Insulin Transiently Increases Tau Phosphorylation
Involvement of Glycogen Synthase Kinase-3β and Fyn Tyrosine Kinase
Abbreviations used : DTT, dithiothreitol ; GSK-3, glycogen synthase kinase-3 ; HRP, horseradish peroxidase ; IGF-1, insulin-like growth factor-1 ; IRS-1, insulin receptor substrate-1 ; MAP kinase, mitogen-activated protein kinase ; PHF, paired helical filament ; SDS, sodium dodecyl sulfate.
Abstract
Abstract : The modulation of tau phosphorylation in response to insulin was examined in human neuroblastoma SH-SY5Y cells. Insulin treatment resulted in a transient increase in tau phosphorylation followed by a decrease in tau phosphorylation that correlated directly with a sequential activation and deactivation of glycogen synthese kinase-3β (GSK-3β). The insulin-induced increase in tau phosphorylation and concurrent activation of GSK-3β was rapid (<2 min) and transient, and was associated with increased tyrosine phosphorylation of GSK-3β. The increase in GSK-3β tyrosine phosphorylation corresponded directly to an increase in the association of Fyn tyrosine kinase with GSK-3β, and Fyn immunoprecipitated from cells treated with insulin for 1 min phosphorylated GSK-3β to a significantly greater extent than Fyn immunoprecipitated from control cells. Subsequent to the increase in GSK-3β activation and tau phosphorylation, treatment of cells with insulin for 60 min resulted in a dephosphorylation of tau and a decrease in GSK-3β activity. Thus, insulin rapidly and transiently activated GSK-3β and modulated tau phosphorylation, alterations that may contribute to neuronal plasticity.
Glycogen synthese kinase-3 (GSK-3), originally identified as a protein kinase that phosphorylates and inactivates glycogen synthase, is widely expressed and has many other cellular functions. GSK-3 is present in the brain where it is expressed at high levels and within neurons (Woodgett, 1990). GSK-3 exists as two highly homologous isoforms, GSK-3α (51 kDa) and GSK-3β (46 kDa), which are encoded by two different genes and appear to be regulated in a similar manner (Woodgett, 1990 ; Hughes et al., 1992). GSK-3β phosphorylates a number of proteins in addition to glycogen synthase, including the regulatory subunit of cyclic AMP-dependent protein kinase (Hemmings et al., 1982), transcription factors such as c-jun, c-myc, c-myb, and cyclic AMP-response element binding protein (Boyle et al., 1991 ; Plyte et al., 1992 ; Nikolakaki et al., 1993 ; Fiol et al., 1994), and synapsin I (Yang et al., 1992).
Recent data indicate that tau protein, which is found predominantly in neurons, is also likely to be an in situ substrate of GSK-3β (Lovestone et al., 1994 ; Hong et al., 1997). Previous studies have shown that GSK-3β phosphorylates tau in vitro (Hanger et al., 1992 ; Mandelkow et al., 1992), and recently transfection studies (Lovestone et al., 1994) and studies using NT2N cells (Hong et al., 1997) have provided strong evidence that tau is an in situ GSK-3β substrate. Because phosphorylation of tau at specific sites reduces its ability to bind and stabilize microtubules (Lindwall and Cole, 1984), and in situ tau phosphorylation results in destabilization of the cytoskeleton and perturbation of axonal transport (Kosik, 1993), GSK-3β has the potential to play an important role in modulating tau function (Craft et al., 1996 ; Wallace et al., 1997).
The activity of GSK-3β is regulated by both serine and tyrosine phosphorylation. Phosphorylation of Ser9 on GSK-3β plays a key role in the down-regulation of GSK-3β activity (Sutherland et al., 1993 ; Stambolic and Woodgett, 1994). Mitogen-activated protein kinase (MAP kinase)-activated protein kinase-1 (also known as 90-kDa ribosomal protein S6 kinase or p90rsk), which is activated through the MAP kinase cascade (Cobb and Goldsmith, 1995), and Akt (also known as protein kinase B or RAC) and p70 S6 kinase (Sutherland et al., 1993), which are activated through the phosphatidylinositol 3-kinase signaling pathway, have been shown to phosphorylate Ser9 residues in vitro, consequently resulting in GSK-3β inactivation (Sutherland et al., 1993 ; Stambolic and Woodgett, 1994 ; Cross et al., 1995). There is also evidence that these kinases phosphorylate GSK-3β and reduce its activity in situ in a cell type-specific manner (Stambolic and Woodgett, 1994 ; Eldar-Finkelman et al., 1995 ; Hong and Lee, 1997). In addition to serine phosphorylation, tyrosine phosphorylation also regulates GSK-3β activity (Villa-Moruzzi, 1989 ; Hughes et al., 1993 ; Wang et al., 1994 ; Murai et al., 1996). In contrast to serine phosphorylation, tyrosine phosphorylation increases GSK-3β activity. In vivo, GSK-3β is phosphorylated on a unique tyrosine residue within an evolutionarily conserved sequence that results in increased activity (Hughes et al., 1993). However, the mechanism(s) by which tyrosine phosphorylation of GSK-3β is regulated in response to extracellular signals remains to be determined.
Insulin and insulin-like growth factor-1 (IGF-1) are likely to play important roles in modulating GSK-3β activity in neurons, as well as in nonneuronal cells. Prolonged activation of the insulin or IGF-1 receptor leads to down-regulation of GSK-3β activity (Cross et al., 1997 ; Hong and Lee, 1997). Additionally, insulin or other growth factors also may influence GSK-3β activity by modulating its tyrosine phosphorylation state (Murai et al., 1996). One possible mediator of GSK-3β tyrosine phosphorylation is Fyn tyrosine kinase, a member of the Src tyrosine kinase family. Recently, it has been demonstrated in vivo and in vitro that Fyn tyrosine kinase binds to insulin receptor substrate-1 (IRS-1) in response to insulin and cytokine stimulation (Sun et al., 1996). In addition, Fyn knockout mice exhibit significant neurological deficits, indicating a role for Fyn in neuronal development (Kojima et al., 1997). However, it remains to be determined if Fyn tyrosine kinase is involved in the regulation of GSK-3β activity by insulin.
In this study, the effects of insulin on tau phosphorylation and the activation of GSK-3β and Fyn were examined in human neuroblastoma SH-SY5Y cells. The results show that insulin induces a transient increase in tau phosphorylation and GSK-3β activity. A short period of insulin treatment (1 min) results in an increase in tau phosphorylation and an increase in GSK-3β activity that is associated with an increase in GSK-3β tyrosine phosphorylation, activation of Fyn, and increased association of Fyn with GSK-3β. Insulin treatment for >2 min decreases GSK-3β activity and tau phosphorylation.
MATERIALS AND METHODS
Materials
Recombinant human insulin and IGF-1 were purchased from Boehringer Mannheim and Bachem, respectively. All tau antibodies were mouse monoclonal except for T3P, a rabbit polyclonal tau antibody. Antibodies 5A6 (Johnson et al., 1997) and Tau5 (Carmel et al., 1996) are phosphate-independent antibodies and detect total tau protein. Tau-1 is a non-phosphorylation-dependent antibody (dephosphorylated epitope, amino acids 189-207) (Szendrei et al., 1993) [amino acids are numbered according to the longest human tau isoform in the brain (Goedert et al., 1989)]. The antibodies PHF-1 (Ser396/404), T3P (Ser396), AT8 (Ser202 and Thr205), AT180 (Thr231), and AT270 (Thr181) are phosphorylation-dependent (phosphorylation sites are indicated in parentheses). AT8, AT180, and AT270 were purchased from Polymedco ; the monoclonal antibodies for GSK-3β and Fyn were purchased from Transduction Laboratories ; monoclonal p-GSK-3β antibody (which recognizes tyrosine-phosphorylated GSK-3β) and purified Fyn were purchased from Upstate Biotechnology ; the polyclonal Fyn antibody was purchased from Santa Cruz Biotechnology. Recombinant GSK-3β was purchased from New England Bio-Labs. Protein G-Sepharose and LiCl were obtained from Sigma ; horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, HRP-conjugated goat anti-rabbit IgG antibodies, and HRP-conjugated protein A were purchased from Bio-Rad. Protein concentrations were determined using the bicinchoninic acid (BCA) assay kit (Pierce). The enhanced chemiluminescence kit and [γ-32P]ATP were purchased from Amersham Corp. Human recombinant tau was prepared from a bacterial expression system as previously described (Fleming et al., 1996).
Cell cultures
SH-SY5Y neuroblastoma cells were grown in RPMI 1640 medium supplemented with 10% horse serum, 5% Fetal Clone II, 2.4 mMl-glutamine, 120 U/ml penicillin G, and 120 μg/ml streptomycin. Cells were grown on 60-mm Corning dishes, and all experiments were carried out on cultures that were 70-80% confluent.
Treatment of SH-SY5Y cells
To determine the effects of insulin and IGF-1 on tau phosphorylation and GSK-3β activity, SH-SY5Y cells were placed in serum-free medium overnight before treatment with 10 μg/ml insulin or 10 nM (83 ng/ml) IGF-1 for 0-60 min. Where indicated, SH-SY5Y cells were pretreated overnight with LiCl (20 mM) before insulin treatment (Bennett et al., 1991).
Immunoblotting
After treatment, SH-SY5Y cells were rinsed once with cold phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 1.47 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.6) and lysed in lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.2 mM NaVO4, 0.5% Nonidet P-40) supplemented with 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, and 0.1 μM okadaic acid. The samples were briefly sonicated and then centrifuged for 20 min at 16,000 g at 4°C, and the protein concentration of the supernatant was determined using bovine serum albumin as the standard. Samples were diluted to a final concentration of 1 mg/ml in 2× Laemmli stop buffer [0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 2% sodium dodecyl sulfate (SDS), 25 mM dithiothreitol (DTT), 10% glycerol, and 0.01% bromophenol blue as tracking dye], incubated in a boiling water bath for 10 min, run on 8% SDS-polyacrylamide gels, and then transferred to nitrocellulose. The membranes were blocked for 5 min in Tris-buffered saline/Tween 20 (137 mM NaCl, 20 mM Tris-base, and 0.05% Tween 20) containing 5% nonfat dried milk, except for the Tau-1 antibody where borate saline (100 mM boric acid, 20 mM sodium borate, 80 mM NaCl) was used. Blots were incubated overnight in the blocking buffer with 5A6/Tau5 (both 1:1,000), Tau-1 (1:1,000), PHF-1 (1:250), polyclonal T3P antiserum (1:1,000), AT8 (1:1,000), AT180 (1:1,000), AT270 (1:1,000), anti-GSK-3β (1:1,000), anti-p-GSK-3β (1:1,000), or polyclonal anti-Fyn (1:500). Blots were rinsed twice in Tris-buffered saline/Tween-20 or borate saline, and then incubated for 4 h with HRP-conjugated goat anti-mouse IgG (H+L) antibody (1 : 1,000) or HRP-conjugated goat anti-rabbit IgG antibody (1:1,000) diluted in the appropriate blocking buffer. The immunoreactive proteins were detected using the enhanced chemiluminescence method. Immunoblots were quantified using a Bio-Rad model GS-670 densitometer.
Co-immunoprecipitation
Cell lysates were prepared as described above. Samples containing 100 μg of protein were precleared for 3 h at 4°C with protein G-Sepharose that had been washed previously three times with the lysis buffer, and were immunoprecipitated overnight at 4°C with 1 μg of the monoclonal GSK-3β antibody or 1.5 μg of the monoclonal Fyn antibody. Protein G-Sepharose (40 μl of suspension beads) was added, and the incubation was continued for 3 h. After the precipitates were washed three times with lysis buffer, 25 μl of Laemmli stop buffer was added to each pellet and the samples were placed in a boiling water bath for 15 min before SDS-polyacrylamide gel electrophoresis and immunoblotting. When the blots were probed with the polyclonal Fyn antibody, HRP-conjugated protein A was used instead of a secondary antibody. In some cases, the blots were stripped by incubation in 100 mMβ-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7, at 50°C for 30 min with agitation, followed by thorough rinsing, blocking, and reprobing with the appropriate antibody.
GSK-3β assay
After treatment, cells were lysed and GSK-3β was immunoprecipitated from 100 μg of protein as described above, except that protein A-Sepharose was used. The resultant precipitates were washed twice with lysis buffer and once with 20 mM Tris-HCl, pH 7.5, 1 mM DTT, and then incubated with 1.5 μg of recombinant tau for 5 min at 30°C in kinase assay buffer (20 mM Tris, pH 7.5, 10 mM MgCl2, 10 mM DTT, 250 μM ATP), containing 400 cpm/pmol [γ-32P]ATP in a final reaction volume of 30 μl. The reaction was terminated by adding 10 μl of 2× Laemmli stop buffer and incubating the samples in a boiling water bath for 15 min. The samples were cooled and then spun briefly, and the proteins in the supernatants were resolved on 8% SDS-polyacrylamide gels. The gels were stained with Coomassie Brilliant Blue, destained, and dried before being exposed to x-ray film. Relative GSK-3β activity was determined by measuring the extent of 32P incorporated into the recombinant tau by densitometric analysis of autoradiographs and the corresponding Coomassie-stained gels.
Fyn kinase assay
Purified Fyn was incubated with recombinant GSK-3β. Fyn (9.9 U) was is incubated with 2 μl of the recombinant GSK-3β in kinase buffer (200 mM Tris-HCl, pH 7.2, 25 mM MgCl2, 5 mM MnCl2, 4 mM EDTA, 0.5 mM NaVO4, 4 mM DTT) containing 200 μM ATP. The reaction was carried out at 30°C for 10 min in a final reaction volume of 20 μl and was stopped by the addition of 10 μl of 2× Laemmli buffer. The samples were incubated in a boiling water bath for 10 min, resolved on 8% SDS-polyacrylamide gels, and immunoblotted with the p-GSK-3β antibody.
To analyze the potential role of endogenous Fyn on tyrosine phosphorylation of GSK-3β, SH-SY5Y cells were incubated in the presence or absence of insulin for 1 min, and Fyn was immunoprecipitated from 100 μg of protein as described above. The resultant precipitate was washed twice with the lysis buffer and once with the kinase buffer (20 mM Tris-HCl, pH 7.5, 10 mM DTT, 10 mM MgCl2, 5 μM phenylarsine oxide, 0.1 μM okadaic acid). Kinase buffer (18 μl) containing 250 μM ATP and 2 μl of the recombinant GSK-3β were added to the resultant immunoprecipitates and then incubated for 30 min at 35°C. The samples were spun briefly, and 10 μl of 2× Laemmli stop buffer was added to the supernatant. The samples were incubated for 15 min in a boiling water bath subsequent to SDS-polyacrylamide gel electrophoresis and immunoblotting with the p-GSK-3β antibody.
Statistical analysis
Statistical significance was determined by using ANOVA with a Tukey-Kramer multiple comparisons test. The comparisons were considered significant if the p value was <0.05. The results are presented as means ± SEM.
RESULTS
Insulin and IGF-1 induce a rapid and transient phosphorylation of tau followed by dephosphorylation
To determine the effects of insulin or IGF-1 stimulation on tau phosphorylation, SH-SY5Y cells were treated with insulin (10 μg/ml) or IGF-1 (10 nM) for 0-60 min, and extracts were immunoblotted with Tau-1 (which recognizes tau dephosphorylated within the epitope 189-207), or with the phosphorylation-independent 5A6/Tau5 antibodies to determine total tau levels. After 1 min of insulin or IGF-1 treatment, a significant decrease in Tau-1 immunoreactivity was observed, indicating an increase of tau phosphorylation at this epitope (Fig. 1). The increase in tau phosphorylation was transient, as Tau-1 immunoreactivity gradually increased with longer times of insulin or IGF-1 treatment, indicating that the epitope was subsequently dephosphorylated. After 60 min of insulin or IGF-1 treatment, the Tau-1 immunoreactivity was greater than in untreated cells (Fig. 1). Thus, insulin and IGF-1 caused a biphasic change in tau phosphorylation within the Tau-1 epitope, consisting of a rapid increase in phosphorylation followed by a slower dephosphorylation. Because insulin (Fig. 1A and B) and IGF-1 (Fig. 1C) gave similar results, all subsequent experiments were carried out with insulin.
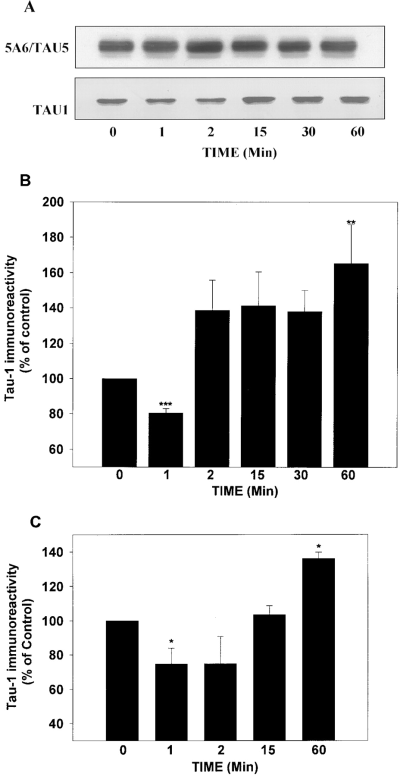
Insulin and IGF-1 induce a rapid and transient increase of tau phosphorylation followed by dephosphorylation in SH-SY5Y cells. SH-SY5Y cells were serum-starved overnight and treated with insulin (10 μg/ml ; A and B) or IGF-1 (10 nM ; C) for 0-60 min. A : Representative immunoblots of the time-dependent effects of insulin on the phosphorylation state of tau. Samples were collected at the times indicated and immunoblotted with the phosphate-independent tau antibodies 5A6/Tau5 or the dephosphorylation-dependent tau antibody Tau-1. Tau-1 immunoreactivity decreased after 1 min of insulin treatment, indicating an increase in tau phosphorylation at this site. At times greater than 1 min of insulin treatment, a reduction in tau phosphorylation occurred as indicated by the increase in Tau-1 immunoreactivity. B : Quantitative analysis of insulin effects on tau phosphorylation. Tau-1 immunoreactivity was normalized to the total amount of tau (5A6/Tau5 immunoreactivity) present in each sample. Insulin treatment induced a rapid (1 min) and significant decrease in Tau-1 immunoreactivity ; this increase in tau phosphorylation was transient, as at times greater than 1 min of insulin treatment increases in Tau-1 immunoreactivity were apparent, indicating tau dephosphorylation. C : Quantitative analysis of IGF-1 effects on tau phosphorylation. The effects of IGF-1 on tau phosphorylation at the Tau-1 site were similar to those obtained with insulin. The results are presented as means ± SEM for three to six different experiments. *p < 0.05 ; **p < 0.01 ; ***p < 0.001.
To identify further the phosphorylation sites on tau that are modulated by insulin, extracts from control and insulin-treated SH-SY5Y cells were probed with antibodies directed against several specific phosphoepitopes of tau. The results from a typical experiment are shown in Fig. 2A, and quantitative values obtained with each phosphate-dependent tau antibody are shown in Fig. 2B. After exposure to insulin for 1 min, increased immunoreactivity was evident with three phosphate-dependent antibodies, PHF-1, T3P, and AT8, indicating a rapid insulin-induced increase in tau phosphorylation at these sites. After 60 min of insulin treatment, the immunoreactivity of PHF-1, T3P, and AT8 was lower than in untreated cells, indicating that tau was dephosphorylated at these sites. In contrast, immunoreactivity with AT180 and with AT270 was not affected by either 1 or 60 min of insulin treatment (Fig. 2). This is in agreement with a previous study that demonstrated in vitro GSK-3β does not phosphorylate sites that are contained within the AT180 and AT270 epitopes (Illenberger et al., 1998).
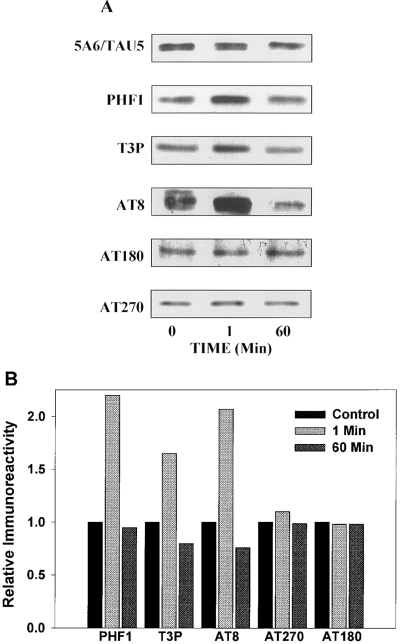
Insulin treatment induces site-specific modifications in tau phosphorylation. To analyze the sites on tau that were phosphorylated or dephosphorylated in response to insulin, SH-SY5Y cells were treated with insulin for 0, 1, or 60 min and lysates were immunoblotted with a panel of phosphate-dependent tau antibodies. A : Representative immunoblots from a typical experiment. Modifications of tau phosphorylation were determined with a panel of antibodies directed against specific phosphoepitopes. B : The densitometric values obtained with each phosphate-dependent tau antibody were normalized to 5A6/Tau5 levels (total tau). One minute of insulin treatment increased tau phosphorylation at sites recognized by PHF-1, T3P, and AT8, and 60 min of insulin treatment resulted in a decrease in tau phosphorylation at the T3P and AT8 sites as compared with cells not exposed to insulin (0 min). In contrast, insulin treatment did not alter phosphorylation on the sites recognized by AT180 and AT270. Results shown are representative of two independent experiments.
Insulin modulates tau phosphorylation through regulation of GSK-3β activity
To test the hypothesis that insulin modulates GSK-3β activity, SH-SY5Y cells were exposed to insulin for 0, 1, or 60 min, GSK-3β was immunoprecipitated, and GSK-3β activity was evaluated using an in vitro kinase assay with recombinant tau protein as the substrate. Figure 3A shows that insulin induced a rapid but transient activation of GSK-3β activity.
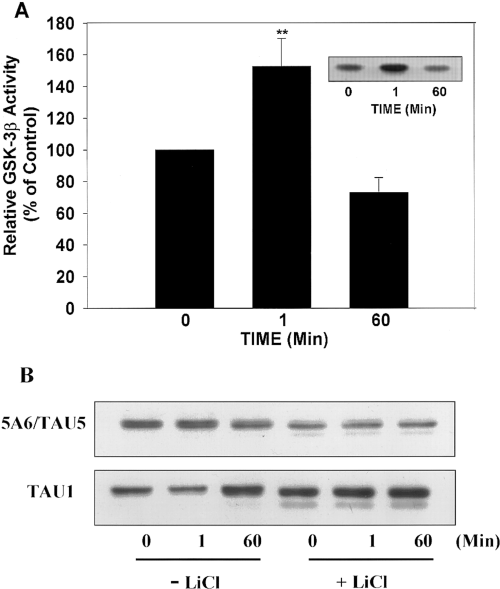
Insulin regulates tau phosphorylation through the modulation of GSK-3β activity. SH-SY5Y cells were treated with insulin for 0, 1, or 60 min, GSK-3β was immunoprecipitated with a monoclonal anti-GSK-3β antibody, and kinase activity was measured using recombinant human tau as a substrate. Incorporation of 32P into tau by GSK-3β was quantified and normalized to the total amount of tau in each reaction. A : A significant increase in GSK-3β activity was detected after 1 min of insulin treatment as compared with non-insulin-treated cells (0 min). This up-regulation of GSK-3β was transient, as 60 min of insulin treatment resulted in a down-regulation in GSK-3β activity. Results are expressed as means ± SEM from three independent experiments. **p < 0.01. Inset : Autoradiograph from a typical experiment. B : Because LiCl is a potent inhibitor of GSK-3β activity, SH-SY5Y cells were incubated in the absence (-LiCl) or in the presence (+ LiCl) of LiCl (20 mM) before treatment with insulin for 0, 1, or 60 min. Tau phosphorylation was analyzed by immunoblotting using the antibody Tau-1, and total tau protein was determined using 5A6/Tau5. In the absence of LiCl, Tau-1 immunoreactivity decreased after 1 min of insulin treatment (increased tau phosphorylation) and increased after 60 min (decreased tau phosphorylation) as compared with cells not treated with insulin (0 min). In cells pretreated with LiCl, no insulin-induced alterations in Tau-1 were observed. However, LiCl pretreatment resulted in an increase in Tau-1 immunoreactivity (indicating dephosphorylation) and in an increase in the electrophoretic mobility compared with untreated cells.
LiCl, a selective inhibitor of GSK-3β (Stambolic et al., 1996), was used to determine if GSK-3β mediated the insulin-induced increase in tau phosphorylation at the Tau-1 epitope. In the absence of LiCl, insulin caused a rapid and transient increase in tau phosphorylation at the Tau-1 epitope followed temporally by a decrease in phosphorylation at 60 min (Fig. 3B). Pretreatment with LiCl blocked the increase in tau phosphorylation at the Tau-1 epitope caused by 1 min of insulin treatment (Fig. 3B). Thus, treatment with LiCl, a selective GSK-3 inhibitor, blocked the insulin-induced phosphorylation of tau at this site, indicating that GSK-3β activity was necessary for the rapid increase in tau phosphorylation in response to insulin treatment. The conclusion that GSK-3β phosphorylates the Tau-1 site is supported further by the finding that inhibition of GSK-3β by LiCl in the absence of insulin resulted in tau dephosphorylation (increased Tau-1 immunoreactivity).
Insulin transiently induces tyrosine phosphorylation of GSK-3β
Insulin treatment induces a rapid and transient activation of GSK-3β and increase in tau phosphorylation in SH-SY5Y cells. Because previous studies reported that tyrosine phosphorylation could play an important role in the activation of GSK-3β (Villa-Moruzzi, 1989 ; Hughes et al., 1993 ; Wang et al., 1994 ; Murai et al., 1996), the effects of insulin on the tyrosine phosphorylation of GSK-3β were examined. SH-SY5Y cells were exposed to insulin for 0, 1, or 60 min, GSK-3β was immunoprecipitated, and tyrosine phosphorylation was analyzed using the anti p-GSK-3β antibody, which specifically recognizes GSK-3β phosphorylated on a tyrosine residue located in its catalytic domain (Fig. 4A). Quantified results are shown in Fig. 4B. One minute of insulin treatment resulted in a significant increase in immuno-precipitated GSK-3β phosphotyrosine immunoreactivity, whereas GSK-3β tyrosine phosphorylation returned to or below control levels after prolonged insulin treatment (60 min). These results demonstrate that insulin induces a rapid and transient tyrosine phosphorylation of GSK-3β.
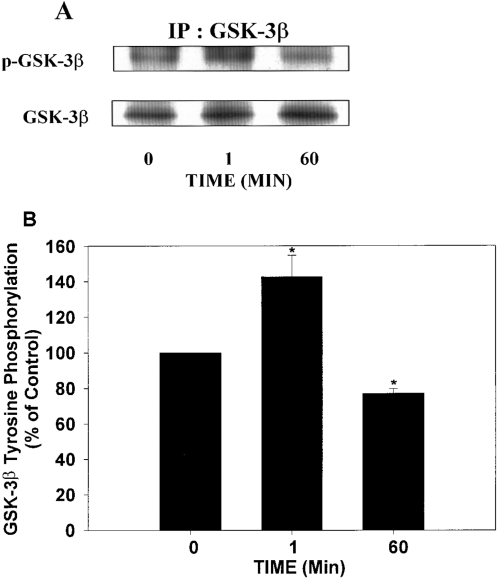
Insulin induces a transient tyrosine phosphorylation of GSK-3β. SH-SY5Y cells were exposed to insulin for 0, 1, or 60 min, GSK-3β was immunoprecipitated with a monoclonal GSK-3β antibody, and the tyrosine phosphorylation of GSK-3β was analyzed with the p-GSK-3β antibody, which recognizes tyrosine-phosphorylated GSK-3β. A : Representative immunoblots. IP, immunoprecipitation. B : The amount of tyrosine-phosphorylated GSK-3β reactivity (p-GSK-3β) was adjusted for the total amount of GSK-3β in the precipitates, and the data were expressed as percentage of control. An increase in tyrosine-phosphorylated GSK-3β was detected after 1 min of insulin treatment as compared with non-insulin-treated cells (0 min). This tyrosine phosphorylation of GSK-3β was transient, as 60 min of treatment resulted in a significant tyrosine dephosphorylation of GSK-3β as compared with cells not exposed to insulin (0 min). Results are expressed as means ± SEM for three different experiments. *p < 0.05.
In vivo association of the Fyn tyrosine kinase with GSK-3β in response to insulin
Because a recent study indicated that Fyn tyrosine kinase may be involved in insulin or IGF-1 signaling (Sun et al., 1996), the in situ association of Fyn with GSK-3β was examined in control and insulin-treated SH-SY5Y cells. Fyn was immunoprecipitated from control and insulin-stimulated cell lysates, and immunoprecipitates were analyzed for the presence of GSK-3β. GSK-3β co-precipitated with Fyn, and treatment with insulin for 1 min increased the amount of GSK-3β that co-precipitated with Fyn (Fig. 5A). Quantification of the data demonstrated that 1 min of insulin treatment significantly increased the association between Fyn and GSK-3β (Fig. 5B).
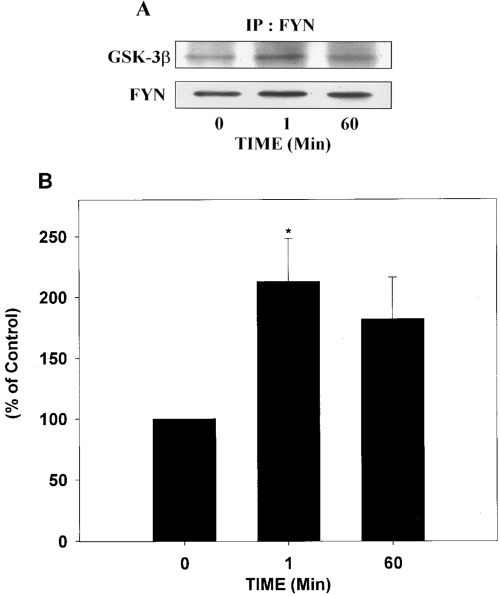
In situ association between GSK-3β and Fyn in response to insulin treatment. Lysates were prepared from cells incubated with insulin for 0, 1, or 60 min, Fyn was immunoprecipitated from each sample, and the precipitates were immunoblotted with the GSK-3β antibody. The blots were then stripped and reprobed with a Fyn antibody. A : Representative immunoblots. IP, immunoprecipitation. B : The amount of GSK-3β associated with Fyn was quantified and expressed as percentage of control (0 min). One minute of insulin treatment resulted in a significant increase in the association between Fyn and GSK-3β as compared with non-insulin-exposed cells (0 min). Results are expressed as means ± SEM for four different experiments. *p < 0.05.
Fyn induces tyrosine phosphorylation of GSK-3β in response to insulin
To confirm that GSK-3β is phosphorylated by Fyn, recombinant GSK-3β was incubated with purified active Fyn or with Fyn immunoprecipitated from SH-SY5Y cells. Purified Fyn and Fyn immunoprecipitated from SH-SY5Y cells readily phosphorylated GSK-3β as evidenced by increased p-GSK-3β immunoreactivity (Fig. 6). Fyn immunoprecipitated from cells stimulated with insulin for 1 min tyrosine-phosphorylated recombinant GSK-3β to a greater extent than Fyn immunoprecipitated from control cells (Fig. 6B).
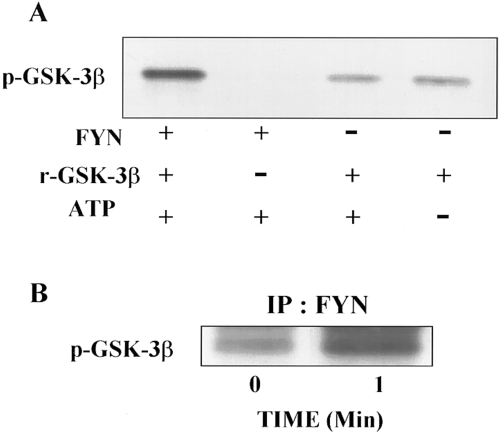
Fyn phosphorylates GSK-3β in response to insulin treatment. A : Purified Fyn was incubated with recombinant GSK-3β (r-GSK-3β) in the presence or absence of ATP (250 μM), and the samples were immunoblotted with p-GSK-3β antibody, which only recognizes GSK-3β when it is tyrosine-phosphorylated. In the presence of ATP, Fyn tyrosine-phosphorylates the r-GSK-3β. Incubation of the r-GSK-3β in the presence of ATP, but in the absence of Fyn, did not result in increased p-GSK-3β immunoreactivity. B : Fyn was immunoprecipitated from SH-SY5Y cells exposed to insulin for 0 and 1 min, incubated with r-GSK-3β in the presence of ATP, and the samples immunoblotted with p-GSK-3β antibody. Fyn precipitated from insulin-stimulated cells tyrosine-phosphorylated r-GSK-3β to a greater extent than Fyn precipitated from control cells. Representative results from two separate experiments are shown. IP, immunoprecipitation.
DISCUSSION
This study identified a novel signaling process induced by insulin in SH-SY5Y cells consisting of a sequential activation and deactivation of GSK-3β that directly correlated with a transient increase in tau phosphorylation and subsequent dephosphorylation. The insulin-induced activation of GSK-3β and increased tau phosphorylation were rapid (<2 min) and transient and were associated with increased tyrosine phosphorylation of GSK-3β. The increase in the tyrosine phosphorylation of GSK-3β occurred concurrently with an increase in the in situ association of GSK-3β with the Src family tyrosine kinase Fyn and with activation of Fyn. The subsequent (60 min) insulin-induced decrease in GSK-3β activity and tau phosphorylation correlated with a decrease in the tyrosine phosphorylation of GSK-3β. Thus, the phosphorylation state of tau is sensitive to changes in the activity of GSK-3β and can be modulated by insulin-induced signaling systems. Indeed, there is a growing body of evidence indicating that insulin plays a significant role during neuronal development and in the maintenance of normal brain function (Craft et al., 1996).
Classically, GSK-3β has been considered to be a constitutive active kinase. However, there is considerable evidence indicating that GSK-3β is activated in response to extracellular physiological signals. For example, epidermal growth factor treatment of A431 cells resulted in a rapid and transient activation of GSK-3β (Yang et al., 1989a). In addition, it has been reported that insulin treatment induces a rapid and transient activation of GSK-3β in rat adipocytes (Yang et al., 1989b) and in 3T3-L1 cells (Villa-Moruzzi, 1989). It is remarkable that, in all of these experiments and in accord with our results, the extracellular signal-induced transient activation of GSK-3β was always extinguished within 3 min. This short duration of activation probably explains why Murai et al. (1996) observed tyrosine dephosphorylation and inactivation of GSK-3β after 5 min of insulin treatment in Chinese hamster ovary cells overexpressing the human insulin receptor. In the present study, only 1-2 min of insulin exposure resulted in an increase in the activity of GSK-3β. In addition, this increase in GSK-3β activity correlated with increased tyrosine phosphorylation of GSK-3β. These results provide strong evidence to support the conclusion that insulin activates GSK-3β through a tyrosine kinase-dependent mechanism.
One candidate protein kinase that may be involved in modulating the tyrosine phosphorylation state of GSK-3β in response to insulin is the tyrosine kinase Fyn. Fyn is a member of the Src family of tyrosine kinases that has been shown to bind IRS-1 in response to insulin (Sun et al., 1996). However, this previous report did not address the biological effects of the recruitment of Fyn to IRS-1. In the present study, insulin induced a rapid (<2 min), significant increase in the association of Fyn with GSK-3β that correlated with increased GSK-3β tyrosine phosphorylation. In addition, Fyn from insulin-treated cells tyrosine-phosphorylated recombinant GSK-3β to a greater extent than did Fyn immunoprecipitated from control cells. These results indicate that Fyn is activated in response to insulin, resulting in the tyrosine phosphorylation and activation of GSK-3β. However, further studies are required to demonstrate unequivocally a role for Fyn in the activation of GSK-3β.
In agreement with previous studies, treatment of SH-SY5Y cells with insulin for a period of >1-2 min resulted in a decrease in tau phosphorylation and a decrease in GSK-3β activity (Hong and Lee, 1997). An increase in phosphotyrosine phosphatase activity is likely to be partially involved in the down-regulation of GSK-3β by inducing tyrosine dephosphorylation (Hughes et al., 1993). However, insulin stimulation of Ser9 phosphorylation is also likely to play a role in the down-regulation of GSK-3β activity (Sutherland et al., 1993 ; Stambolic and Woodgett, 1994 ; Cross et al., 1995). In several cell types, insulin stimulation of the phosphatidylinositol 3-kinase pathway and subsequent activation of the serine/threonine kinase Akt result in phosphorylation and inactivation of GSK-3β (Hong and Lee, 1997). However, other studies have shown that epidermal growth factor-induced GSK-3 inactivation can be mediated by the MAP kinase signaling pathway (Eldar-Finkelman et al., 1995). These results suggest that the activity of GSK-3β is likely to be down-regulated by several different intracellular signaling pathways.
Insulin stimulation resulted in a transient increase in tau phosphorylation that correlated directly with a transient increase in GSK-3β activity, which agrees with previous reports that GSK-3β associates with microtubules and phosphorylates tau (Ishiguro et al., 1992). A primary function of tau is to modulate microtubule dynamics, a function that is negatively regulated by increased tau phosphorylation. It is also likely that phosphorylation mediates the less classical functions of tau, especially those involved in neurite outgrowth (Black et al., 1996 ; Mandell and Banker, 1996). The question now remains as to what could be the functional consequences of the biphasic alterations in tau phosphorylation induced by insulin. An answer to this question may be linked to the role of tau in neuritogenesis. Insulin and IGF-1 both play an important role in cell motility through receptor-mediated regulation of the activities of specific kinases and phosphotases (Leventhal et al., 1997). It is also clear that coordinated tyrosine phosphorylation of specific proteins is required for neurite outgrowth and cell motility (Leventhal et al., 1997). GSK-3β (Takahashi et al., 1994) and Fyn (Bixby and Jhabvala, 1993) are both enriched in growing axons. Tau is likely to be involved in the initial development of neurites, and is also enriched in growth cones and in the distal regions of growing axons (Black et al., 1996). Suppression of tau expression in cultured neurons using antisense oligonucleotides significantly reduced growth cone area and filopodia number concomitant with considerable changes in the phalloidin staining of assembled actin (DiTella et al., 1994). In addition, tau interacts with the plasma membrane via its N-terminal region, and membrane-associated tau is enriched in growth cones (Brandt et al., 1995), suggesting that tau may participate in maintaining the structural organization of growth cones. The mechanism by which tau influences axonal growth and guidance remains incompletely elucidated ; however, several studies have shown that alterations in tau phosphorylation occur during early neuronal development. For example, tau phosphorylated at the PHF-1 epitope is associated with the early stages of axon formation in the developing nervous system (Pope et al., 1993). In addition, a recent study demonstrated the presence of a gradient of phosphorylated tau within nascent axons (Mandell and Banker, 1996). This gradient was dynamic and potentially regulated by upstream signals involving tyrosine kinases (Mandell and Banker, 1996). We hypothesize that GSK-3β and tau may contribute to the increase of the neuronal cytoskeleton dynamics required for neuronal plasticity and growth during development. By transiently activating GSK-3β and increasing tau phosphorylation, insulin stimulation may contribute to the initial and transient instability of the neuronal cytoskeleton necessary for the extension and retraction of neurites, or leading to the increased flexibility of the cytoskeleton-plasma membrane interface to enable neurite outgrowth.
Although appropriately coordinated tau phosphorylation plays an important role during development, abnormal tau phosphorylation is likely to contribute to the neurodegeneration in Alzheimer's disease. Since the discovery that the paired helical filaments (PHFs) in Alzheimer's disease are composed of abnormally hyperphosphorylated tau, many studies have focused on the regulation of tau phosphorylation. GSK-3β phosphorylates tau in vitro (Mandelkow et al., 1992 ; Hanger et al., 1992) and in vivo (Lovestone et al., 1994 ; Hong et al., 1997), at some of the sites that are hyperphosphorylated in PHF-tau. A previous study also reported an increase in Fyn immunoreactivity in Alzheimer's disease brains compared with controls (Shirazi and Wood, 1993). In addition, in hippocampal slices prepared from Fyn knockout mice, diffusible β-amyloid oligomers (ADDLs) did not induce cell death, whereas ADDL treatment of hippocampal slices from control mice resulted in significant cell death (Lambert et al., 1998). These findings suggest that the neurotoxic effect of β-amyloid in Alzheimer's disease brain may be mediated in part by Fyn. Because Fyn is likely to tyrosine-phosphorylate and activate GSK-3β, an increase in Fyn expression and/or activation could result in increased GSK-3β activity and, subsequently, an abnormal increase in tau phosphorylation in Alzheimer's disease brain. However, further research is necessary to elucidate the possible roles of Fyn and GSK-3β in the abnormal phosphorylation of tau in Alzheimer's disease brain.
Acknowledgements
The authors would like to thank Scott Jenkins for technical assistance, Dr. V. Lee for the T3P antibody, Dr. L. Binder for the antibodies Tau-1 and Tau5, and Dr. S. Greenberg and Dr. P. Davies for the PHF-1 antibody. This work was supported by NIH grants NS27538 and MH38752.




