Pattern and process in the geographical ranges of freshwater fishes
Abstract
North American freshwater fishes were studied to determine whether they displayed the same relationships between log (geographical range size) and log (body size) and the same pattern of range shape as found among North American birds and mammals. The forces that produce these patterns were also investigated. The log (geographical range size) : log (body size) relationship was analysed for 121 North American freshwater fish species. Thirty-two imperilled species were compared with 89 non-imperilled species to determine if the overall relationship could result from differential extinction. Range geometries were analysed, within and among habitat guilds, to determine if general patterns could be detected. The log (geographical range size) : log (body size) pattern among freshwater fish species was triangular and qualitatively similar to that found for North American birds and mammals. The results suggest that below a minimum geographical range, the likelihood of extinction increases dramatically for freshwater fishes and that this minimum range size increases with body size. The pattern of fish species’ range shapes differs from that found for other North American vertebrate taxa because, on average, fish possess much smaller ranges than terrestrial species and most fish species’ geographical ranges extend further on a north–south axis than on an east–west axis. The log (geographical range size) : log (body size) pattern reveals that fish species’ geographical ranges are more constrained than those of terrestrial species. The triangular relationship may be caused by differential extinction of species with large bodies and small geographical ranges as well as higher speciation rates of small-bodied fish. The restricted geographical ranges of freshwater fishes gives them much in common with terrestrial species on oceanic islands. Range shape patterns within habitat guilds reflect guild-specific historical and current ecological forces. The overall pattern of range shapes emerges from the combination of ecologically different subunits.
Introduction
Analyses of ecological patterns across taxa allow biogeographers to uncover universal forces that constrain and promote the abundance, distribution and diversity of life (‘macroecology’; Brown & Maurer, 1987, 1989; Brown, 1995; Maurer, 1999). Macroecological analyses of body size, geographical range size and range shape in North American birds and mammals have revealed similarities between the two groups. For example, the relationship between log (body size) and log (range size) produces a distinctive triangular pattern in both taxa (Brown & Maurer, 1987, 1989). Also, in both groups geographical range shapes are North–South biased (NS biased; extend further on a NS-axis than on an EW-axis) among species with small ranges and East–West biased (EW biased) among species with large ranges. Here, macroecological analyses are presented of the geographical ranges of North American freshwater fish species to determine if they display patterns found among other continental vertebrate taxa.
Fish are believed to preserve ancient geographical patterns because the colonization of new habitats is limited by the extent and continuity of their aquatic habitat in addition to biotic and abiotic tolerances within that habitat (Smith, 1978; Hocutt & Wiley, 1986). Because freshwater fish have dispersal limitations not found among terrestrial taxa, historical influences on geographical range patterns may be more important among freshwater fish than they are among other continental vertebrates. Also, because freshwater fish are poikilothermic and aquatic, they may not display the same response to climatic conditions as terrestrial homeotherms. For these reasons, we may expect fish species to display patterns in geographical range geometries that differ from birds and mammals.
Body size, range size and conservation status
Many of the organismal attributes that influence species’ geographical ranges (e.g. individual home range size and dispersal capability) scale with body size (e.g. Schmidt-Nielsen, 1984; West et al., 1997). Geographic range sizes of continental bird and mammal species are not a function of average or maximum body sizes. Instead, small-bodied species occupy the entire span of geographical range sizes while large-bodied species are restricted to large range sizes (Brown & Maurer, 1987, 1989; Gaston & Blackburn, 1996). This relationship was also found among 144 North American freshwater fish species (McAllister et al., 1986). Likewise Taylor & Gotelli (1994) found a triangular relationship between log (body size) and log (range size) in the North American fish genus Cyprinella.
The current distribution of fish ranges reflects past speciation and extinction events. Brown & Maurer, 1987, 1989) proposed an extinction-driven mechanism to explain the triangular log (geographical range size) : log (body size) pattern. They argued that the constraints underlying the energetic relationship between body mass and metabolic requirements cause large species to have low population densities (Damuth, 1981). Populations of large species with small ranges are more likely to be extirpated, both because of low effective population sizes resulting from low population density and because populations with small ranges are more susceptible to catastrophic events. Conversely, small-bodied species can maintain high populations in small areas; thus, they may be less vulnerable to the effects of demographic and genetic stochasticity (although they are still susceptible to local catastrophes). Differential extinction was detected among large-bodied fish species in the Great Basin desert of the United States (Smith, 1981). In addition, interpopulation maximum adult body size was positively correlated with habitat area, particularly among large-bodied species. This suggests that energetic or life historical constraints limit large fishes’ persistence in small habitats (Smith, 1981). If this process produces among fish the same triangular log (geographical range size) : log (body size) relationship as seen among North American terrestrial vertebrates, a disproportionate number of species along the hypotenuse of the triangular distribution should be recently extinct or in jeopardy of extinction. Here, I investigate the hypothesis that body size and range size interact to form an extinction threshold constraining the overall range size–body size relationship.
Geometry of fish species’ ranges
If freshwater fish distributions are constrained mainly by their historical dispersal opportunities, the shape of species’ ranges should reflect the shape of the drainage basins they occupy. Because river networks elongate (grow along the major axis of flow) faster than they widen (Hack, 1957), the geographical ranges of riverine fish species may be expected to extend further along the major axis of drainages they inhabit than in a direction orthogonal to the major axis of flow. For example, most of the largest North American Rivers flow from north to south (e.g. Mississippi, Rio Grande, Fraser, Hudson) or south to north (San Joaquin). If fish species’ range orientations reflect drainage orientation, fishes that live in these large rivers should have NS biased distributions.
In contrast, fish that dwell primarily in lakes, especially northern lakes, are expected to have EW biased geographical ranges. This expectation results from the way that the Wisconsin glacial advance and retreat created, shaped and connected most of the lake basins of northern North America. Presumably, glacial fronts were distributed along isotherms that extend from east to west. During phases of rapid glacial retreat, lakes at the glacial margin were probably connected in EW bands by floodwaters. Fish living close to the glacial front probably dispersed through floodwaters to colonize new habitats. Colonization to the north was limited by the glacier itself and extirpation may have increased to the south due to increasing temperatures as the glacial period ended.
Methods
The geographical range, maximum NS range extension and maximum EW range extension of North American freshwater fish species were calculated using the species collection maps provided by Lee et al. (1980). These maps document the locality of capture sites for specimens held in numerous museum collections prior to 1980. They provide an estimate of the historic distribution of most species as they may include samples collected in the early twentieth and late nineteenth centuries. Lee et al. (1980) included detailed range maps (those showing actual sampling localities) only for populations in the United States, Canada, and Northern Mexico (hereafter, the study area). Excluded from my sample were species whose ranges extended beyond the study area and species that were designated as imperilled by Williams et al. (1989) specifically because their natural range was small (e.g. Cyprinodon diabolus Baird & Girard).
One hundred and twenty-one endemic species were selected, representing each of the 27 families with at least one species endemic to the study area. Random sampling, stratified across families, was used to select species in approximate proportion to each family's representation in the North American ichthyofauna. The sample includes approximately 18% of the species endemic to the study area. Because I wanted to include at least one representative of each of the 27 families, those families with very few endemic species are relatively over-represented in the sample.
Species range and body size measures
Any method of determining the geographical range or body size will be controversial because both measures are necessarily subjective (Brown et al., 1996), particularly among fishes (McAllister et al., 1986; Pyron, 1999). Geographic ranges are an imperfect measure of fish species’ distributions because fish exist only within the aquatic portion of the area measured. A more accurate measure of distribution for riverine species might be linear river miles, but such data would be extremely tedious to collect for widespread fishes and irrelevant for lake-dwelling species. A similar problem exists for terrestrial species as few species exist throughout the entire area encompassed by geographical extremities (the ‘hull’ of their range).
Maps presented in Lee et al. (1980) were scanned using an Epson ES-12200C scanner and ScanTastic ps software (Version 4; Second Glance Software). These digitized maps were imported into NIH Image 1.57 (NIHI; National Institutes of Health, 1995). NIHI calculates the area encompassed by any polygon drawn on an image with an image scale provided by the user. State and province borders were used to specify a scale for each map; border lengths were measured from the Rand McNally road atlas for the United States, Canada and Mexico (Rand McNally, 1996). As a check of scale accuracy, I outlined a state or Canadian province, calculated its area using NIHI, and compared the area calculated to the area provided in Rand McNally (1996). The map scale was modified until the calculated area of the measured state or province was within 5% of the area reported by Rand McNally for that state or province. Maximum NS range extension was calculated as the difference between the vertical coordinate of the most southerly point and that of the most northerly point. Maximum EW extension was calculated by subtracting the horizontal coordinate of the western-most collection point from that of the eastern-most collection point.
The polygon used to measure a species’ range was created by connecting collection points on the species collection maps. Sixteen of these collection points (four in each cardinal direction) were chosen to represent the extremities of each species’ range. These points were chosen so that lines drawn between them encompassed all other collection points while minimizing the total area. Populations of native species established via anthropogenic translocations were excluded from the sample. To minimize the error caused by disjunct or largely disjunct populations, such populations were measured separately (using the method described above). Population range estimates were then summed to determine a species’ overall geographical extent. For four species with exceptionally small ranges (i.e. those represented by one or two sampling locations), range sizes were approximated from measures provided in written range descriptions (Lee et al., 1980).
Each species’ characteristic body size was quantified using the largest standard length in the adult body size ranges presented by Lee et al. (1980). In some cases they reported maximum sizes in addition to the body size ranges, but these measures were not used because they were not available for all species. When only total length or fork length ranges were presented, those measures were converted to standard length using a species-specific total (or fork) length to standard length conversion estimate made by measuring the images of the species presented by Lee et al. (1980). Identifying a single size that characterizes a fish species is difficult. Maximum adult size, size at sexual maturity and other potentially important measures differ across populations, environments and years and sometimes between the sexes (Lee et al., 1980; Smith, 1981; Page & Burr, 1991). Average body mass measures, used in similar studies on birds (Brown & Maurer, 1987) and mammals (Brown & Maurer, 1989), are not available for non-game freshwater fish species, while length measures of some type are available for all species documented in Lee et al. (1980). Differences in standard length should be correlated closely with body mass and, on a log scale, accurately portray size differences between species.
Habitat guilds
Species were grouped into one of five habitat categories that described the type of water body in which they most commonly occur (Appendix I). Species that occur predominantly in lakes were classified as ‘Lake’ species. Species that occur principally in rivers were placed in one of three river-size categories, based on the largest river systems in which they were collected (Lee et al., 1980). The ‘Headwaters’ category included fish that inhabit only inland, small streams. Fish in the ‘Medium rivers’ category were found in larger order streams (those with many tributaries) even if they were also collected in smaller streams. Fish from ‘Large rivers’ included those with documented collection sites in the central and southern Mississippi River, the lower mainstems of its major tributaries (Missouri, Ohio, etc.), the lower Rio Grande, lower Columbia and other similar waterways. Fish whose maximum range extension was best explained by an ability to migrate through marine waters were classified as ‘Coastal’ species; these included anadromous and catadromous species and those whose entire freshwater distributions occurred within a short distance (< c. 150 km) of the ocean. Three species, pirate perch (Aphredoderus sayanus Gilliams), bowfin (Amia calva Linnaeus) and brook silverside (Labidesthes sicculus Cope), were included in the Coastal group because, while they have been sampled throughout much of the Mississippi drainage, their EW extensions are clearly due to their ability to disperse through coastal waters. For the same reason, the chain pickerel (Esox niger Lesueur) was included in the Coastal group rather than the Lake group. These species demonstrate that current habitat use is only an approximation of habitats used for dispersal in the past.
Conservation status
Species were grouped into conservation status categories using the American Fisheries Society's 1989 conservation evaluation of North American fish species (Miller et al., 1989; Williams et al., 1989). One species, Acipenser transmontanus (Richardson), was classified as an endangered species in my sample because it was added to the US Federal Endangered Species List (50 CFR §17.11) after the AFS list was published. Descriptions in Lee et al. (1980) and the AFS list were used to create five conservation categories: extinct, endangered, threatened, special concern or not of concern. Thirty-two of the species in my sample fell in the first four categories (hereafter, endangered and extinct species).
Analyses
Species’ geographical range and body size were log-transformed and plotted against each other to determine if any constraints on the log (geographical range size) : log (body size) relationship were apparent (Fig. 1). Endangered and extinct species were identified on this plot to determine if extinction might be a force producing constraints on the log (geographical range size) : log (body size) relationship. Since the log (geographical range size) : log (body size) data did not meet the assumptions of parametric statistical tests, an index was created by dividing species’ range size by body size. The log of this index satisfied the assumptions of parametric statistics and it was used to test the hypothesis that endangered and extinct fish species have smaller ranges relative to their body size than do non-imperilled species.
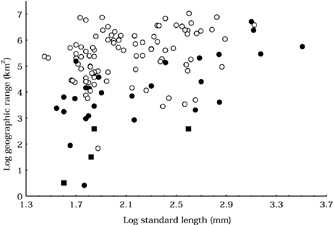
The relationship between log (standard length) and log (geographical range) for 121 North American freshwater fish species. The triangular pattern resembles that found among North American mammals and birds (Brown & Maurer, 1987, 1989). Species for which the American Fisheries Society (Miller et al., 1989; Williams et al., 1989) did not report conservation concerns are represented by open circles. Imperilled species are represented by other symbols (extinct =▪; endangered, threatened, or of special concern =•). These species have smaller ranges on average than non-imperilled species. The plot suggests an interaction between body size and geographical range size.
Species’ maximum NS range extents were plotted against maximum EW extents (Fig. 2). Habitat classes (Coastal, Headwaters, etc.) were analysed separately to determine if fishes in the different guilds obey different range shape limitations. Regression analyses were performed on the overall and within-guild relationships between NS and EW extent to determine the shape and strength of the relationship within guilds and to uncover differences in this relationship among habitat guilds. To determine if the NS : EW relationships were linear, simple linear regressions were compared to a quadratic polynomial model using an F-test to determine which model best described the data while meeting the assumptions of parametric statistics. In addition to the result of the F-test and adherence to statistical assumptions, models with small y-intercepts were favoured because, on average, geographical ranges should have both NS and EW components.

Range geometry of 121 North American freshwater fish species (a). Ranges that are symmetrical in their north–south (NS) and east–west extensions fall along the line of equality in each plot. Most species display ranges that are NS biased. Lake species (b) account for the EW bias of species with the largest geographical ranges in (a). Riverine species (c) and coastal species (d) display range goemetries that are predominantly NS biased. In each guild, ranges become gradually more EW-biased as range size increases. Subgroups within the Riverine guild were studied but a single quadratic relationship fit all three groups the best, revealing that fish from different river classes represent a continuum of change in range geometry.
A comment on phylogeny
Phylogenetic history and shared ancestral characters may mean that related species are not independent sampling units (Felsenstein, 1985; Harvey & Pagel, 1991). When a phylogenetic hypothesis of evolutionary relationships exists, it is possible (to some extent) to account for the potential effect of phylogeny on ecological patterns (Felsenstein, 1985). Phylogenetic contrasts were not performed here because (1) the endemic North American freshwater fishes are not monophyletic (even within many families) and, as a result, there is no good estimate of phylogenetic relatedness; (2) many of the 27 families sampled here are so distantly related that ecological constraint is a much more likely explanation for biogeographical patterns than phylogenetic constraint (Westoby et al., 1995); and (3) my stratified sampling approach was designed to minimize the relatedness of species in my sample (65 genera were sampled; Table 1). Finally, the questions addressed here do not lend themselves to phylogenetically independent contrasts. For example, I did not ask whether body size is correlated with range size as have previous studies (e.g. McAllister et al., 1986; Pyron, 1999). The overall log (geographical range size) : log (body size) relationship, the pattern of endangerment within that relationship and the distribution of range shapes are not likely to result from retention of primitive characteristics. That said, it is clear that body size, geographical range size and conservation status of individual species are not independent of phylogeny (Appendix I, Table 1).
| Family | Genera sampled | Species sampled | Endangered species sampled |
|---|---|---|---|
| Acipenseridae | 2 | 3 | 3 |
| Amblyopisdae | 3 | 3 | 1 |
| Amiidae | 1 | 1 | 0 |
| Aphredoderidae | 1 | 1 | 0 |
| Atherinidae | 2 | 2 | 0 |
| Catastomidae | 5 | 10 | 5 |
| Centrarchidae | 5 | 6 | 0 |
| Clupeidae | 1 | 3 | 0 |
| Cottidae | 2 | 4 | 1 |
| Cyprinidae | 13 | 29 | 7 |
| Cyprinodontidae | 2 | 5 | 1 |
| Esocidae | 1 | 1 | 0 |
| Gadidae | 1 | 1 | 0 |
| Gasterosteidae | 2 | 2 | 0 |
| Gobiidae | 1 | 1 | 0 |
| Hiodontidae | 1 | 2 | 0 |
| Ictaluridae | 3 | 6 | 2 |
| Lepsisosteidae | 1 | 3 | 0 |
| Moronidae | 1 | 2 | 0 |
| Osmeridae | 2 | 2 | 0 |
| Percidae | 2 | 17 | 5 |
| Percopsidae | 1 | 1 | 1 |
| Petromyzontidae | 2 | 4 | 0 |
| Poecillidade | 3 | 4 | 3 |
| Polyodontidae | 1 | 1 | 1 |
| Salmonidae | 4 | 5 | 2 |
| Umbridae | 2 | 2 | 1 |
| Total | 65 | 121 | 32 |
Results
Body size, range size, and conservation status
The log (geographical range size) : log (body size) relationship revealed a triangular distribution (Fig. 1). Small-bodied species displayed the complete range of geographical range sizes while large-bodied species possessed only large geographical ranges. Applying the AFS interpretation of conservation status (Miller et al., 1989; Williams et al., 1989) to the log (geographical range size) : log (body size) distribution revealed that endangered and extinct species have small range sizes relative to their body size (Fig. 1). However, some of the largest species (e.g. Acipenser fulvescens Rafinesque and Polyodon spathula Walbaum) with large geographical ranges (5 138 468 and 2 395 672 km2, respectively) are also imperilled. In my sample, the log of the ratio (geographical range size) : (body size) was significantly lower for endangered and extinct species than it was for non-imperilled species (P < 0.0001; t= 7.865; d.f. = 119).
Range geometry
Most of the species in my sample had NS biased ranges (Fig. 2). For the entire sample, a simple linear regression of NS extent on EW extent revealed a highly significant relationship with a slope that was significantly less than 1.0 and a positive intercept (Table 2). In simple linear models, slopes less than 1.0 indicate a decreasing NS bias (increasing EW bias) as range size increases. A second-order polynomial provided a significantly better fit to the data than the simpler model. A small negative quadratic term revealed a gradual decrease in NS bias as range size increased. Deviations from model assumptions were not considered serious for either model.
| Category | Model | Relationship (NS =) | d.f. | p slope | p intercept | R 2 | MSE | p model |
|---|---|---|---|---|---|---|---|---|
| Entire sample | Linear | 0.795(EW) + 350.67 | 119 | < 0.001 | 0.00002 | 0.730 | 328834 | |
| Quadratic | 1.295(EW) − 0.0001(EW2) + 83.3 | 118 | 0.782 | 269432 | < 0.00001 | |||
| Lake | Linear | 0.753(EW) + 229.5 | 16 | < 0.01 | 0.342 | 0.844 | 416434 | |
| Quadratic | 1.282(EW) − 0.0010(EW2) − 89.6 | 15 | 0.896 | 248768 | 0.025 | |||
| All Rivers | Linear | 0.901(EW) + 169.3 | 72 | < 0.02 | 0.0006 | 0.866 | 90132 | |
| Quadratic | 1.338(EW) − 0.00015(EW2) + 25.7 | 71 | 0.909 | 66556 | < 0.00001 | |||
| Coastal | Linear | 0.709(EW) + 841.4 | 27 | > 0.20 | 0.010 | 0.242 | 708692 | |
| Quadratic | 2.592(EW) − 0.00064(EW2) − 167.5 | 26 | 0.388 | 640764 | 0.032 | |||
| Headwaters | Linear | 1.204(EW) + 40.24 | 14 | > 0.20 | 0.454 | 0.479 | 16387.5 | |
| Quadratic | 2.429(EW) − 0.004(EW2) − 5.89 | 13 | 0.560 | 15968 | 0.263 | |||
| Medium rivers | Linear | 0.951(EW) + 98.27 | 32 | > 0.20 | 0.155 | 0.628 | 56934 | |
| Quadratic | 1.752(EW) − 0.00067(EW2) − 50.3 | 31 | 0.673 | 53274.25 | 0.083 | |||
| Large rivers | Linear | 0.662(EW) + 697.8 | 22 | < 0.001 | 0.0002 | 0.742 | 123522 | |
| Quadratic | 1.145(EW) − 0.00012(EW2) − 311.3 | 21 | 0.796 | 107386 | 0.050 |
Quadratic models were not significantly better than linear models at describing the pattern of range geometries within riverine classes. However, the pattern of increasing intercept and decreasing slope as river size-class increased suggested that the distribution of NS : EW geometries was curvilinear among river-dwelling species as a whole (Fig. 2). Indeed, a quadratic model produced a significantly better fit than a linear model when riverine classes were combined. The quadratic analysis of combined riverine classes explained a higher proportion of the variance than linear or quadratic analyses of individual riverine classes (Table 2). The quadratic produced an homogeneous distribution of residuals and improved adherence to the assumption of normality compared with linear models. Finally, the y-intercept of the quadratic model was an order of magnitude smaller than that found in the simple linear model of all riverine species.
Quadratic relationships described the pattern of range geometries seen among Lake and Coastal species significantly better than linear models (Table 2). As with riverine species, quadratic models produced a smaller y-intercept than linear models and revealed a decreasing NS bias with increasing range size for both of these groups. Coastal species displayed greater variation in range shape than species in the other groups; thus, linear and quadratic models failed to explain more than 40% of the variation in range geometries in this group.
Discussion
Body size, range size and conservation status
The triangular pattern in the log (geographical range size) : log (body size) relationship (Fig. 1) is qualitatively equivalent to that found by McAllister et al. (1986; Fig. 2.7) in a study using similar data. This relationship has also been uncovered in terrestrial birds and mammals of North America (Brown & Maurer, 1987, 1989) and other taxa (Taylor & Gotelli, 1994; for review see Gaston & Blackburn, 1996). In previous analyses, triangular distributions were caused by (1) the physiological limits of body size (left and right boundaries); (2) the size of the study area (upper boundary); and (3) some force(s) that prevented the persistence of large species in relatively small areas (the hypotenuse). Fish in my sample displayed the full range of body sizes and range sizes seen among the North American freshwater fish fauna. The smallest body sizes in my sample (28–40 mm SL) approximated that of the smallest freshwater fish (Cyprinodon diabolis, not in my sample). The largest fish in my sample (Acipenser transmontanus; 3220 mm SL) is the largest freshwater fish species endemic to North America and one of the largest freshwater fishes in the world. Thus, the left and right limits on the log (geographical range size) : log (body size) relationship probably reflect physiological limits on the body size of freshwater fish. Unlike the relationship found in terrestrial fauna (Brown & Maurer, 1987, 1989), the triangle's upper limit was not the size of the study area (c. 19.5 × 106 km2), as the largest geographical range size in my sample was 10.6 × 106 km2 (Salvelinus namycush Walbaum).
The hypotenuse of the triangular distribution has been interpreted as an extinction threshold caused by the relationship between body size and maximum sustainable population density (Brown & Maurer, 1987, 1989; Brown et al., 1993; Gaston & Blackburn, 1996). Results of this study are consistent with the operation of this mechanism among freshwater fishes. Imperilled species occurred across the entire range of body sizes and geographical ranges studied (Fig. 1). The ratio log (geographical range) : log (body size) was significantly lower among endangered and extinct species than it was in the rest of my sample. At a given size, endangered and extinct fish species typically have the smallest geographical ranges and, within area classes, the largest species are more likely to be endangered than smaller species. Indeed, the largest species within the study area, A. transmontanus and A. oxyrhynchus Mitchill (not included in my sample) both display relatively large geographical ranges (> 5 × 105 km2) and both are listed on the US Federal Endangered Species list. Another recent macroecological study of two North American fish families (Centrarchidae and Catastomidae) found that ‘extinction prone’ fishes had much smaller geographical ranges than non-endangered members of their families; an interaction between body size and range size was particularly evident among Catastomidae (Pyron, 1999).
Geographic range appears to have an effect on conservation status beyond its interaction with body size. Most of the species in my sample with geographical ranges < 50 000 km2 are endangered or extinct. This pattern is not unexpected because species with small geographical ranges can be jeopardized by local disturbances that have nothing to do with population density or effective population sizes (Goodman, 1987). The relationship between geographical range and conservation status has important implications for conservation biology. While it is axiomatic among conservation biologists that ‘large’ reserves are better at protecting species than ‘small’ reserves, little work has been done to determine just what ‘large’ and ‘small’ mean for any set of organisms. The data presented here suggest that areas < 50 000 km2 may be too small to ensure long-term persistence of freshwater fish species exposed to anthropogenic habitat disturbance. As body size increases, the minimum area needed to support a viable population increases as well. The minimum geographical distribution necessary for species’ long-term persistence likely depends on aspects of the fish's ecology in addition to body size. As a result, certain groups of fishes may require larger geographical ranges for persistence than predicted by body size alone. Within Centrarchidae, for example, Pyron (1999) considered all species with geographical ranges < 1 × 105 km2 to be ‘extinction prone’.
In addition to revealing potential endangerment and extinction thresholds, analysis of the log (geographical range) : log (body size) relationship may allow identification of particular taxa requiring conservation attention. Extant freshwater fish species with geographical ranges < 1.0 × 105 km2 that were not identified as endangered or extinct are prime candidates for study. Also, the conservation status of large-bodied species with ranges > 1.0 × 105 km2 that are surrounded by endangered and extinct species on a log (geographical range) : log (body size) plot should be regularly re-evaluated (Fig. 1). Clearly, a species’ absolute or relative position on a bivariate plot is not sufficient to warrant conservation action. A species’ conservation status results from both species-specific characteristics and chance; thus, I do not expect all species with absolute or relatively small geographical range sizes to be at risk of extinction. However, a log (geographical range) vs. log (body size) plot is a useful tool for identifying species that might need additional protection.
The geographical ranges of freshwater fish are, on average, much smaller than those of terrestrial continental vertebrates. Brown & Maurer (1989) found no bird species in North America or Europe with range sizes < 1.0 × 105 km2. More than 20% of the freshwater fish species in my sample occupy ranges < 1.0 × 105 km2. Among North American birds and mammals, species with the largest geographical ranges occupy the entire continent. The largest geographical range among species in my sample was c. 55% of the size of the study area. Similarly, the most widespread endemic species of the 144 sampled by McAllister et al. (1986) had a range covering < 30% of North America. These restricted ranges reveal that freshwater fish species are ‘island’ fauna in the same way as terrestrial species endemic to islands of land. Given this similarity, it is not surprising that the conservation threats, and the relative importance of those threats, are the same for freshwater fish and terrestrial island species. Both freshwater fish conservation biologists and those who specialize in the study and conservation of terrestrial island biota may benefit from an exchange of information and techniques based on these commonalities.
Among North American freshwater fishes, the triangular log (geographical range size) : log (body size) is probably caused by both differential extinction of large-bodied species and higher speciation rates among small-bodied species. Clearly, there are more small-bodied species than large-bodied species among freshwater fish species (Fig. 1). This pattern is consistent among vertebrates (e.g. Brown et al., 1996). If endangerment carries with it a certain constant risk of extinction we might expect erosion of the lower left corner of the triangular distribution. Faster speciation rates among small species would counter such erosion. Body size constrains dispersal of most vertebrates (Schmidt-Nielsen, 1984) and this may make it easier for populations of small-bodied fish species to become genetically isolated from closely related populations. Body size is also correlated positively with generation length and the shorter generation time of small-bodied species may lead to rapid genetic divergence and speciation in isolation. Differential speciation and differential extinction are not exclusive hypotheses; both forces probably contribute to the overall pattern.
Range geometry
The overall NS : EW relationship in fishes has a shape and aspect similar to that found for North American birds and mammals, but the distribution has been shifted to the left and down (i.e. to smaller NS and EW extensions). Brown & Maurer (1987, 1989) found that birds and mammals with the largest geographical ranges had EW biased ranges while those with smaller ranges were NS biased. While species with the largest ranges in my sample had EW biased ranges, most species had NS biased ranges. In part, this may result from the general truncation of freshwater fish range sizes as compared to those of birds and mammals (see discussion above). An EW bias in range geometries was apparent among the few freshwater fish with EW extensions > 2200 km; this is approximately the range size where North American bird and mammal species’ ranges begin to display an EW bias (Brown & Maurer, 1987, 1989).
Dividing the fishes into habitat guilds allows a finer scale inspection of the general pattern and may provide some clues into its genesis. As predicted, most of the Lake species in my sample had EW biased ranges. This bias probably stems from the dispersal opportunities available to these species at the end of the Wisconsin glaciation. The lake-dwelling species represented the largest range sizes in my sample. The fact that these fishes had EW biased ranges enhances the impression of a general trend toward EW biased ranges as range size increases. Analysis of the group of species with EW biased ranges among North American birds and mammals might reveal whether the positive relationship between range size and EW bias is caused by the historical effect of glaciers, the surficial geology of North America, present-day climate patterns or some other force.
Fish species restricted to headwaters displayed a NS bias that increased as range size increased (i.e. the NS : EW slope is positive). My definition of Headwater species included those found in high elevation headwaters but not those found in small streams of the coastal plain (these were in the Coastal group). Thus, the NS bias seen in the Headwaters group may be explained by the NS orientation of most North American mountain chains. This orientation means that high elevation areas, where headwater streams exist, occur in a band with a NS orientation. Fish species restricted to headwaters habitats are also restricted to (locally) high elevation habitats and their range geometries should reflect the orientation of high elevation isoclines (NS in North America). Limitation to high elevation habitats could potentially explain the NS bias seen in North American bird and mammal species with small geographical distributions (Brown & Maurer, 1987, 1989).
When riverine fishes were considered together, a quadratic relationship described the NS : EW relationship better than simple linear models described any of the groups alone. The quadratic model accounts for > 90% of the variation in river species’ range geometries, shows no substantial deviation from underlying statistical assumptions, and displays a y-intercept that is indistinguishable from the origin. Range geometries of Medium river fishes represent a transition between the increasing NS bias of headwater fish and the decreasing NS bias seen in Large river fish. The quadratic model demonstrates that there are not discontinuities in the NS : EW relationship across riverine habitat guilds (although the forces producing range geometry may change as range size changes).
Most species in the Coastal guild displayed NS biased ranges (Fig. 2). Range geometries in this guild were more variable than those seen in other habitat guilds. This variability reflects variability in the orientation of the coastline itself. The Atlantic and Pacific coastlines of North America are strongly NS biased. However, the Gulfs of Mexico and Alaska represent strongly EW biased coastlines. The distribution of Coastal species’ range geometries reveals that their shapes were determined by the shape of the coastline and that this is a function of species’ range size and location along the coast. The degree of freshwater colonization also influenced range shape.
The negative quadratic terms seen in the distribution of range geometries (which produces a decline in NS bias with increasing range size) may reflect an ecological response to modern-day climatic variables that vary with latitude (e.g. temperature). Climatic limitations on species’ NS range extensions have been proposed for North American endotherms (Root, 1988; Brown, 1995). The quadratic terms in models of guild-specific range geometries (Table 2) are very small, suggesting that any NS limitation imposed by increasing latitude is slight. Indeed, except for fishes in the Lake guild, most fishes have NS biased ranges even when those ranges are large.
Freshwater fish ranges appear to be constrained by the historical forces that shaped particular habitats (e.g. lake habitats) as well as current ecological limitations (e.g. elevation), and drainage interconnectedness. The restricted ranges of North American freshwater fish species (compared to birds and mammals) are consistent with the idea that fish ranges are constrained by historical dispersal opportunities. However, the majority of North American fish species do not occupy the entire area into which they could disperse. Clearly, forces other than dispersal ability (e.g. philopatry, physiological limits, competition) limit the ranges of fish species within the drainage networks they occupy. The combination of these forces produce different patterns of range geometries in different guilds of fish. When employing a macroecological approach, biogeographers should determine whether patterns found across an entire taxonomic group are consistent among ecologically and taxonomically distinct subgroups or whether larger patterns arise only from the combination of subgroup-specific patterns.
Acknowledgments
The author thanks J.H. Brown and A. Kodric-Brown for many valuable discussions of fish and their geographical ranges and for comments on several versions of the manuscript. C. Stockwell and W.A. LaRue provided valuable insights into the forces limiting geographical range size and shape. D. Schluter, K. Howard, S. Maruca and two anonymous referees read and improved the manuscript with their suggestions. NSF grant no. IBN-9806483 funded this research. The author was supported by the US Environmental Protection Agency's STAR fellowship programme during the writing of the manuscript.
Supplementary material
The following material is available from http://www.blackwell-science.com/products/journals/suppmat/GEB/GEB287/GEB287sm.htm
Appendix: Names, authorities and data for all species used in this study.
References
Biosketch
Jonathan Rosenfield earned his PhD in Biology at the University of New Mexico, Albuquerque, NM, USA. His research interests include the forces that cause speciation and extinction. He applies information gathered through research to efforts to conserve native biological diversity, particularly freshwater fish of North America. Currently, he is using behavioural and ecological experiments to determine what forces promote hybridization and introgression between two species of fish.




