The role of nitric oxide in morphine dependence and withdrawal excitation of rat oxytocin neurons
Abstract
Magnocellular oxytocin neurons develop morphine dependence after intracerebroventricular infusion for 5 days as revealed by their profound excitation following naloxone-induced withdrawal. Oxytocin neurons strongly express nitric oxide synthase (NOS) and nitric oxide (NO) inhibits their activity. This study investigated whether excitation of oxytocin neurons during morphine withdrawal involves reduced activity of NOS and NO. Neuron activity was measured in urethane-anaesthetized rats with blood sampling for oxytocin radioimmunoassay and extracellular single unit firing rate recording of supraoptic nucleus oxytocin neurons. To compare morphine-dependent and -naive rats oxytocin secretion was measured during stimulation by intravenous hypertonic saline infusion. Prior treatment with Nω-nitro-l-arginine methyl ester, a NOS inhibitor, facilitated osmotically stimulated oxytocin secretion in both morphine-dependent and -naive rats. The facilitation was not different between these groups when corrected for the slower responses observed in morphine-dependent rats. Treatment of morphine-dependent rats with Nω-nitro-l-arginine methyl ester also enhanced oxytocin secretion during naloxone-precipitated withdrawal. Oxytocin neurons excited by withdrawal were recorded during microdialysis application to the supraoptic nucleus of the NO donor sodium nitroprusside alone and in combination with the GABAA antagonist bicuculline. Sodium nitroprusside inhibited oxytocin neurons during naloxone-precipitated morphine withdrawal and, while bicuculline alone increased firing rate, it did not reduce the inhibition by sodium nitroprusside, in contrast with previous findings in naive rats. Together, these findings indicate that NO restraint of oxytocin secretion is not curtailed during morphine dependence and remains a potent inhibitor of withdrawal excitation despite reduced effectiveness on GABA innervation of the supraoptic nucleus. Hence there is no evidence that changes in NO regulation underlie excitation of oxytocin neurons during opiate withdrawal in morphine dependence.
Introduction
Magnocellular oxytocin neurons in the supraoptic and paraventricular nuclei in the hypothalamus project to the posterior pituitary where they release oxytocin into the circulation. Oxytocin cells are strongly inhibited by acute exposure to µ-opioids (Pumford et al., 1991) and chronic treatment with morphine, a µ-opioid receptor agonist, leads to the development over 5 days of dependence in oxytocin neurons (Bicknell et al., 1988; Pumford et al., 1991). Dependence is revealed as a marked, sustained hyperexcitation of oxytocin neuron activity following naloxone-precipitated morphine withdrawal (Bicknell et al., 1988). Naloxone administration directly into the supraoptic nucleus (SON) of morphine-dependent rats indicates that changes in intrinsic mechanisms in oxytocin neurons or their immediate synaptic inputs underlie the withdrawal excitation and hence dependence (Johnstone et al., 2000). This may involve increased effectiveness of excitatory mechanisms or reduced activity of inhibitory mechanisms that compensate for the opiate action (for review see Russell et al., 1995; Brown et al., 2000). Here we investigated potential changes in inhibitory mechanisms involving nitric oxide (NO), an autocrine inhibitor of magnocellular oxytocin neurons.
Nitric oxide is produced by NO synthases (NOS) and neuronal NOS (nNOS) is strongly expressed in oxytocin neuron cell bodies and terminals (Kadowaki et al., 1994; Hatakeyama et al., 1996).
Almost all studies of NO actions on oxytocin neurons show inhibitory effects. Thus, the NO donor sodium nitroprusside (SNP) and the NO precursor l-arginine inhibit SON neurons, whereas NOS inhibitors increase the activity of stimulated oxytocin neurons in vivo (Liu et al., 1997; Srisawat et al., 2000). Centrally, NO inhibits the firing of oxytocin neurons by direct actions and by potentiating presynaptic GABA activity and/or inhibiting postsynaptic glutamate-mediated excitatory inputs (Ozaki et al., 2000; Stern & Ludwig, 2001) and, in the posterior pituitary, NO inhibits secretion (Kadowaki et al., 1994).
In dependent rats withdrawal increases Fos expression in SON neurons immunoreactive for nicotinamide dinucleotide phosphate-diaphorase (a marker for NOS; Bredt et al., 1991; Jhamandas et al., 1996). As oxytocin cells express NOS, and express Fos during withdrawal (Johnstone et al., 2000; Srisawat et al., 2000), it is certain that these nicotinamide dinucleotide phosphate-diaphorase-positive cells include oxytocin neurons. Indications of a more widespread involvement of NO mechanisms in the brain in morphine withdrawal come from studies showing increased Ca2+-dependent NOS activity in the cerebellum (Leza et al., 1996), increased numbers of nNOS-positive cells in the locus coeruleus and hypothalamus (Cuellar et al., 2000) and increased Fos expression in nicotinamide dinucleotide phosphate-diaphorase-positive cells in the brainstem (Jhamandas et al., 1996).
The expression of nNOS in oxytocin neurons is regulated and related to their activity; thus, quiescence of oxytocin neurons near the end of pregnancy is associated with reduced nNOS mRNA expression and reduced endogenous NO action (Okere & Higuchi, 1996; Srisawat et al., 2000) whilst the stimulation of oxytocin neurons by parturition, lactation and dehydration increases nNOS expression and action (Sagar & Ferriero, 1987; Pow, 1992; Kadowaki et al., 1994; Villar et al., 1994; Srisawat et al., 2000). We, therefore, investigated whether chronic morphine exposure leads to a downregulation of NO restraint of oxytocin neurons that subsequently facilitates withdrawal excitation.
Materials and methods
Virgin female Sprague-Dawley rats were used (body weight 250–350 g; Bantin & Kingman, UK), kept under standard conditions (12 h dark : 12 h light cycle; ambient temperature 22 ± 1 °C) with continuous access to food and water. All procedures were performed in accordance with UK Home Office regulations governing animal experimentation.
Induction of morphine tolerance and dependence
Rats were anaesthetized with 5% halothane in a mixture of oxygen and nitrous oxide and a 21-gauge stainless steel cannula was stereotaxically implanted in the right lateral cerebral ventricle (3 mm posterior to bregma, 2 mm lateral, 4 mm below the skull surface) and secured with two small screws fixed in the skull and dental cement. This was connected by polythene tubing to a subcutaneously implanted osmotic minipump (Alzet 2001; Alza Corp., Palo Alto, CA, USA) to continuously deliver morphine sulphate solution (Edinburgh Royal Infirmary, Edinburgh, UK) at 10 µg/µL for 40 h then 20 µg/µL for 40 h and finally 50 µg/µL, or vehicle (pyrogen-free distilled water; 1 µL/ h) in naive rats, over 5 days (Rayner et al., 1988). After surgery was completed rats were caged singly.
Blood sampling experiments
To ascertain whether NO mechanisms are altered as a consequence of morphine dependence, morphine-dependent and -naive rats were pretreated with the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) or the inactive enantiomer Nω-nitro-d-arginine methyl ester (d-NAME) and challenged with a hyperosmotic stimulus. Six days after minipump implantation morphine-dependent and naive rats were anaesthetized with intraperitoneal (i.p.) urethane (1.25 g/kg, 25% w/v) and then given an i.p. injection of either l-NAME (50 mg/kg, n = 5 and 6, respectively; Sigma, UK) or d-NAME (50 mg/kg, n = 6 and 7, respectively; Sigma). A femoral vein was cannulated for intravenous (i.v.) injections and a femoral artery for blood sampling; the other femoral vein was cannulated for infusion of hypertonic saline (via a slow infusion syringe pump). Blood samples (0.3 mL) were withdrawn into heparinized syringes, centrifuged and the plasma separated and stored at − 20 °C for subsequent oxytocin radioimmunoassay. Blood cells were resuspended in 0.9% saline and infused via a femoral venous cannula.
A basal blood sample was taken from all rats 240 min after l-NAME or d-NAME administration. Rats were then infused with i.v. hypertonic saline (2 m, 2.3 mL/h) and five further blood samples taken at 30, 45, 60, 75 and 90 min, at which point the infusion was stopped. All rats then received an i.v. injection of naloxone (5 mg/kg) and a final sample was taken 5 min later.
In a separate experiment to investigate whether endogenous NO is involved in the expression of withdrawal excitation, morphine-dependent rats were treated with the NOS inhibitor, l-NAME or vehicle before naloxone was given. Six days after mini-pump implantation morphine-dependent and -naive rats were prepared for blood sampling as described above. A first basal blood sample was drawn 120 min after the injection of either l-NAME or vehicle (0.9% NaCl, 0.5 mL/kg). Two further basal samples were obtained at 30 and 5 min prior to injection of naloxone (5 mg/kg i.v.; Sigma) at t = 0 min (240 min from l-NAME/vehicle treatment). Three further blood samples were taken at 5, 15 and 60 min after precipitation of morphine-withdrawal with naloxone.
Radioimmunoassay
Oxytocin content in unextracted plasma samples was measured by specific radioimmunoassay (Higuchi et al., 1985). The sensitivity was less than 4.8 pg/mL and intra-assay variation was less than 15% at standard concentrations of 10, 50, 100, 250, 500 and 1000 pg/mL. All samples were measured in the same assay and postnaloxone samples were diluted 1 : 10 in assay buffer prior to assay to ensure measurement on the reliable part of the standard curve.
Electrophysiology
Morphine-treated rats were anaesthetized with urethane (1.25 g/kg, i.p.), a femoral vein and the trachea cannulated and the pituitary stalk and right SON exposed transpharyngeally (Leng & Dyball, 1991). A locally made U-shaped dialysis probe (2 mm membrane length, Spectra/Por RC Hollow Fibres; Spectrum Medical Inc., Great Falls, MT, USA) was bent to position the loop of the membrane flat onto the exposed ventral glial lamina of the SON. A glass micropipette filled with saline (20–40 MΩ) was introduced into the centre of the dialysis probe loop to record extracellular single neuron activity within the SON (Ludwig & Leng, 1997) via a CED 1401 interface attached to a personal computer running Spike 2 software (Cambridge Electronic Design, Cambridge, UK). A bipolar stimulating electrode (Snex-200X; Clarke Electromedical Instruments, Reading, UK) was placed on the pituitary stalk and set to deliver single matched biphasic pulses (1 ms, < 1mA peak to peak) for antidromic identification of SON neurons. Oxytocin neurons were distinguished from vasopressin neurons by their continuous firing pattern and transient excitation in response to i.v. cholecystokinin (20 µg/kg: Renaud et al., 1987). Artificial cerebrospinal fluid was dialysed at 3 µL/min throughout the experiment (pH 7.2; in mm: NaCl, 138; KCl, 3.36; NaHCO3, 9.52; Na2HPO4, 0.49; CaCl2, 1.26; MgCl2, 1.18 and urea, 2.16). After withdrawal excitation had been induced by naloxone the dialysis fluid was changed to artificial cerebrospinal fluid containing the NO donor SNP (50 mm; Sigma), then the GABAA antagonist bicuculline methiodide (2 mm; Sigma) and finally SNP combined with bicuculline (see Fig. 3A). Based on previous experiments we estimate the concentrations of drugs achieved within the SON using this method to be 1 × 10−3 of that in the dialysate (Ludwig & Leng, 1997). Sodium nitroprusside has previously been shown to inhibit SON neurons solely through the generation of NO (Liu et al., 1996). One neuron was recorded from each rat.
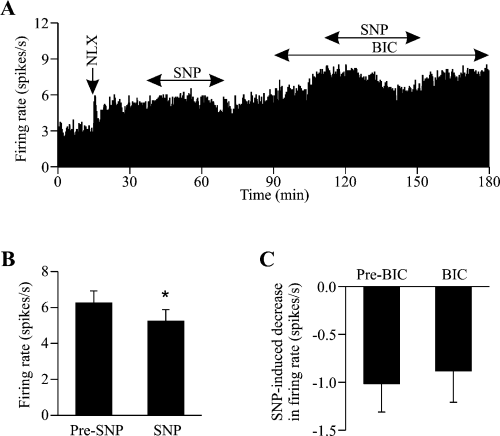
Effects of sodium nitroprusside (SNP) on the firing rate of oxytocin cells during morphine withdrawal excitation. (A) Extracellular single unit recording of the firing rate (averaged in 10-s bins) of an oxytocin cell (identified by transient excitation by i.v. cholecystokinin, 20 µg/kg, not shown) recorded from the supraoptic nucleus of a urethane-anaesthetized (1.25 g/kg, i.p.) rat administered morphine intracerebroventricularly for 5 days prior to recording. The cell shows a robust and sustained increase in firing rate following i.v. injection of the opioid receptor antagonist naloxone (5 mg/kg, NLX), typical of morphine withdrawal excitation in oxytocin cells. Microdialysis application of the nitric oxide donor SNP (50 mm, solid double-headed arrow) reduced the firing rate of the cell before and during microdialysis application of the GABAA receptor antagonist, bicuculline (5 mm, BIC); bicuculline alone increased firing rate. (B) The mean firing rates (± SEM, analysed in 5-min bins) of seven oxytocin cells recorded before administration of, and during maximal inhibition by, SNP (50 or 100 mm). *P < 0.05, paired t-test. (C) The mean maximal decrease in firing rate (± SEM) induced by SNP (50 or 100 mm) in the absence (n = 7) and presence (n = 4) of bicuculline, showing that bicuculline did not alter the SNP-induced inhibition of firing rate (P = 0.78, Student's t-test).
Statistical analysis
Blood sampling data were analysed using two-way repeated measures analysis of variance (anova) to isolate differences within and between groups. Where the F ratio was significant this was followed by posthoc analysis with Student-Newman-Keuls tests, unless stated otherwise, using SigmaStat® software (SPSS Science, Chicago, IL, USA). All values are expressed as the mean ± SEM and differences were considered statistically significant if P ≤ 0.05.
The firing rates of identified oxytocin cells were downloaded onto a personal computer using the Spike 2 software package (Cambridge Electronic Design) and analysed using SigmaStat® software. The mean firing rate of each cell was calculated for 5-min periods immediately before and during peak effects of each treatment unless stated otherwise. Differences were considered significant at P ≤ 0.05 values following paired and Student's t-tests.
Results
Blood sampling experiments
Effects of the nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester on hypertonic saline-stimulated oxytocin secretion in morphine-dependent and -naive rats
To allow comparison of the activity of NO mechanisms on stimulated oxytocin neurons in dependent and naive rats, an i.v. infusion of hypertonic saline (Brimble & Dyball, 1977) was given to morphine-dependent and -naive rats. There were no differences in basal plasma oxytocin concentrations between morphine-dependent and -naive rats pretreated with either l-NAME or d-NAME, indicating lack of endogenous NO generation and action on basal oxytocin secretion in naive as well as dependent rats [Fig. 1; before hypertonic saline the mean values were, respectively, 22.5 ± 5.4 and 32.9 ± 6.8 pg/mL, n.s., in d-NAME- and l-NAME-treated naive rats and 24.8 ± 5.5 and 17.9 ± 3.8 pg/mL, n.s., in d-NAME- and l-NAME-treated dependent rats; Fig. 2; before naloxone, the mean values were, respectively, 40.1 ± 7.7 and 46.8 ± 7.4 pg/mL, n.s., in vehicle- and l-NAME-treated dependent rats].
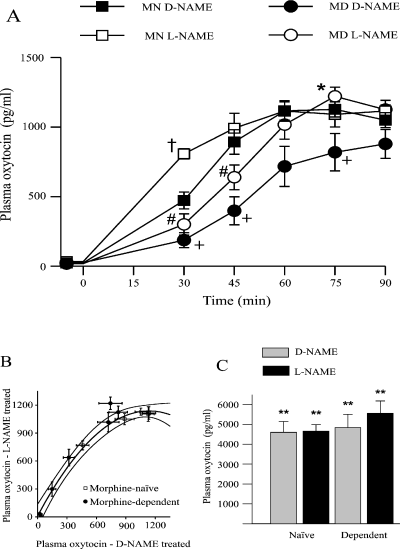
Effects of the nitric oxide synthase (NOS) inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME), on hypertonic saline-induced secretion of oxytocin in morphine-naive and -dependent rats. (A) Plasma oxytocin concentration (± SEM) in anaesthetized morphine-naive (MN) and morphine-dependent (MD) rats given Nω-nitro-d-arginine methyl ester (d-NAME) or l-NAME (both 50 mg/kg, i.p.) 240 min prior to a 2-m NaCl infusion at 2.3 mL/ h over 90 min. *P < 0.05 MD l-NAME vs. MD d-NAME; #P < 0.05 MD l-NAME vs. MN l-NAME; †MN l-NAME vs. MN d-NAME; +P < 0.05 MN d-NAME vs. MD d-NAME. (B) The data from A plotted as plasma oxytocin concentration in l-NAME-treated rats against the d-NAME-treated rats at the same time point during 2 m NaCl infusion. The data from morphine-naive rats was best fit by a second order regression (plotted with 95% confidence limits). Note that the data from the morphine-dependent group, except 75 min from the start of infusion, fall within the 95% confidence limits of the morphine-naive data. (C) Plasma oxytocin concentrations 5 min after naloxone injection (5 mg/kg, i.p.) at the end of the 2 m NaCl infusion in the rats from A. **P < 0.005 vs. prenaloxone sample (paired t-test).
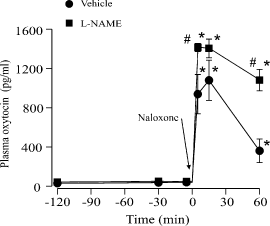
Effects of Nω-nitro-l-arginine methyl ester (l-NAME) on morphine withdrawal-induced hypersecretion of oxytocin. Plasma oxytocin concentrations (± SEM) in morphine-dependent rats injected with naloxone (5 mg/kg, i.p. at time = 0) to induce morphine withdrawal in rats pretreated with l-NAME or vehicle (0.9% saline) 240 min before naloxone administration. *P < 0.05 vs. basal within the same treatment group and #P < 0.05 vs. the time-matched data from vehicle-treated rats.
anova showed a significant increase in oxytocin secretion during hyperosmotic stimulation (P < 0.0001) which was also significantly different between treatment groups (P = 0.003). Thirty minutes after the start of the hypertonic saline infusion oxytocin secretion was increased significantly more in the naive l-NAME-treated rats than in the naive d-NAME-treated rats (by 639.4 ± 148.7 vs. 449.6 ± 56.6 pg/mL, P < 0.05). The oxytocin secretory response to the hypertonic saline infusion in the morphine-dependent rats was delayed in comparison with the naive rats, taking 45–60 min to reach the level at 30 min in naive rats (Fig. 1A). At 45 min, the plasma oxytocin concentration in the l-NAME-treated dependent rats was greater than in the d-NAME-treated dependent rats (Fig. 1A; increases from basal were 620.5 ± 99.4 and 373.2 ± 102.1 pg/mL, respectively; P < 0.05).
To take into account the different time courses of responses in the dependent and naive rats, the plasma oxytocin concentrations at each time point during l-NAME treatment were plotted against the oxytocin values at the equivalent time point during d-NAME treatment for the respective dependent and naive groups. The naive rat data were best fitted by a second order regression (r2 = 0.998, P = 0.0001; Fig. 1B). It is clear that l-NAME was no less effective in increasing oxytocin secretion during hyperosmotic stimulation in dependent than in naive rats.
Naloxone given at the end of the hypertonic saline infusion further greatly increased oxytocin secretion within 5 min in all groups, with no differences in plasma concentrations among groups (Fig. 1C).
Effects of the nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester on oxytocin secretion during naloxone-precipitated morphine withdrawal
As expected from previous studies (Bicknell et al., 1988), the i.v. injection of naloxone produced a large and sustained increase in plasma oxytocin concentration in the morphine-dependent rats (pretreated with an i.p. injection of vehicle as a control procedure in the present study; Fig. 2; P < 0.05). As noted above, the NOS inhibitor l-NAME given 240 min before injection of naloxone had no effect on basal plasma oxytocin concentration. However, l-NAME potentiated naloxone-induced oxytocin secretion in morphine-dependent rats (Fig. 2; P < 0.05).
Electrophysiology
Extracellular single unit recordings were made from eight antidromically identified SON cells, each from a different morphine-dependent rat, for between 1 and 6 h; all cells recorded displayed spontaneous activity and were transiently excited by i.v. administration of 20 µg/kg cholecystokinin (1.0 ± 0.2 spikes/s increase in firing rate, averaged over 5 min before and 5 min after cholecystokinin), typical of oxytocin cells in morphine-naive and -dependent rats (Brown et al., 1996). As previously demonstrated (Bicknell et al., 1988), i.v. administration of 5 mg/kg naloxone caused a robust and sustained increase in the firing rate of five oxytocin cells (by 2.9 ± 0.4 spikes/s). Recordings from the three remaining cells were started after naloxone administration when these cells displayed fast continuous firing at 8.3 ± 1.2 spikes/s, typical of oxytocin cells during morphine withdrawal excitation.
Effects of sodium nitroprusside and bicuculline on the firing rate of oxytocin cells during naloxone-precipitated morphine withdrawal
After naloxone administration, seven oxytocin cells were challenged with microdialysis application of 50 mm (n = 6) or 100 mm (one cell where 50 mm was ineffective) of the NO donor SNP at 3 µL/min, for 6–35 min, which reduced firing rate from 6.2 ± 0.7 to 5.2 ± 0.6 spikes/s (P = 0.013, paired t-test; Fig. 3A and B), a much smaller effect of SNP than is evident in morphine-naive rats under basal conditions (Stern & Ludwig, 2001). Microdialysis application of bicuculline alone (5 mm at 3 µL/min for > 60 min) increased the firing rate of four of these cells from 5.9 ± 0.7 to 8.3 ± 1.2 spikes/s (P = 0.022, paired t-test), similar to our previous experiments in naive rats (Ludwig & Leng, 2000). During bicuculline administration, SNP re-application (50 mm, n = 3) again inhibited firing to an extent that was not different from the level of inhibition as seen prior to bicuculline (Fig. 3C). This contrasts with oxytocin cells in morphine-naive rats in which bicuculline reduces the inhibition by SNP (Stern & Ludwig, 2001).
Discussion
The results reported here demonstrate that NO restrains oxytocin neuron excitation and secretion in morphine-dependent rats during both hyperosmotic stimulation and naloxone-precipitated withdrawal. The studies with the NOS inhibitor, l-NAME, indicate that endogenous NO was at least as effective in morphine-dependent rats as in naive rats in restraining oxytocin secretion when the neurons were strongly stimulated by hypertonic saline infusion. These findings are consistent with previous studies showing inhibitory actions of NO on oxytocin neurons in naive rats but are contrary to studies indicating excitatory actions of NO on some other morphine withdrawal phenomena.
Nω-nitro-l-arginine methyl ester and oxytocin secretory responses to hyperosmotic stimulation
As found previously in anaesthetized rats, there was no evidence for significant endogenous NO restraint of oxytocin neurons under basal conditions (Srisawat et al., 2000) and l-NAME treatment clearly did not precipitate withdrawal excitation of oxytocin neurons in dependent rats (1, 2). Similarly, a NOS inhibitor applied directly onto the SON increased oxytocin neuronal firing rate by only 13%, although intracerebroventricularly administered l-NAME in conscious rats increases basal oxytocin secretion (Kadekaro & Summy-Long, 2000; Srisawat et al., 2000).
Other studies indicate that if stimulation is brief, as with a systemic injection of cholecystokinin (Brown et al., 1996), NO inhibition of oxytocin neurons is still not significantly activated (Srisawat et al., 2000). The hypertonic saline stimulus used in the present study produces a continual increase in oxytocin neuron firing rate in naive rats, by c. 7 spikes/s at 30 min (Leng et al., 2001). At this time in the naive rats l-NAME pretreatment enhanced the oxytocin secretory response by 41% compared with the d-NAME-treated rats, indicating activation of NO restraint on oxytocin neurons (Srisawat et al., 2000) which became ineffective with further osmotic stimulation (Fig. 1). In the morphine-dependent rats the delayed increase in oxytocin secretion with hypertonic saline infusion is likely to reflect incomplete tolerance to inhibitory actions of morphine. Although hyponatraemia reduces the initial response to hypertonic saline infusion (Leng et al., 2001), plasma [Na+] is actually increased in dependent rats (140.1 ± 0.5 vs. 135.8 ± 1 mmol/L in naive rats, P < 0.05, t-test). Nor was the delayed response due to greater NO inhibition as l-NAME did not advance the response (Fig. 1). The action of l-NAME in further increasing oxytocin secretion in only the dependent rats with further hypertonic saline infusion indicates that inhibition by NO was at least as effective as in the naive rats.
Nitric oxide and morphine withdrawal-induced oxytocin secretion
We have shown here that the direction of action of NO on oxytocin neurons is not altered in morphine dependence either before or after naloxone-precipitated withdrawal. Hence, for withdrawal-induced hyper-excitation of oxytocin neurons, a robust feature of naloxone-precipitated morphine withdrawal in rats (Brown et al., 2000), inhibition of NOS accentuated the excitation (Fig. 2). Morphine withdrawal increases mean arterial blood pressure only slightly (Rayner et al., 1988) although we expect l-NAME to have had a more pronounced effect. However, oxytocin neurons are hardly affected by increases in blood pressure (Leng et al., 1991; Renaud & Bourque, 1991) so the effects of l-NAME on oxytocin neurons is not likely to be a result of blood pressure changes.
Nω-nitro-l-arginine methyl ester increased oxytocin secretion during withdrawal and hypertonic saline infusion in morphine-dependent rats to a similar extent (by 34% at peak of withdrawal and by 33–38% from 45 to 75 min of osmotic stimulation, respectively). Naloxone-precipitated morphine withdrawal resulted in a sustained increase in oxytocin neuron firing rate of 2.9 ± 0.4 spike/s (Fig. 3), similar to increases expected during hypertonic saline infusion (Leng et al., 2001). Hence, the effect of l-NAME (and thus NO) on oxytocin neurons during withdrawal seems to be similar to that in dependent rats stimulated osmotically and, in turn, was not less than the effects of l-NAME in osmotically stimulated naive rats.
The present experiments measuring oxytocin secretion could not distinguish effects of l-NAME on NO production and action on distant or local inputs to oxytocin neurons or on their terminals in the posterior pituitary. The mechanism by which NO production is induced by osmotic stimuli and withdrawal stimulation may involve enhanced glutamate release within the SON (Brown et al., 2000; Leng et al., 2001). As activation of glutamate receptors induces NO production in other systems (for reviews see Fedele & Raiteri, 1999; Kiss & Vizi, 2001), enhanced glutamatergic drive on oxytocin cells during these stimuli may increase NO production to restrain further excitation. Nonetheless, the aggregate effects of NO on secretion by excited oxytocin neurons were evidently not less in morphine-dependent than in naive rats.
In naive rats naloxone has no effect on the firing of oxytocin neurons during hyperosmotic stimulation but increases oxytocin secretion by antagonizing endogenous κ-opioid actions in the posterior pituitary in a similar way in both naive and dependent rats (Coombes et al., 1991; Leng et al., 1997). The similar terminal plasma oxytocin concentrations in all groups after hyperosmotic saline infusion for 90 min, before and after naloxone (Fig. 1), indicate that the maximal firing and secretory capacity of the neurons had been reached and NO inhibition overwhelmed.
Nitric oxide and morphine withdrawal-induced oxytocin cell excitation
The actions of NO on the electrical activity of oxytocin neurons in morphine naive rats are inhibitory (Ozaki et al., 2000; Srisawat et al., 2000; Stern & Ludwig, 2001), as shown here to be the case in dependent rats. The inhibitory actions of NO on oxytocin neurons in naive rats include direct actions on the neurons (Stern & Ludwig, 2001) as well as increasing the frequency and amplitude of fast GABAergic inhibitory postsynaptic currents (Ozaki et al., 2000; Stern & Ludwig, 2001). Oxytocin neurons express soluble guanylyl cyclase subunits, a target for NO (Furuyama et al., 1993), but activating cGMP mechanisms in SON neurons causes excitation (Yang & Hatton, 1999). In mice, histochemical studies indicate that NO produced by oxytocin neurons can act via cGMP on both inhibitory GABA and excitatory glutamate and catecholamine terminals on the neurons rather than directly on the neurons (Vacher et al., 2003). In rats, electrophysiological functional studies have found only facilitatory actions of NO on GABA terminals (Ozaki et al., 2000; Stern & Ludwig, 2001). Nonetheless, if the cGMP signalling mechanism is also present, and appropriately coupled as the major mechanism of NO action (Prast & Philippu, 2001) in excitatory terminals on oxytocin neurons, then it is possible that NO could act to excite oxytocin neurons rather than inhibit them in some circumstances. However, no such circumstance has yet been found and morphine dependence does not, as shown here, ablate or reverse the inhibitory action of NO on oxytocin neurons.
In contrast, the electrical excitation of locus coeruleus neurons, somato-dendritic release of noradrenaline and local release of glutamate during morphine withdrawal are suppressed by systemic or local infusion of NOS inhibitors (Hall et al., 1996; Pineda et al., 1998; Javelle et al., 2002) or by local infusion of a soluble guanylyl cyclase inhibitor (Sullivan et al., 2000). Thus, it is envisaged that withdrawal excitation of locus coeruleus neurons involves glutamate release and action through N-methyl-d-aspartate receptors, increased intracellular [Ca2+] and hence nNOS activation (Garthwaite et al., 1989; Hall et al., 1996). The action of NO in further stimulating excitatory synapses and supporting withdrawal excitation of locus coeruleus neurons (Manzoni et al., 1992; Uzbay & Oglesby, 2001) contrasts with the present study showing inhibitory actions of NO on oxytocin neurons during withdrawal.
There may be a modification of the interaction of NO with the GABA input onto oxytocin neurons in morphine-dependence. Firstly, the reduction by SNP of the firing rate of withdrawn oxytocin neurons (Fig. 3) was less marked than previously reported for SNP inhibition of basal oxytocin neuron firing rate in naive rats (Stern & Ludwig, 2001). This may well simply be a consequence of a reduced effect of NO per se as the neurons are strongly excited, as indicated by the oxytocin secretion data (Fig. 1). Alternatively, or additionally, NO generated by SNP may be less effective because of competition with endogenous NO generated by NOS as the neurons are excited. Secondly, despite the increased firing rate during morphine withdrawal, bicuculline increased oxytocin neuron firing rate to an extent (28% increase; Fig. 3) similar to that seen in naive rats (24% increase), under identical recording conditions, previously reported by Stern & Ludwig (2001). Thus, there is continuing GABA restraint of oxytocin neuron firing even during withdrawal excitation, similar to the GABA input to oxytocin neurons during osmotic stimulation (Leng et al., 2001). Thirdly, bicuculline did not diminish the inhibitory action of SNP on firing rate during withdrawal excitation (Fig. 3), in contrast with a significant reduction in the inhibitory effect of SNP on the firing rate of oxytocin neurons in naive rats in the presence of bicuculline (Stern & Ludwig, 2001). However, this may, like the less marked effect of SNP alone, reflect that, under the conditions of strong withdrawal excitation, endogenous NO is maximally effective on the GABA terminals and the additional NO generated by SNP acts only directly on the oxytocin neurons for this reason.
In conclusion, it seems that the effects of inhibiting NOS generation in neurons excited by precipitated morphine withdrawal will depend upon whether the action of NOS and NO in the normal regulation of these neurons is excitatory, as in the locus coeruleus, or inhibitory, as in the regulation of oxytocin neurons. The excitation of oxytocin neurons during morphine withdrawal activates NOS which then restrains the excitation, as in other conditions in which they are strongly excited. Our evidence does not indicate that changes in NOS and NO mechanisms underlie morphine dependence and withdrawal excitation in oxytocin neurons. The hyper-secretion of oxytocin in the rat model of precipitated morphine withdrawal is accentuated by a NOS inhibitor and this questions whether NOS inhibitors should be considered for use in controlling other withdrawal phenomena (Uzbay & Oglesby, 2001). The present study cautions that NOS inhibitors should not be expected to prevent all opiate withdrawal symptoms and, indeed, that some may be enhanced.
Acknowledgements
The authors would like to thank Prof. G. Leng for helpful discussions and advice. This research was supported by The Wellcome Trust.
Abbreviations
-
- d-NAME
-
- Nω-nitro-d-arginine methyl ester
-
- l-NAME
-
- Nω-nitro-l-arginine methyl ester
-
- nNOS
-
- neuronal nitric oxide synthase
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthase
-
- SNP
-
- sodium nitroprusside
-
- SON
-
- supraoptic nucleus.