous gift from DR. Wolfgang Sadée (University of California at San Francisco, San Francisco, CA, U.S.A.) and were plated at an initial density of 5.0 × 105 cells in 75-cm2 flasks. SH-SY5Y cells, which are a subclone of the SK-N-SH cell line, express both μ- and δ-opioid receptors in a ratio of 4:1, and these sites have been previously shown to be susceptible to both desensitization and down-regulation by repeated or chronic opioid exposure (Carter and Medzihradsky, 1993 ; Zadina et al., 1994 ; Wang et al., 1996b). Cells were maintained in medium consisting of a 1:1 mixture of Dulbecco's modified Eagle's medium/F-12 medium (GibcoBRL), supplemented with 2 mMl-glutamine, 10% fetal bovine serum (Gemini Bio-Products, Calabasas, CA, U.S.A.), 50 μg/ml gentamicin sulfate (Gemini Bio-Products), and 1 mM nonessential amino acids (GibcoBRL) at 37°C in 7% CO2/humidified air. After 24 h, SH-SY5Y cells were differentiated toward the neuronal phenotype by addition of 10 μM retinoic acid (Sigma), and the retinoic acid-containing medium was changed every 3 days until the cell monolayer reached confluency (5-7 days). On reaching confluency, the cells were incubated in the above medium without fetal bovine serum and exposed to drugs and/or phorbol esters at the indicated concentrations for various intervals. PTX was added to some cultures at a concentration of 100 ng/ml for 24 h before addition of opioid agonists.
Cell harvesting and membrane preparation
After drug incubation, the culture media were aspirated, and the cells were washed twice with sterile phosphate-buffered saline (PBS ; pH 7.4). Cells were harvested in a solution of PBS containing 1 mM EDTA and centrifuged at 3,000 g at 4°C for 10 min in a Sorvall centrifuge using an SS-34 rotor. The supernatant was discarded, and the pellet was resuspended in a receptor preparation buffer containing (mM) Tris-HCl (38.5), Tris base (11.5), EDTA (2.0), EGTA (0.5), dithiotheritol (0.8), phenylemthylsulfonyl fluoride (100 μM), leupeptin (2 μg/ml), and aprotinin (2 μg/ml) at pH 7l.4. The lysed cells were homogenized for 5 s using a Brinkmann tissue homogenizer at a setting of 2 and centrifuged at 45,000 g 4°C for 20 min. The nellet (P2 fraction), containing the washed membrane preparation, was resuspended at a protein concentration of 0.2-0.4 mg/ml (as measured by the BCA method ; Pierce) in a receptor binding buffer containing (mM) Tris-HCl (38.5), Tris base (11.5), NaCl (100), and CaCl2 (1.3), pH 7.4. This memebrane preparation was stored at — 70°C until used.
[3H]DIPR, [3H]DSLET, [3H]DAMGO, and [3H]PDBu binding
The nonselective opiate antagonist [3H]DIPR was used to quantifgy the basal level of opioid receptors in washed membrane homogenates. An antagonist wasused instead of a radiolabeled agonist becasuse its binding kinetics are unaffected by the presence of Na+ in the assay buffer, which are necessary for simultaneous 3H-probol ester binding experiments (Simon et al., 1975). For 3H-opioid binding (opioid binding sites), 590 μl of membranes were allowed to equilibrate in the absence or presence of 10 μM (—)-naloxone (National Institute on Drug Abuse) to determine specific binding. Scatchard analyses of saturation binding data were performed to determine]3H]DIPR (Amersham ; specific activity, 39.0 Ci/mmol), [3H]DAMGO (47 Ci/mmol ; Multiple Peptide Systems, San Diego, CA, U.S.A.), or [3H]DSLET (21.6 Ci/mmol ; Multiple Peptide Systems) bidings parameters (KD and Bmax) in a total well volume of 1 ml for 120 min at room temperature. In some experiments, single-point determinations were used to assay the number of opioid binding sites using a saturating concentration of [3H]DIPR (2.0 nM). The tissue was harvested onto Titertek filtermats (coated with 0.1% polyethylenimine to reduce non-specific bining) using a Brandel cell harvester, and the filters were placed in scintillation vials containing 3.0 ml of Liquiscint (National Diagnostics). Samples were counted for radioactivity for 5.o min in a Beckman liquid scintillation counter (efficientcy, 50%). The cpm data were converted to femtomoles of [3H]DIPR bound per milligram of protein. For [3H]PDBu binding (PKC binding sites), 300 μl of membranes were allowed to equilibrates with receptor binding buffer in the absence of presence of 10 μM PMA (Sigma) to determine specific binding. Scatchard analyses of saturation binding data were performed to determine [3H]PDBu binding parameters (KD and Bmax) using [3H]PDBu (NEN ; specific activity, 18.0 Ci/mmol) in a total well volume of 1 ml for 120 min at room temperature. In some experiments, single-point determinations were used to assay the number of PKC binding sites using a single concentration of [3H]PDBu (1.0 nM). This [3H]PDBu concentration near its KD wasused to minimize the nonspecific binding of this highly lipophilic molecules. This tissue was harvested, and radioactivity was quantified as described above. The cpm data were converted to femtomoles of [3H]PDBu bound per milligram of protein.
Immunoblotting of PKC isoforms in SH-SY5Y neuroblastoma cells
Cells were incubated in serum-free medium, or medium plus drug, as indicated. Cell monolayers were rinsed twice with sterile PBS and twice with 20 mM Tris-HCl and 0.15 M NaCl, pH 7.5 (buffer A). Soluble proteins were extracted by incubation for 10 min with a PKC extraction buffer (buffer B ; 20 mM Tris-HCl, 0.15 M NaCl, 2mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiotheitol, and 2.5 μg/ml leupeptin, pH 7.5). This preparation was centrifuged at 45,000 g for 20 min and the supernatant, which contained cytosolic PKC isoforms, was removed and stored at —70°C until used. The P1 fraction was resuspected in buffer B supplemented with 0.5% Triton X-100, Shaken for 60 min at 4°C to elute the particulate PKC, and then centrifuged at 100,000 g for 20 min. The resulting supernatant, containing membrane-bound PKC isoforms, was stored at —70°C until used. Cytosolic and membrane-bound proteins were prepared for sodium dodecuyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) by boiling them in a smaple buffer of 4% SDS, 37.5 mM Tris-HCl, 20% glycerol, and 0.-02% bromophenol blue (pH 6.8) containing 0.2 M dithiothretiol. Proteins (2-10 μg per lane) were separated by SDS-PAGE (8% acylamide running gel, 4% acrylamide staking gel) and electroblotted to nitrocellulose (pore size, 0.45 μm) at 250 mV for 90 min. Nitrocellulose membranes were rinsed threee times with PBS and blocked overnight in a solution of 50 mM Tirs-HCl, 100mM NaCl, 3% bovine serum ablumin, and 0.05% Tween-20, pH 7.5. Membranes were exposed for 12 h at 4°C to rabbit polyclonal antibodies against the carboxyl terminus of the PKC-α, -ε, or -ξ isofomr (1:10,000 dilution in blocking solution ; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). After three washes with locking buffer without bovine serum albumin, the membranes were incubated for 60 min at room temperature in a biotinvlated secondary antibody (goat anti-rabbit IgG at a dilution of 1:8,000). The nitrocellulose was washed three times in serum-free buffer consisting of 50 mM Tirs-HCl, 100 mM NaCl, 3% bovine serum albumin, and 0.05% Tween-20 (pH 7.5) and incubated with a 1:20,000 solution of streptavidin-peroxidase for 1 h at room temperature. Immunoreactive proteins were visualized using a horseradish peroxidase-linke enhanced chemiluminescent western blotting kit (Pierce). Images of immunoreactive bands were captured on x-ray film and analyzed using the MICD morphometric system (Imaging Research, St. Catharines, Ontario, Canada).
Data analysis and statistical methods
[3H]PDBu and [3H]DIPR binding curves were analyzed using an interative curve-filing program (LIGAND) (Munson and Rodbard, 1980). All graphs were produced using Sigmaplot for Windows (ver. 4.0), and all regression analyses were done using the Sigmaplot curve-fitting program. In displacement studies, IC/EC50 values were determined by the equation of Cheng and Prusoff (1973). One- and two-way ANOVA and the post hoc Tukey's test were used for multiple comparisons at a minimal significance level of p≤ 0.05. Student's t test was used when applicable for simple two-sample tests at the same minimal significance level. Satistical data were expressed as mena ± SE values of the indicated number of observation. In some figures, a representative graph is used to illustrate the results of a particular experiment that was repeated at least four times.
RESULTS
As stated in our accompanying article (Kramer and Simon, 1999)k, μ-opioids induced a bimondal change in the density of membrane-bound [3H]PDBu binding sites. These opposite changes are exemplified at 4 and 24 h after addition of DAMGO (Table 1). The current series of experiments focuses on gathering further information about the mechanisms by which μ-opioids produce this response, which of the expressed PKC isozymes are sensitive to μ-opioid agonists, and gaining evidence as to why opioid receptor down-regulation continues in cells depleted of PKC after prolonged phorbol ester exposure.
| [3H]PDBu | [3H]DIPR | ||||
|---|---|---|---|---|---|
| Condition | Inhibitor/±DAMGO incubation time (h) | K D (nM) | B max [pmol/mg] (% control) | K D (nM) | B max [fmol/mg] (% control) |
| Control | NA | 1.2 ± 0.4 | 2.0 ± 0.2 (100) | 0.58 ± 0.02 | 273.3 ± 33.3 (100) |
| 1 μM DAMGO | 4 | 1.1 ± 0.1 | 3.9 ± 0.5 (195)a | 0.71 ± 0.04 | 192.3 ± 15.6 (70)a |
| 1 μM DAMGO | 24 | 1.8 ± 0.2 | 0.87 ± 0.03 (43)a | 0.73 ± 0.1 | 76.0 ± 9.9 (32)b |
| 1 μM naloxone | 2 | 1.3 ± 0.1 | 1.8 ± 0.9 (90) | 0.52 ± 0.02 | 291.2 ± 25.1 (106) |
| 100 ng/ml PTX | 24 | 1.4 ± 0.1 | 2.1 ± 0.1 (105) | 0.64 ± 0.09 | 251.4 ± 15.6 (92) |
| 1 μM nimodipine | 2 | 1.3 ± 0.1 | 2.2 ± 0.4 (110) | 0.45 ± 0.08 | 244.0 ± 22.6 (89) |
| Naloxone/DAMGO | 2/4 | 0.9 ± 0.2 | 1.8 ± 0.5 (90)c | 0.75 ± 0.03 | 307.4 ± 50.2 (112)c |
| PTX/DAMGO | 24/4 | 1.4 ± 0.4 | 3.0 ± 0.3 (150)a,c | 0.53 ± 0.06 | 200.3 ± 28.2 (73)a |
| Nimodipine/DAMGO | 2/4 | 1.2 ± 0.3 | 2.9 ± 0.1 (145)a,c | 0.68 ± 0.07 | 243.6 ± 21.5 (87)a,c |
| PTX/DAMGO | 24/24 | 1.6 ± 0.9 | 0.96 ± 0.5 (48)a | 0.57 ± 0.06 | 73.1 ± 16.1 (31)a |
| Nimodipine/DAMGO | 2/24 | 1.5 ± 0.7 | 1.9 ± 0.1 (95)d | 0.80 ± 0.07 | 210.4 ± 31.1 (77)a,d |
- a p≤ 0.01 by the post hoc Tukey's test from control (q0.01,50,10 = 5.45) ;
- b p≤ 0.001 by the post hoc Tukey's test from control (q0.001,50,10 = 8.27) ;
- c p≤ 0.01 by the post hoc Tukey's test from 4-h DAMGO alone (q0.01,50,10 = 5.45) ;
- d p≤ 0.01 by the post hoc Tukey's test from 24-h DAMGO alone (q0.01,50,10 = 5.45).
DAMGO-induced PKC translocation and its effect on opioid receptor down-regulation
DAMGO exposure for 4 h. The sensitivity of DAMGO-mediated PKC translocation to the plasma membrane and opioid receptor down-regulation to regulators of receptor activity is presented in Table 1. A total inhibition of DAMGO-mediated PKC translocation was observed after pretreatment with the opioid antagonist naloxone. Naloxone pretreatment also prevents the loss of membrane μ-opioid receptors. The presence of PTX attenuated DAMGO-mediated PKC translocation (DAMGO alone, 3.9 ± 0.46 pmol of [3H]PDBu bound/mg of protein ; PTX/DAMGO, 3.0 ± 0.31 pmol/mg of protein ; -23% ; p≤ 0.01). However, PTX did not significantly alter the loss of [3H]DIPR sites, which accompanied PKC translocation after a 4-h DAMGO exposure. PTX had no appreciable effect on either PKC translocation or opioid receptor down-regulation in the absence of DAMGO.
Nimodipine, an L-type Ca2+ channel antagonist, likewise attenuated the increase in PKC translocation mediated by DAMGO, although nimodipine had no effect on PKC translocation by itself. Nimodipine also partially prevented DAMGO-induced receptor down-regulation, maintaining the density of [3H]DIPR binding sites at 87% of control (Table 1 ; p≤ 0.01 by post hoc Tukey's test vs. DAMGO-treated cultures).
DAMGO exposure for 24 h. In cultures treated with DAMGO for 24 h, nimodipine pretreatment completely abolished PKC “reverse translocation” and maintained the level of [3H]DIPR binding sites to 77% of control compared with 35% of control after DAMGO alone (Table 1). In contrast to its partial effect at the shorter interval (4 h), PTX did not significantly modulate DAMGO's effect on either [3H]PDBu or [3H]DIPR binding at the 24-h time point (Table 1).
Modulation of opioid receptor down-regulation by activators and inhibitors of PKC
Effect of prior PKC activation by PMA. SH-SY5Y human neuroblastoma cells were preincubated with the PKC activator PMA 60 min before addition of DAMGO for 4 h. We wished to see whether the combination of PMA- and opioid-stimulated PKC activation potentiates opioid receptor down-regulation. The 4-h time point was chosen because it represented the period in which maximal opioid-mediated PKC translocation to the membrane is observed. The results of this experiment are presented in Fig. 1. A 60-min preincubation with PMA (≥100 nM) significantly potentiates the DAMGO-induced loss of [3H]DIPR binding sites. DAMGO alone decreased [3H]DIPR binding to 51.9% of the value in medium-treated controls (control, 275 ± 17.3 fmol/mg of protein ; DAMGO, 150 ± 6.7 fmol/mg of protein ; p≤ 0.01), whereas the presence of 1 μM PMA with DAMGO (PMA/DAMGO) enhanced μ-receptor loss from the membrane to 25.3% of control (PMA/DAMGO, 70.2 ± 10.2 fmol/mg of protein ; p≤ 0.01 vs. DAMGO alone). In contrast, 1 μM PMA had no measurable effect on [3H]DIPR binding by itself. The inactive phorbol ester 4α-PMA produced no additive effect on agonistinduced down-regulation (data not shown).
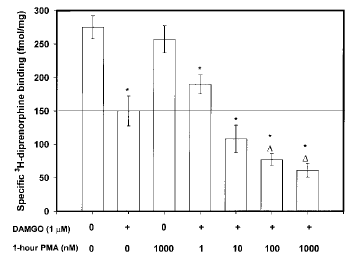
Effect of PKC preactivation on 4-h DAMGO-mediated opioid receptor down-regulation in SH-SY5Y neuroblastoma cells. Confluent cells were incubated with PMA (1-1,000 nM) for 60 min before addition of 1 μM DAMGO for 4 h. At the end of DAMGO treatment, cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding to quantify opioid receptor number. Nonspecific binding, determined in the presence of 10 μM naloxone, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments. *p≤ 0.01 by ANOVA and the post hoc Tukey's test compared with control ; ▵p≤ 0.01 by ANOVA and the post hoc Tukey's test compared with DAMGO (1 μM) alone.
Effect of PKC inhibition by chelerythrine chloride on opioid receptor down-regulation. The presence of chelerythrine chloride, an inhibitor of PKC's catalytic subunit, attenuated agonist-mediated down-regulation of opioid receptors at concentrations of ≥100 nM (Fig. 2). Chelerythrine was added 60 min before addition of DAMGO (1 μM) for 24 h. At the highest concentration tested (1 μM), chelerythrine reduced the magnitude of μ-opioid receptor down-regulation and maintained the number of opioid receptor sites at 75% of control (1 μM chelerythrine/DAMGO, 310.2 ± 57.8 fmol/mg ; DAMGO alone, 153.1 ± 22.3 fmol/mg ; p≤ 0.05). It should be noted, however, that chelerythrine was unable to restore fully the number of [3H]DIPR binding sites to control levels at any concentration tested.
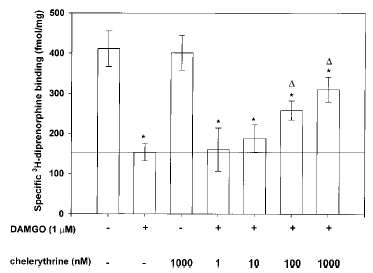
Effect of PKC inhibition on DAMGO-mediated opioid receptor down-regulation in SH-SY5Y neuroblastoma cells. Confluent cells were incubated with chelerythrine chloride (1-1,000 nM) for 60 min before addition of 1 μM DAMGO for 24 h. At the end of DAMGO exposure, cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding to quantify opioid receptor number. Nonspecific binding, determined in the presence of 10 μM naloxone, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments. *p≤0.01 by ANOVA and the post hoc Tukey's test compared with control ; ▵p≤0.05 by ANOVA and the post hoc Tukey's test compared with DAMGO (1 μM) alone.
Chronic phorbol ester treatment fails to modify agonist-mediated opioid receptor down-regulation
A second method that has been used to examine the role of PKC in G protein-coupled receptor regulation is depletion of the kinase from the cell. This technique has been used to study the PKC dependency of opioid receptor desensitization and down-regulation and has provided much of the data that suggest that PKC has a limited influence on these processes (Pei et al., 1995). A 48-h exposure to PMA produces a concentration-dependent decrease in both membrane-bound (Fig. 3, inset) and cytosolic (data not shown) PKC content. The IC50 was determined by a nonlinear curve-fitting program to be 98.1 nM for the elimination of membrane [3H]PDBu binding sites, and concentrations of PMA of ≥1 μM depleted membrane PKC levels to 10% of control. For the opioid receptor down-regulation studies, SH-SY5Y cells were incubated in the presence of increasing amounts of PMA (1-1,000 nM) for 48 h before addition of DAMGO for 24 h. Neither basal [3H]DIPR nor [3H]DAMGO binding is altered in cultures that are devoid of >90% of their total cellular PKC (data not shown). Moreover, in spite of the highly significant decrease in cellular PKC level, the degree of DAMGO (1 μM for 24 h)-induced opioid receptor down-regulation is not changed compared with non-PMA-treated cells (Fig. 3).
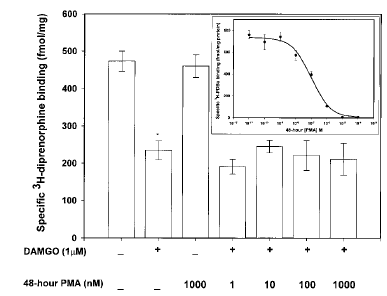
Effect of PKC-α and -ε down-regulation on DAMGO-induced opioid receptor down-regulation. Confluent cells were incubated with PMA (1-1,000 nM) for 48 before addition of 1 μM DAMGO for 24 h. Fresh PMA was readded at the beginning of DAMGO treatment to prevent PKC regeneration. At the end of DAMGO treatment, cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding to quantify opioid receptor number. Nonspecific binding, determined in the presence of 10 μM naloxone, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments. *p≤ 0.01 by ANOVA and the post hoc Tukey's test compared with control. Inset : Prolonged exposure to phorbol ester depletes SH-SY5Y neuroblastoma cell membranes of DAG-sensitive PKC isoforms. Confluent cells were incubated with PMA (10-11-10-4M) for 48 h before preparation of cell membranes for [3H]PDBu binding as described in Materials and Methods. Nonspecific binding, determined in the presence of 10 μM PMA, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments.
Analysis of PKC isozyme expression after chronic phorbol ester treatment
As stated earlier, data have emerged on the differences among the 10 known PKC isozymes and their dependencies on calcium, DAG, and phorbol esters for activation. We used immunoblotting techniques to assess whether all of the expressed PKC isozymes are depleted by prolonged PMA treatment. SH-SY5Y neuroblastoma cells express PKC isoforms α, ε, and ξ in both the cytosol and particulate fraction (Turner et al., 1996). Confluent cultures were exposed to either serum-free medium or medium supplemented with 0.1-1,000 nM PMA for 48 h. PKC was extracted from the plasma membrane, and 5 μg of protein was separated by SDS-PAGE, followed by immunoblot analysis using isozyme-specific polyclonal antibodies. A purified, mixed-isozyme PKC enzyme standard was assayed as a positive control. In control cultures, all three antibodies produced immunopositive bands, which corresponded to molecular masses of 76, 83, and 67 kDa for the purified α, ε, and ξ isoforms, respectively (Fig. 4). Representative immunoblots show that anti-PKC-α (Fig. 4, upper panel) and ε (Fig. 4, middle panel) immunoreactivity was markedly decreased in cells exposed to PMA (concentrations of ≥100 nM) for 48 h. In contrast, the expression of PKC-ξ was not changed relative to that in control cultures after PMA treatment (Fig. 4, lower panel).
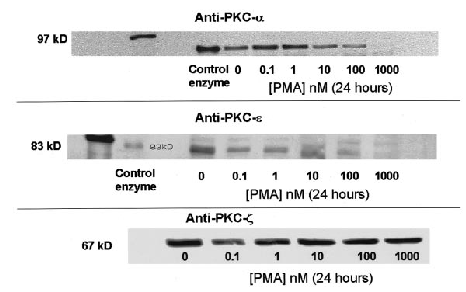
Effect of 48-h treatment with PMA on protein levels of PKC isoforms α (upper panel), ε (middle panel), and ξ (lower panel) in SH-SY5Y neuroblastoma cells. Confluent cultures were exposed to either serum-free media or media supplemented with 0.1-1,000 nM PMA for 48 h. After drug treatment, the cells were washed extensively and prepared for PKC extraction as explained in Materials and Methods. Whole-cell PKC was extracted from these cells, and 5 μg of protein was separated via SDS-PAGE (8% running gel, 4% stacking gel), followed by immunoblot analysis using polyclonal antibodies raised against PKC-α -ε, and -ξ as described in Materials and Methods. Nitrocellulose membrane strips were incubated with anti-PKC antibodies, and immunopositive bands were detected using enhanced chemiluminescence. As a positive control in all experiments, 2.5 μg of a purified, mixed-isozyme PKC standard was electrophoresed.
Immunoblot analysis of opioid-mediated translocation of individual PKC isozymes
Because PKC-ξ remains in the cytosol and membrane fractions of cells chronically treated with PMA, we hypothesized that this atypical isoform may also be sensitive to translocation by μ-opioid receptor activation. If so, this may contribute to the maintenance of agonist-mediated μ-opioid receptor down-regulation in cells depleted of PKC-α and -ε. Quantification of this isoform's translocation to the plasma membrane cannot be performed via [3H]PDBu binding, because this protein does not express a phorbol ester binding site. Consequently, we used immunoblotting techniques to determine if each of the expressed PKC isoforms is similarly responsive to 4- and 24-h DAMGO exposure. Extracts from the cytosol and membrane of control and DAMGO-treated SH-SY5Y human neuroblastoma cells were separated by SDS-PAGE, and the presence of PKC-α, -ε, and ξ was determined using the procedures explained in Analysis of PKC isozyme expression after chronic phorbol ester treatment (above). In control cells, each isoform (α, ε, and ξ) had a measurable presence in both the cytosol and membrane. Increasing concentrations of DAMGO (1 nM-1 μM for 4 h) elicited a redistribution of PKC-α, -ε, and ξ from the cytosol to the membrane fraction (Fig. 5 ; data for PKC-ε not shown). At a concentration of 1 μM, DAMGO produced a complete disappearance of the immunopositive signal from the cytosol and an increase within the membrane. The use of isozyme-specific PKC antibodies also enabled us to investigate whether individual PKC isoforms undergo “reverse PKC translocation” with time. Previously, we showed that [3H]PDBu binding is decreased in the membrane, as the time of DAMGO exposure is increased (Kramer and Simon, 1999). At 4 h, each PKC isoform is exclusively localized to the membrane fraction (Fig. 6 ; data for PKC-ξ not shown). At 12 h, there is a return of the PKC-α and -ξ immunopositive signal to the cytosol and a concomitant decrease in the membrane (data for PKC-ξ not shown). From 24 to 48 h, there is a progressive loss of PKC-α and -ξ protein expression from both pools and an almost complete disappearance of PKC-α and -ξ immunoreactivity from the cytosol and membrane by 48 h (data for PKC-ξ not shown). In contrast, PKC-ξ remains exclusively within the membrane fraction at all time points examined (4-48 h ; Fig. 6).
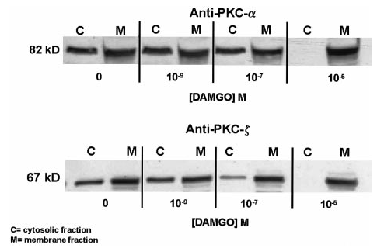
Effect of 4-h treatment with DAMGO on cytosolic and membrane protein levels of PKC isoforms α (upper panel) and ξ (lower panel) in SH-SY5Y neuroblastoma cells. Confluent cultures were exposed to either serum-free medium or medium supplemented with 1 μM DAMGO for 4 h. After drug treatment, the cells were washed extensively and prepared for PKC extraction as explained in Materials and Methods. PKC was separated from the cytosolic and particulate fractions, and 5 μg of protein was separated via SDS-PAGE (8% running gel, 4% stacking gel), followed by immunoblot analysis using polyclonal antibodies raised against PKC-α and -ξ as described in Materials and Methods. Nitrocellulose membrane strips were incubated with anti-PKC antibodies, and immunopositive bands were detected using enhanced chemiluminescence.
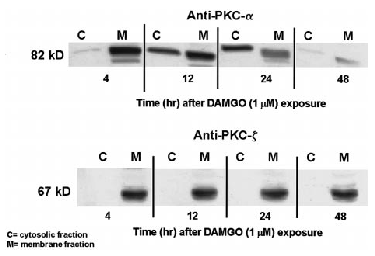
Effect of 4-48-h treatment with DAMGO on cytosolic and membrane protein levels of PKC isoforms α (upper panel) and ξ (lower panel) in SH-SY5Y neuroblastoma cells. Confluent cultures were exposed to either serum-free medium or medium supplemented with 1 μM DAMGO for 4, 12, 24, or 48 h. After drug treatment, the cells were washed extensively and prepared for PKC extraction as explained in Materials and Methods. PKC was separated from the cytosolic and particulate fractions, and 5 μg of protein was separated via SDS-PAGE (8% running gel, 4% stacking gel), followed by immunoblot analysis using polyclonal antibodies raised against PKC-α and -ξ as described in Materials and Methods. Nitrocellulose membrane strips were incubated with anti-PKC antibodies, and immunopositive bands were detected using enhanced chemiluminescence.
DISCUSSION
The PKC (ATP : protein-phosphotransferase, EC 2.7.1.37) family of serine/threonine kinases is composed of at least 10 isoforms (α, β1, βII, γ, δ, ξ, ξ, η, θ, and λ), which are encoded by nine individual genes (Nishizuka, 1992). These 10 members have been subdivided into groups based on their common structural characteristics, regional localization, and dependencies on Ca2+, phospholipids, and DAG for activity. Individual PKCs are classified into the following groups : conventional (α, β1, βII, and γ ; Ca2+ - and DAG-regulated), novel (γ, ε, η, and θ ; DAG-regulated), and atypical (ξ and λ ; regulated by neither DAG nor Ca2+). PKC isoforms exist in an inactive state within the cytosol but become translocated to the plasma membrane by various stimuli, including activation of G protein-coupled receptors (Nishizuka, 1992 ; Tippmer et al., 1994). Similar to what has been described for β-adrenergic receptor kinase, the translocation of PKC is believed to be its primary mode of activation in mammalian cells (Nishizuka, 1986 ; Favaron et al., 1988 ; Lefkowitz, 1993).
Classically, PKC translocation follows the Gq/11 protein-mediated activation of phospholipase C (PLC) and the hydrolysis of phosphatidylinositol 4,5-bisphosphate, producing DAG and inositol 1,4,5-trisphosphate (IP3) (Conn and Sanders-Bush, 1985, 1986 ; Kendall and Nahorski, 1985 ; Nishizuka, 1986). Membrane-bound DAG binds to PKC directly, whereas IP3 stimulates the release of calcium from intracellular stores and stabilizes PKC's association with DAG (Kendall and Nahorski, 1985). DAG and Ca2+ are essential for the maximal translocation of the conventional PKC isoforms, whereas the novel PKCs only require DAG for activation (Berridge, 1984 ; Manev et al., 1990 ; Nishizuka, 1992). The conventional and novel PKC isoenzymes can also be directly activated by tumor-promoting phorbol esters, which are analogues of DAG (Castanga et al., 1982 ; Wolf et al., 1985 ; Cardell et al., 1990). The mechanism(s) that control the translocation of the atypical PKC isoforms (ξ and λ) are not understood.
Several interesting lines of evidence may explain how opioids induce translocation of multiple PKC isoforms in SH-SY5Y cells. In general, phosphatidylserine, Ca2+, and DAG are required to activate PKC-α and -ε but not PKC-ξ (Chen et al., 1995). Therefore, given the significant translocation of all PKC isoenzymes by DAMGO, some of these important modulators of PKC activity may be produced by μ-opioid receptor stimulation. μ-Opioids induce a PTX- and Ca2+-sensitive activation of PLC (possibly the PLC-β2 or -β3 isoform) and increase the formation of IP3 in SH-SY5Y cells (Smart and Lambert, 1995a, 1996 ; Smart et al., 1997). Opioids can also increase intracellular Ca2+ levels (Smart and Lambert, 1995b) despite their well-characterized action as inhibitors of voltage-sensitive calcium channels (Porzig, 1990 ; Tokuyama et al., 1995b ; Wilding et al., 1995). Opioid-mediated increases in intracellular calcium levels have been shown to arise from Ca2+ entry via N- and L-type calcium channels, through channels linked to the N-methyl-D-aspartate-sensitive glutamate receptor, and from the release of Ca2+ sequestered in IP3-sensitive stores (Jin et al., 1994 ; Tang et al., 1994 ; Smart and Lambert, 1995a, 1996 ; Connor and Henderson, 1996 ; Cai et al., 1997). The positive modulation of intracellular calcium levels and PLC activity by μ-selective opioids may combine and result in prolonged PKC translocation. PKC translocation is normally a transient event (lasting seconds) but can become prolonged in the presence of elevated intracellular calcium levels or other products of phosphatidylinositol 4,5-bisphosphate hydrolysis (Melloni et al., 1985 ; Berridge, 1993 ; Kramer et al., 1997).
PLC-β can be activated via the β/γ subunits of heterotrimeric (α and βγ subunits) Gi proteins (Ueda et al., 1995a,b ; Tsu and Wong, 1996), and this mode of action may be involved in PKC translocation by μ-opioids. The stimulation of Gi-linked μ-opioid receptors will liberate “free”β/γ subunits after the dissociation of the α subunit. It has been estimated that a single agonist-bound μ-receptor can activate up to 10 Gi proteins, greatly increasing the availability of free β/γ subunits (Breivogel et al., 1997). Therefore, agonist stimulation of μ-opioid receptors may produce enough catalytic β/γ subunits to activate the PLC-PKC pathway. Enhanced calcium entry into SH-SY5Y cells via voltage-sensitive calcium channels may explain why nimodipine, an L-type Ca2+ channel antagonist, was effective at reducing 4-h DAMGO-induced PKC translocation and opioid receptor down-regulation. Nimodipine also attenuated opioid receptor down-regulation after longer (24 h) DAMGO exposures and prevented or slowed the development of reverse PKC translocation, which peaked at this time point (Table 1). Therefore, calcium influx through the L-type channel may be important in the mechanism(s) that support opioid-mediated PKC translocation, receptor down-regulation, and possibly behavioral tolerance to morphine (Tokuyama et al., 1995a). Preventing calcium flux through L-type Ca2+ channels—and limiting PLC activation and PKC translocation—may be a mechanism by which nimodipine attenuates DAMGO-mediated decreases in number of opioid binding sites.
The significant attenuation of DAMGO (4-h)-mediated PKC translocation by preexposure of the cultures to PTX ( 1) suggests that both PTX-sensitive and -insensitive effector systems contribute to this response. Therefore, opioid receptors coupled to PTX-sensitive G proteins represent only a portion of those necessary to activate PKC and initiate down-regulation. In contrast to its partial effect on PKC translocation, PTX was unable to prevent opioid receptor down-regulation at any time point (Table 1), which is consistent with previous reports (Carter and Medzihardsky, 1993). The EC50 for PKC translocation by a 4-h treatment with DAMGO was 89.3 nM, which is ninefold higher than its IC50 for the inhibition of adenylyl cyclase in the same cell line (Carter and Medzihradsky, 1993). This difference is consistent with PLC activation occurring by a different mechanism (via Giβ/γ) compared with the inhibition of adenylyl cyclase via the Giα subunit. μ-Opioid receptors may also increase PLC/PKC activation and IP3 formation by coupling to Gzα or Gqα (Shen et al., 1991 ; Sarne and Gafni, 1996 ; Sarne et al., 1996), both of which are not sensitive to PTX.
Prior activation of PKC, before addition of opioid agonists, has been shown to enhance the magnitude of opioid receptor desensitization (Gucker and Bidlack, 1992 ; Narita et al., 1997). Our studies provide similar results for receptor down-regulation, which are shown in Fig. 1. It has been shown that the μ-opioid receptor protein becomes hyperphosphorylated in the presence of active PKC or after agonist stimulation of the μ-receptor expressed in Chinese hamster ovary cells (Wang et al., 1996b ; Zhang et al., 1996). It is possible that coapplication of opioids and phorbol esters activates different PKC isozymes or distinct pools of a single isoform to enhance the magnitude of receptor phosphorylation and down-regulation. In contrast, PKC activation in the absence of opioid agonist altered neither the affinity nor the density of μ-opioid receptors in SH-SY5Y cells (data not shown). Simultaneous activation of N-methyl-D-aspartate and δ-opioid receptors enhanced the phosphorylation and desensitization of the latter through a PKC-dependent pathway (Fan et al., 1998). However, NMDA receptor stimulation, by itself, which increases PKC translocation and activity, had no effect on the phosphorylation state of the δ-opioid receptor in the absence of an opioid agonist. It appears from these converging lines of evidence that agonist binding is required to change the conformational state of the receptor protein, which would allow the phosphorylation of specific serine/theronine residues required for down-regulation.
Chelerythrine chloride, an isozyme-nonspecific inhibitor of the catalytic subunit of PKC (Herbert et al., 1990), partially prevented the loss of [3H]DIPR binding sites, further supporting a role for PKC in DAMGO-mediated receptor down-regulation (Fig. 2). In examining the short-term (20-min) desensitization of opioid receptors, Zhang et al. (1996) argued against the involvement of PKC in receptor phosphorylation, because the PKC inhibitor staurosporine prevented phorbol ester- but not morphine-mediated phosphorylation of the cloned μ-receptor. These results are inconclusive because staurosporine has been shown to induce the membrane translocation and activation of several PKC isoforms (α, β, δ, ε, θ, and ξ) in different cell types (Jones et al., 1997 ; Mahon et al., 1997 ; O'Connell et al., 1997). This raises the possibility that activation of some isoforms of PKC by saturosporine may offset its inhibition of others. Other catalytic PKC inhibitors such as calphostin C—applied in vivo or in culture—effectively attenuate homologous opioid receptor desensitization, prevent tolerance to the analgesic effects of repeated morphine injections, and reduce the somatic symptoms of naloxone-precipitated opiate withdrawal (Narita et al., 1994 ; Ueda et al., 1995b ; Fundytus and Coderre, 1996 ; Wang et al., 1996a).
In our earlier study (Kramer and Simon, 1999), the loss of membrane PKC-α and -ε after extended DAMGO exposure (as measured by [3H]PDBu binding) did not halt the progression of opioid receptor down-regulation. In addition, the almost complete removal of phorbol ester-sensitive PKC isoforms, produced by a 2-day exposure to 1 μM PMA, did not attenuate DAMGO-mediated receptor down-regulation (Fig. 4). Similar findings have led some investigators to conclude that PKC is not involved in opioid receptor regulation (Pei et al., 1995 ; Trapaidze et al., 1996). However, the depletion of PKC-α and -ε (by PMA) in the SH-SY5Y cell line does not abolish such PKC-mediated biochemical events as K+- and carbochol-stimulated [3H]noradrenaline release (Turner et al., 1996). SH-SY5Y neuroblastoma cells express three of the known 10 PKC isoforms, α (conventional), ε (novel), and ξ (atypical) (Nakanishi and Exton, 1992 ; Turner et al., 1994), and only those PKC isoforms (α and ε) that contain a DAG/phorbol ester binding site are substrates for [3H]PDBu binding and can be down-regulated by chronic PMA exposure (Hong et al., 1995 ; Keenan et al., 1995). Therefore, this treatment should not affect the expression of the phorbol ester-insensitive PKC-ξ protein. Using immunoblots, our results clearly show that prolonged PMA (1 μM for 48 h) exposure depletes PKC-α and -ε from SH-SY5Y cells. The loss of these isoforms accounts for the dramatic decrease in [3H]PDBu binding (>90%) depicted in Fig. 3. As expected, PKC-ξ is resistant to the degradative effects of chronic PMA exposure (Fig. 4). Consequently, the continued presence of PKC-ξ may be sufficient to compensate for the loss of the other PKC isoforms and catalyze PKC-dependent processes within the cell, including μ-opioid receptor down-regulation. It cannot be ruled out, however, that the small amount of PKC-α and -ε that remains after chronic PMA exposure has an influence on agonist-induced opioid receptor down-regulation.
For PKC-ξ to be involved, it would have to be sensitive to translocation by opioids. We show in Fig. 6 that PKC-ξ is translocated to the membrane fraction by DAMGO, and this occurs with pharmacokinetics similar to those observed for PKC-α and -ε. It is interesting that no appreciable reverse translocation of PKC-ξ occurs over time, which is in contrast to what happens to PKC-α and -ε (Fig. 6). PKC-ξ appears to be resistant to the factors that cause reverse translocation of the calcium-and DAG-dependent isoforms (α and ε). The continued activity of this atypical PKC isoform may be sufficient to contribute to opioid receptor down-regulation during periods when the expression of the other isoforms is reduced, i.e., during reverse translocation. The existence of a pathway that uses multiple but differentially regulated subtypes of an enzyme may explain the effectiveness of catalytic PKC inhibitors and the ineffectiveness of phorbol ester-induced PKC depletion in preventing opioid receptor desensitization and down-regulation (Pei et al., 1995 ; Narita et al., 1996 ; Trapaidze et al., 1996).
It remains unknown which of the expressed PKC isoforms is involved in agonist-mediated opioid receptor down-regulation in SH-SY5Y neuroblastoma cells. One, two, or all three of these opioid-sensitive PKC isoforms may participate in this regulatory mechanism after their translocation to the plasma membrane. It is similarly unknown where PKC enters the pathway of μ-opioid receptor regulation, although at least two possibilities exist (a) Direct phosphorylation of the μ-opioid receptor may be necessary to initiate down-regulation and degradation via a lysosomal pathway, or (b) PKC may modify the activity of other enzymes known to contribute to opioid receptor plasticity such as β-adrenergic receptor kinase or mitogen-activated protein kinase (Winstel et al., 1996 ; Mullaney et al., 1997 ; Polakiewicz et al., 1998). Additional studies will be required to answer these questions.
In summary, as shown in our accompanying article (Kramer and Simon, 1999), a μ-selective opioid agonist is capable of producing prolonged PKC translocation to the plasma membrane of SH-SY5Y neuroblastoma cells, an effect that roughly parallels opioid receptor down-regulation. Prior activation of PKC potentiates, whereas inhibition of PKC's active site attenuates, the agonist-induced loss of membrane opioid receptors. Therefore, PKC activation may be one of the mechanisms involved in agonist-dependent μ-receptor down-regulation. In addition, more than one PKC isoform in this cell line appears to be involved in this process. All three PKC isoforms (α, ε, and ξ) expressed in SH-SY5Y cells translocate to the plasma membrane in response to prolonged DAMGO treatment ; however, the total cellular expression of the α and ε isoforms decreases as the incubation period is extended. The continued activity of PKC-ξ may explain why opioid receptor down-regulation is not reduced after the loss of DAG-regulated PKC isoforms. Overall, these lines of evidence suggest that multiple PKC isoforms may have a significant influence on the complicated mechanisms controlling agonist-mediated opioid receptor plasticity.
Acknowledgements
The authors are grateful for the help provided by Drs. Theresa Gioannini, Jacob Hiller, and Matthew Andria in the completion of the manuscript. This work was supported by training grant T32-DA07254 and research grant DA00017 to E.J.S. from the National Institute on Drug Abuse.




