The effects of productivity and seasonality on life history: comparing age at maturity among moose (Alces alces) populations
Abstract
The productivity hypothesis in respect of an animal species’ geographical range predicts that whereas higher productivity at the equatorial periphery of a species’ range favours superior competitors, lower productivity at the centre of a species’ range favours high reproduction and reduced competitive traits. I test whether life-history patterns follow this hypothesis, using demographic data from 15 Canadian moose (Alces alces) populations. Two models are contrasted; the first assumes that intraspecific variation in age at maturity is explained proximately by density and juvenile mortality. Age at maturity was found to increase with decreasing juvenile mortality (P = 0.01) and increasing density (P = 0.006). To test the productivity hypothesis, the second model additionally included primary productivity and seasonality as geographical explanatory variables that would ultimately influence age at maturity via juvenile mortality and density. Path analysis indicated that including productivity and seasonality improved the model predictions of variation in age at maturity (Ra2 0.56 and 0.85). In bivariate comparisons, seasonality was negatively associated (P = 0.01) with age at maturity. In the best model, however, primary productivity was the environmental variable that explained 25% of the variance in age at maturity, and forest cover replaced seasonality as an explanatory variable. The positive association between primary productivity and age at maturity is consistent with the productivity hypothesis. Relative to populations that lived at the centre of the species’ range (51°N), moose populations living in relatively high productivity and low seasonality environments (equatorial periphery of species’ range; 48°N) experienced less juvenile mortality, more variable year-to-year density, higher relative density and slower life history (slower growth rate, later age at maturity, lower fecundity).
Introduction
Can the selective forces responsible for the evolution of specific life-history traits be identified (Maynard Smith, 1978)? Important environmental selection pressures include primary productivity and variation in energy, such as seasonality (Stevens, 1989; Rosenzweig & Abramsky, 1993; Ferguson et al., 1996; Ferguson & McLoughlin, 2000). For example, trophic theory predicts that with increasing primary productivity herbivore populations should change from resource limitation to predator regulation (Hairston et al., 1960; Oksanen et al., 1981; Fretwell, 1987; Crête & Manseau, 1996). Also, differences in seasonality influence life-history traits (Boyce, 1979; Zeveloff & Boyce, 1988; Adolph & Porter, 1996; McLoughlin et al., 2000). As well, a varying and/or unpredictable environment can result in limited food availability and favour fewer young and delayed maturity (Boyce, 1979; Krementz & Handford, 1984; Ferguson et al., 1996; Badyaev, 1997).
Life-history traits may also vary with environmental and density changes associated with the geographical locations of populations (Ferguson & McLoughlin, 2000). Age at reproductive maturity is a pivotal life history trait as selection pressures and trade-offs change dramatically with maturation. At maturity, the energy that had previously gone into growth is instead used for the production of offspring. Natural selection optimizes allocation of resources to growth and reproduction depending on relative mortality rates (Kozlowski & Weiner, 1997) that are determined environmentally (Tuljapurkar, 1990). For mammals, the physical environment exerts selection most strongly by determining adult mortality and density-dependent juvenile mortality (Fowler, 1981; Sinclair, 1989; Charnov, 1993; Gaillard et al., 2000). Thus, mammals mature at the age that maximizes lifetime reproductive success according to local environmental conditions. Different processes may account for variation in age at maturity among species and among populations within a species (Stearns, 1992). After controlling for the effects of size and phylogeny, bird and mammal species that delay maturity have longer lives and lower fecundities (Harvey & Zammuto, 1985; Gaillard et al., 1989). However, the process of adaptation occurs at the population level (Wilson, 1996; Kozlowski & Weiner, 1997) and therefore intraspecific comparisons are the favoured approach to describe trade-offs between timing of maturation, growth rate and juvenile mortality (e.g. Sæther & Haagenrud, 1985; Elowe & Dodge, 1989; Gaillard et al., 1992; Lunn et al., 1994; Festa-Bianchet et al., 1995; Hewison, 1997; McLoughlin & Ferguson, 2000). Individuals within populations vary in growth pattern for particular environments and selection ensures maturity at an age that maximizes fitness. For example, animals with high juvenile mortality rates (e.g. due to predation) are predicted to have correspondingly rapid development and early age at maturity (Cole, 1954; Williams, 1966; Law, 1979; Reznick & Endler, 1982; Abrams & Rowe, 1996; Reznick et al., 2001).
The productivity–diversity model (Grime, 1973) predicts species richness based on the effects of primary productivity on the competitive outcome of multi-species dynamics (Huston, 1994; Kondoh, 2001). An intraspecific test of this productivity model would include changes in life-history traits that determine competitiveness of individuals among populations living in different productivity environments within a species’ geographical range. Temperate ungulates that have increased age at maturity as a response to density-related resource limitation include reindeer (Rangifer tarandus: Reimers, 1983; Skogland, 1985), moose (Sæther & Haagenrud, 1985), white-tailed deer (Odocoileus virginianus: Woolf & Harder, 1979; Verme, 1991), and mule deer (Odocoileus hemionus: Thomas, 1983) among others (Putman et al., 1996). The moose (Alces alces L.) is an appropriate species by which to test these predictions, as females show considerable variation in age and size at maturity (Sæther & Heim, 1993; Sand & Cederlund, 1996) and reproductive effort (e.g. yearling pregnancy and twinning rates) with environment (Geist, 1974, 1987; Boer, 1992). Moose have a circumpolar distribution and have adapted a diversity of life-history traits that probably correspond with a wide variety of energy availability and environmental variability (Franzmann & Schwartz, 1997). The geographical range model predicts: (a) that animal populations living at high latitudes display slow life histories (late maturity) as phenotypic adaptations to abiotic stress; (b) that populations living at the centre of the geographical range display fast life histories (early maturity); and (c) that equatorial periphery populations display slow life histories (late maturity) as phenotypic adaptations to intraspecific competition (Ferguson & McLoughlin 2000).
Here, the influences of environment and population dynamics on age at maturity are described by comparing moose populations living near the centre (b) and equatorial periphery (c) of their North American geographical range that display a wide range of juvenile mortality, population density, primary productivity and seasonality. I compare the fit of one model that predicts increasing age at maturity with decreasing juvenile mortality and increasing density (Stearns & Koella, 1986; Kozlowski, 1992; Abrams & Rowe, 1996); and the same model with the addition of two geographical explanatory variables: primary productivity and seasonality. It is predicted (1) that populations that live in environments at the centre of the species’ geographical range, characterized by highly seasonal primary productivity, should have fast life-history phenotypes (e.g. early age at maturity) that increase reproductive rate; and (2) that populations living in environments at the equatorial periphery, characterized by less seasonality and low forest cover, should have life-history phenotypes of later age at maturity that increase intraspecific competitive abilities.
Methods
Study areas
This study is based on 15 populations in two study areas: (1) four populations in Ontario and (2) 11 populations in Newfoundland, all of which have been subject to hunting (Ferguson et al., 2000). Both study areas lie within the boreal forest region (Rowe, 1972) dominated by conifer species (Picea mariana (Mill.) B.S.P., P. glauca (Moench) Voss, Abies balsamea (L.) Mill. and Pinus banksiana Lamb.) and less abundant deciduous trees (Populus tremuloides Michx. and Betula papyrifera Marsh.). The climate of Newfoundland is strongly influenced by the surrounding ocean. This applies particularly to coastal communities that are characterized by heavy precipitation (1200–1700 mm/year), intermittent snow cover and frequent freezing rain in late winter, and by low forest cover (Banfield, 1983). However, moose populations in the interior of the island of Newfoundland experience colder and drier winters, with 75% of winter precipitation falling as snow and with snow cover remaining more continuous. Also, interior areas are characterized by moderately warmer summers similar to the northern Ontario climate. Whereas the considerable seasonal pulse in productivity during the growing season has favoured the development of extensive boreal forests in the interior, the coastal region of Newfoundland is relatively barren (Banfield, 1983). The northern Ontario climate is humid continental with a mean annual precipitation of 650–800 cm, including a mean January snowfall of 55 cm (Baldwin et al., 2000).
Newfoundland moose density has varied from 0.5 to 4.0 moose/km–2 over the period 1969–91 (Ferguson, 1993). Humans harvest between 3% and 22% of the moose population per year (Ferguson & Messier, 1996a). Black bears (Ursus americanus) are the only natural predators of moose. Moose were introduced to Newfoundland from Nova Scotia in 1878 and 1904 (Pimlott, 1953). The Ontario moose populations were located > 1000 km to the east of Newfoundland and here moose density varied from 0.08 to 0.5 km–2 over the period 1979–93 (Timmermann & Whitlaw, 1992). Ontario moose experience high rates of juvenile predation from wolves and black bears (Ferguson et al., 2000).
Age at maturity
Age at maturity was defined as age at the inflection point (highest mortality rate) of annual survivorship curves estimated from cohort analysis of hunter kill data (Ferguson, 2002) and relates to the proportion of breeding 2-year-old females. Kill data indicate that first-time reproducers suffer the highest mortality during the hunting season. The inflection point thus demarcates the change in mortality rate between juvenile and adult life stages associated with hunting mortality of inexperienced females accompanied by 6-month-old calves.
The cohort analysis model CAGEAN (Deriso et al., 1985) was used to estimate sex- and age-specific moose numbers and annual survival of cohorts (as Ferguson, 1993). Moose demography can be modelled using cohort analyses because of synchronized spring breeding and the availability of age-specific kills and age-specific natural mortality rates from time series data provided by hunters. Age of moose was determined from tooth eruption pattern for juveniles (< 18 months old) and by counting incremental growth rings in the cementum layer on the first incisor from older animals (Sergeant & Pimlott, 1959).
Juvenile mortality
Percentage of juvenile mortality was calculated using the survivorship at the inflection point. Log-transformed juvenile mortality was the dependent variable and age was the independent variable in regression analysis. Values for juvenile mortality given in tables and figures are percentages of total population mortality that occurred before age at maturity.
Unpredictability of population size
The variance of a population over a time-series typically increases as a power-law with increasing time lag, indicating long-range correlation in population size fluctuations (Keitt & Stanley, 1998). Hurst exponents (H), a measure of fractal dimension, were used to evaluate the unpredictability of total population size for each moose population (Arino & Pimm, 1995). The Hurst exponent was estimated using a Turbo Pascal program written by Hastings & Sugihara (1993: 57–61). H close to 0.5 describes a close to random series of events that are uncorrelated (i.e. random walk). H approaching 1.0 indicates a persistent, or trend-reinforcing, series. The strength of the trend-reinforcing behaviour increases as H approaches 1.0, or 100% correlation.
Primary productivity, seasonality and growing season
Measures of primary productivity and seasonality were calculated, respectively, as the sum and the coefficient of variation (CV) of monthly (n = 12) values of actual evapotranspiration (AET). The calculations of AET were made with a water budget analysis that is based on observed average monthly precipitation and an estimate of potential evapotranspiration derived from observed average monthly surface temperature, using a modified version of the method of Thornthwaite (Willmott et al., 1985). The mean and CV values were calculated for weather stations located within or close to moose populations (n = 15). Actual evapotranspiration is correlated highly with primary productivity (Rosenzweig, 1968). Primary productivity (g/m–2/year) was estimated from annual AET using a power function (Leith, 1976; Ferguson & McLoughlin, 2000). Larger mean primary productivity values thus represent greater annual energy availability within a populations’ range. Larger CV values indicate greater seasonality (Zeveloff & Boyce, 1988). Total primary productivity during the growing season may be as important as total annual primary productivity in development of moose life history (Geist, 1987). Therefore, the total primary productivity during the months of June, July and August was also calculated. The distribution of weather stations followed the spatial pattern of human habitation of coastal communities in Newfoundland. Therefore for four of 11 moose populations (Table 1) located along the Newfoundland coastline, data from coastal weather stations were used. In contrast, for moose populations located within the centre of Newfoundland, weather data were derived from weather stations located inland.
| Population | Years | Sample size* | Area (km2) | Latitude | Longitude | Age at maturity (y) | Density† | Juvenile mortality‡ | Unpredictability§ | Forest cover | Primary productivity¶ | Seasonality | Productivity of growing season |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WMU 13** | 1971–91 | 2421 | 13325 | 48°40′ | 89°23′ | 2.54 | 0.36 | 60.2 | 0.75 | 75.9 | 1094 | 109.2 | 690.3 |
| Dist. 13 | 1975–93 | 1952 | 9759 | 49°40′ | 90°21′ | 2.21 | 0.25 | 70.0 | 0.89 | 83.5 | 1025 | 122.1 | 752.0 |
| Dist. 15 | 1975–93 | 2284 | 57670 | 51°31′ | 90°50′ | 2.72 | 0.28 | 60.6 | 0.81 | 89.8 | 1096 | 115.0 | 733.1 |
| Dist. 16 | 1975–93 | 2780 | 103023 | 51°49′ | 93°28′ | 2.57 | 0.08 | 58.0 | 0.80 | 89.1 | 1068 | 123.3 | 757.0 |
| MMU 3†† | 1973–91 | 299 | 3580 | 50°25′ | 56°44′ | 2.88 | 2.35 | 29.3 | 0.47 | 25.8 | 1051 | 110.8 | 663.0 |
| MMU 7 | 1973–91 | 576 | 1720 | 49°06′ | 57°30′ | 2.94 | 1.37 | 50.5 | 0.62 | 57.0 | 1072 | 110.6 | 690.3 |
| MMU 9†† | 1970–91 | 496 | 809 | 48°07′ | 58°52′ | 3.32 | 4.54 | 39.9 | 0.43 | 59.0 | 1063 | 103.4 | 627.8 |
| MMU 11 | 1973–91 | 502 | 2844 | 48°10′ | 58°08′ | 3.30 | 1.21 | 38.1 | 0.71 | 26.9 | 1127 | 104.8 | 706.1 |
| MMU 16 | 1966–91 | 1007 | 1676 | 48°39′ | 56°04′ | 3.09 | 1.93 | 41.1 | 0.97 | 51.8 | 1109 | 105.6 | 693.0 |
| MMU 18 | 1973–91 | 548 | 3871 | 48°14′ | 56°23′ | 3.00 | 1.12 | 35.9 | 0.46 | 13.5 | 1140 | 103.9 | 714.2 |
| MMU19†† | 1973–91 | 327 | 2228 | 47°53′ | 57°48′ | 3.13 | 1.24 | 28.1 | 0.42 | 6.2 | 1076 | 101.7 | 630.6 |
| MMU 22 | 1975–91 | 312 | 2015 | 49°14′ | 54°48′ | 3.14 | 3.77 | 51.4 | 0.66 | 65.5 | 1078 | 109.9 | 690.2 |
| MMU 23 | 1973–91 | 311 | 4302 | 49°11′ | 53°58′ | 3.49 | 2.21 | 46.3 | 0.66 | 54.4 | 1124 | 106.0 | 711.7 |
| MMU 24 | 1966–91 | 1882 | 910 | 48°49′ | 55°03′ | 3.33 | 2.57 | 50.7 | 0.63 | 64.1 | 1064 | 108.7 | 666.0 |
| MMU 36†† | 1973–91 | 1210 | 3469 | 47°02′ | 53°15′ | 2.75 | 1.94 | 54.9 | 0.85 | 20.2 | 1104 | 98.9 | 638.7 |
- * Number of known-aged females;
- † moose km–2 from Ferguson (1993) and Whitlaw et al. (1993);
- ‡ calculated as inflection point of survivorship data (see Methods);
- § Hurst exponent measures unpredictability of total population size (see Methods);
- ¶ g/m–2/year;
- ** Wildlife Management Unit and District for Ontario and Moose Management Unit for Newfoundland;
- †† moose populations occurring in coastal environments characterized by low forest cover.
Statistical analyses
Statistical tests included Pearson product-moment correlations and partial correlation analysis (Sokal & Rohlf, 1981) to analyse association among variables, and were performed using SAS (PROC REG, SAS Institute Inc., 1987) statistical software for microcomputers. All data were normally distributed (Wilk's test: P > 0.30) with the exception of juvenile mortality, which was log-transformed to ensure normality (P = 0.87). The best model was selected by examining Aikaike information criteria (AIC) that ranked the possible models of age at maturity based on the best compromise between parsimony and bias (Burnham & Anderson, 1998) and comparing R2 with forward stepwise selection with entry criteria and model acceptance set at P < 0.05.
Many important ecological and evolutionary processes are influenced by multiple interacting factors (Quinn & Dunham, 1983) that are best analysed using path analysis and related techniques (Petraitis et al., 1996). Predictions are possible if models are based on a mechanistic understanding of relationships between the variables (Zmyslony & Gagnon, 2000). Two models were compared using path analysis. Whereas Model 1 predicts that density and juvenile mortality cause changes in age at maturity, Model 2 makes the additional prediction that the addition of the variables primary productivity and seasonality as causal effects on age at maturity will improve the model fit. In Model 2, it is assumed that primary productivity does not influence juvenile mortality and that seasonality does not influence density (i.e. correlation coefficients were set to 0 regardless of the observed correlation; Petraitis et al., 1996). Models were compared based on explanatory power (R2). Here, I use the a priori path model approach that describes formal hypothesis testing in the presentation of contrasting path models. Hence, the causal models are separate from the data used to estimate strengths of paths in the model.
Results
Age at maturity was related to both population demography and environment (Table 1). First, age at maturity was correlated positively with density (r = +0.67, P= 0.006) and correlated negatively with juvenile mortality (r = −0.64, P= 0.01) and unpredictability of population size (r = −0.47, P= 0.07; Fig. 1). Age at maturity was correlated negatively with seasonality (r = −0.64, P= 0.01) but not correlated with primary productivity (r = +0.43, P= 0.11; Fig. 2). Also, greater seasonality was associated with greater forest cover (r = +0.77, P < 0.001). Multiple regression analysis found that density explained most (45%) of the variation in age at maturity, followed by primary productivity (25%), juvenile mortality (9%) and forest cover (4%; Table 2).
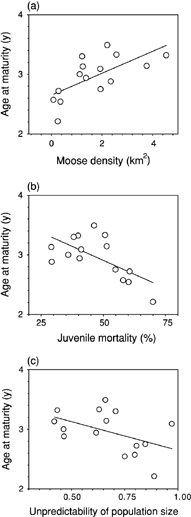
Demographic variables that correlate with age at maturity in moose: (a) density of moose (km2); (b) percentage of juvenile mortality; and (c) unpredictability of population size.

Bivariate relationships among environmental variables (primary productivity, seasonality and forest cover) and age at maturity in moose: (a) primary productivity and age at maturity; (b) seasonality and age at maturity; and (c) percentage of forest cover and seasonality.
| Independent variable | Coefficient value | Standard error | Partials R2 | Model R2 | P |
|---|---|---|---|---|---|
| Intercept | −1.273 | 2.124 | – | – | – |
| Density* | +0.161 | 0.044 | 0.447 | 0.445 | 0.006 |
| Primary productivity* | +0.0487 | 0.00170 | 0.250 | 0.697 | 0.02 |
| Juvenile mortality* | −0.344 | 0.184 | 0.092 | 0.789 | 0.05 |
| Forest cover* | +0.00880 | 0.00506 | 0.037 | 0.811 | 0.13 |
| Seasonality | −0.00763 | 0.0198 | 0.026 | 0.843 | 0.21 |
| Unpredictability | −0.242 | 0.417 | 0.012 | 0.863 | 0.32 |
- * Independent variables for best model based on AIC criteria.
Growing season and seasonality were highly correlated (r = +0.77, P < 0.001) and both were correlated positively with juvenile mortality and forest cover and correlated negatively with density. I chose to enter only seasonality in the path analysis. Grouping moose populations according to geographical regions (Ontario, coastal NF, inland NF) did not suggest differences in primary productivity (F2,11 = 2.24, P = 0.16). However, Ontario was characterized by greater seasonality (253 vs. 214; F2,11 = 15.2, P= 0.001), and coastal moose populations in Newfoundland were characterized by less forest (28 vs. 51%) and less primary productivity during the growing season relative to inland populations (640 vs. 698 g/m2/year; F2,11 = 15.2, P= 0.001).
Two models that explain variation in age at maturity were compared using path analysis (Fig. 3). The first model assumes that including density and juvenile mortality best explains age at maturity. The second model (Model 2) assumes that the same model (Model 1) with the addition of primary productivity and seasonality better explains age at maturity. Path analysis indicated that consideration of primary productivity and seasonality improved the model (R2 0.62 vs. 0.89; Table 3).
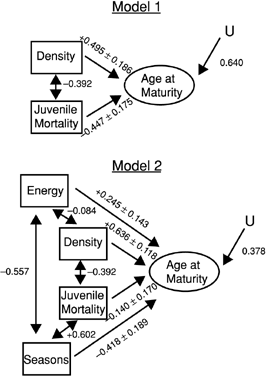
Path diagram comparing two models that describe the direct and indirect effects of environmental and demographic variables on age at maturity in moose: whereas Model 1 predicts that density and juvenile mortality adequately explain variation in age at maturity, Model 2 combines the same predictions of Model 1 with the additional explanatory variables of primary productivity (energy) and seasonality (seasons). Correlation coefficients and path coefficients and their standard errors are compared (multiple regression models in Table 3). Double-headed arrows represent the correlations among the predictor variables and single-headed arrows are the standardized partial regression coefficients. Sample size is 15 North American moose populations. All paths are significant, except the correlation between primary productivity and density in Model 2. U represents the unexplained variance in the complete model.
| Statistic | Model 1 | Model 2 |
|---|---|---|
| Degrees of freedom | 2,15 | 4,15 |
| F-value | 10.57 | 21.81 |
| Probability | 0.002 | 0.0001 |
| Root MSE | 0.640 | 0.378 |
| R 2 | 0.619 | 0.888 |
| R 2 adjusted | 0.561 | 0.847 |
| Parameter estimate | ||
| Juvenile mortality | −0.447 ± 0.186 | −0.140 ± 0.170 |
| Density | +0.495 ± 0.186 | +0.636 ± 0.118 |
| Primary productivity | +0.245 ± 0.143 | |
| Seasonality | −0.418 ± 0.189 | |
Primary productivity and seasonality differed in their relationships with the demographic and life-history variables. Bivariate correlation analyses found that seasonality, rather than primary productivity, was correlated positively with age at maturity (above). Also, seasonality was correlated positively with juvenile mortality (r = +0.46, P = 0.09) and percentage of forest cover (r = +0.77, P < 0.001) and correlated negatively with density (r = –0.49, P = 0.06) and primary productivity (r = –0.56, P = 0.03). However, primary productivity rather than seasonality was associated positively with age at maturity in the best model (rp = +0.25, P = 0.02). These findings suggest that whereas seasonality and growing season may act more directly on age at maturity, primary productivity and forest cover act indirectly.
Discussion
Moose populations that occur across different environments in North America vary in life-history responses to environmental gradients in primary productivity and seasonality. The population response along the two environmental gradients suggests that increased productivity and reduced seasonality favour later age at maturity, a life-history trait associated with superior competitors (Chase, 1999). This pattern can be explained as follows: (1) greater seasonality associated with forested environments produces empty patches due to frequent forest fires (Flannigan et al., 2001 is high, such as occur in coastal Newfoundland environ>) and encourages superior colonizers (i.e. early age at maturity and high rate of increase). In contrast (2), higher productivity encourages superior competitors and the associated life histories of later age at maturity and low population rate of increase. If forest cover is low and productivity ments, life-history characteristics of individuals in such populations favour delayed reproduction (Grime, 1973). Thus, results support the hypothesis that productivity and seasonality are important environmental selection pressures influencing the phenotypic expression of population and life-history characteristics.
Results also support the geographical range model predictions that higher productivity at the equatorial periphery of a species’ range favours superior competitors while lower productivity at the centre of a species’ range favours high reproduction and reduced competitive traits. The geographical range model extends the Dobzhansky (1950) and MacArthur (1972) predictions that the poleward limits of a species range are set by abiotic factors and stresses that include competition for limiting resources, while the equatorial periphery limits are set by interspecific interactions that include predation (Haldane, 1956; Brown, 1995; Crête & Courtois, 1997; Loehle, 1998; Ferguson & McLoughlin, 2000). A further test of the geographical range model is the prediction that moose populations occurring at the poleward limits of their geographical range will be characterized by slower life histories (delayed age at maturity and lower juvenile mortality). Swedish moose prolong growth as winter harshness increases poleward (and with increasing altitude; Sand et al., 1995). The threshold in body mass for ovulation is higher for slow-growing moose facing harsh winters than for fast-growing moose occurring in environments characterized by milder winters (Sand, 1996). Slow-growing moose reach sexual maturity later than fast-growing moose (Sand & Cederlund, 1996) via a trade-off between growth (body reserves) and reproduction as winter length increases (Lesage et al., 2001).
Density and juvenile mortality are likely the proximate factors associated with variation in age at maturity (Langvatn et al., 1996; Sæther, 1997). Moose populations located more centrally within the species’ continental distribution (i.e. northern Ontario) are less influenced by abiotic conditions such as snow conditions and more influenced by natural predation of adults and juveniles by wolves (Messier, 1991, 1994). Here, predation by wolves on juveniles (Pimlott, 1967; Keith, 1974, 1983) leads to populations characterized by increased individual growth rate, increased fecundity and earlier age at maturity. Specifically, predators maintain relatively low prey density thereby ensuring high food supply for the prey. The relationships between densities, juvenile mortality by wolves and age at maturity indicate trade-offs linked with whether moose population dynamics are dominated by density dependent predation or density-independent variation via food limitation (Haugen, 2000). In contrast, populations existing along the extremities of the moose's continental distribution (e.g. coastal Newfoundland) are influenced more strongly by environmental conditions, such as snowfall and ice storms during winter (Albright & Keith, 1987; Post & Stenseth, 1999). Despite the high primary productivity of coastal habitats, the low seasonal variation results in a less productive summer growing season and thus less forest growth. Environments of reduced forest cover support fewer moose and probably fewer natural predators. As a result, moose in coastal environments live at higher densities relative to available forest cover, have later age at maturity, slower growth rates and lower reproduction (Ferguson et al., 2000). Hence, by this reasoning primary productivity and growing season (seasonality) played a role in selecting particular life histories via the proximate mechanisms of density and juvenile mortality.
Life history models that predict age at maturity require consideration of geographical location of populations (i.e. abiotic environment) within the species’ distribution. I have shown elsewhere (Ferguson et al., 2000) that moose populations varied in body growth rate, fecundity and recruitment relative to density and environmental region. Moose density relative to forest cover increased from 0.28 moose/km–2 in the central geographical range (Ontario), to 4.6 moose/km–2 in inland Newfoundland populations living in areas covered mainly by forest, to 9.4 moose/km–2 for coastal Newfoundland moose populations living in the periphery of the geographical range, largely without forests. For example, pregnancy rates decreased from 97% to 87% to 77%; percentage of twins decreased from 49% to 41% to 5%; and percentage of yearlings found to be pregnant decreased from 54% to 64% to 38%, respectively (Ferguson et al., 2000).
Unpredictability in population size was also found in this study to affect age at maturity. Variation and unpredictability of environmental variables may be as important as their mean values in selecting for optimal life history traits (Schaffer, 1974; Gillespie, 1977; Tuljapurkar, 1982; Boyce & Perrins, 1987; Benton et al., 1995). For example, certain climatic variables, such as the timing of seasons, show a latitudinal gradient in interannual variation, with high latitude environments characterized as more unpredictable (Ferguson & Messier, 1996b; Knapp & Smith, 2001). Juvenile mortality and population density of moose, at least for populations living in coastal habitats near the extremes of the continental range, are probably influenced by variability in the timing of seasonal events (e.g. onset of the spring growing season). For some Newfoundland moose populations living in less seasonal habitats (low forest cover), density independent food limitation due to winter severity strongly influences population demography (Albright & Keith, 1987; Ferguson, 2002). Newfoundland moose populations experience unpredictable year-to-year variations in densities, relatively high densities and variable food availability due to climatic effects and low primary productivity. In contrast, Ontario moose populations are regulated at low densities by wolf predation (Bergerud et al., 1983) and live in more forested environments (highly seasonal). Possibly as a result, Ontario moose populations showed more predictable year-to-year changes in density.
The results indicate two extreme groups of moose populations incorporating differences in environment and life history: (1) an area of relatively uniform primary productivity across seasons (less productivity during the growing season) that includes moose populations occurring along the coast of Newfoundland; and (2) areas of relatively high seasonality with a highly productive growing season favouring forest growth and including moose populations living at low densities under predator regulation in northern Ontario. Individuals in different populations responded to varying environmental conditions with different life histories. My analyses of the correlates of age at maturity cannot uncover causal relationships; however, using path analysis, I have shown moose populations that varied in life-history traits were associated with different environments. Resources limit populations occurring in productive and low seasonal environments (e.g. coastal Newfoundland), and life-history responses appear to equate to the maximization of competitive abilities. A change in timing of reproduction (i.e. later age at maturity) and smaller offspring size and number improves competitive abilities such that the geometric mean fitness is greater (Yoshimura & Jansen, 1996). In contrast, populations living at the other extreme in geographical range (i.e. central) are regulated by predation, and here the environment selects for individuals that maximize reproduction (i.e. early age at maturity, faster growth rate, and high fecundity) and dispersal as adaptations to temporal and spatial variability (e.g. frequent forest fires).
Acknowledgments
I benefited from the constructive comments and criticisms of D. Joly, the journal editors and two anonymous reviewers. Financial support was provided by a NSERC Industrial Post-doctoral fellowship. Thanks are extended to the many individual moose hunters who volunteered specimens and information from the provinces of Ontario and Newfoundland and Labrador. I also thank the Newfoundland and Labrador Inland Fish and Wildlife Division and the Ontario Ministry of Natural Resources for collection of the Newfoundland and Ontario data, respectively.
References
Biosketch
Steve Ferguson received his BSc in Zoology at the University of Guelph in 1979, his MSc in Biology from the University of Victoria in 1983, his PhD at the University of Saskatchewan in 1997, and is currently studying forestry–wildlife relations as postdoctoral research at Lakehead University. His long-range research goal is to understand how and why traits evolve in response to trade-offs in morphology (body size, ontogeny), time (phylogeny, history), space (geography, landscape) and environment (amount and variation in energy).




