Estradiol modulates acetylcholine-induced Ca2+ signals in LHRH-releasing GT1-7 cells through a membrane binding site
Abstract
Estrogen regulation of the female reproductive axis involves the rapid inhibition (< 30 min) of luteinizing hormone-releasing hormone (LHRH) secretion from hypothalamic neurons. This fast time-course suggests interactions with potential plasma membrane binding sites that could result in short-term effects on LHRH neurons. Because LHRH release is calcium dependent, we have studied the acute effects of 17β-estradiol (E2) and estradiol-peroxidase (E-HRP) on the elevations of intracellular calcium ([Ca2+]i) induced by acetylcholine (ACh) in LHRH-producing GT1-7 cells. Exposure to ACh (1–100 µm) induced transient increases of [Ca2+]i, whereas pretreatment with E2 or E-HRP (10 nm) for 2 min reduced this response by 50–60%. The effect was specific for E2 as neither 17α-estradiol (1 µm) nor the synthetic antiestrogens ICI182 780 (1 µm) or tamoxifen (1 µm) elicited any change on the ACh-induced Ca2+ signal. Both the latency of the effect and the response to the membrane impermeant conjugate suggested a membrane-mediated mechanism. Such membrane binding sites for E2 in GT1-7 cells were demonstrated by visualizing the binding of E-HRP and estradiol-BSA-fluorescein isothiocyanate (E-BSA-FITC) conjugates. Competition studies showed that E-HRP binding was blocked by preincubation with E2, but not with 17α-E2, ICI182 780, tamoxifen or progesterone, indicating that the plasma membrane binding site is highly specific for E2 and exhibits a pharmacological profile different from classical estrogen receptors. We conclude that ACh-induced increase in [Ca2+]i in GT1-7 cells is modulated acutely by physiological E2 concentrations in a manner which is compatible with the existence of an estrogen-specific membrane binding site.
Introduction
Hypothalamic luteinizing hormone-releasing hormone (LHRH) neurons play a central role in the control of mammalian reproduction. LHRH is secreted from nerve terminals into the hypophyseal portal veins in the median eminence in a pulsatile manner, which in turn controls the episodic release of gonadotropins from the anterior pituitary and generates the oscilatory profile of LH secretion observed during the estrous cycle (Freeman, 1994). However, the investigation of LHRH neurons and the identification of the intracellular signals that participate in their regulation has been extremely difficult because of their scattered distribution. Therefore, immortalized LHRH-producing cell lines constitute a valuable tool to explore the functional properties of LHRH neurons. In this respect, GT1-7 cells, obtained from a hypothalamic tumour in a transgenic mouse (Mellon et al., 1990), secrete LHRH in a pulsatile pattern and exhibit many of the biochemical and biophysical characteristics of typical LHRH neurons (Wetsel et al., 1992; Nuñez et al., 1998; Martínez-Morales et al., 2001; Nunemaker et al., 2001; Vazquez-Martínez et al., 2001).
It is now clear that the oscillatory pattern of LHRH secretion can be controlled by the feedback action of estradiol (E2) at different levels within the LHRH network (Herbison, 1998). For several decades, estrogen regulation of LHRH secretion was thought to be mediated by presynaptic inputs from estrogen-sensitive neurons. However, the demonstration that the human LHRH gene contains an estrogen response element (Radovick et al., 1991, 1994) and the identification of intracellular estrogen receptors in LHRH neurons (Butler et al., 1999; Hrabovszky et al., 2001) have raised the possibility that estrogen can affect LHRH secretion from these cells by directly modulating gene expression (Shen et al., 1998). Remarkably, estrogen administration to ovariectomized mammals reduces LHRH (Sarkar & Fink, 1980) and LH (Yamaji et al., 1972; Condon et al., 1988) levels in portal blood and peripheral plasma, respectively, within minutes. Furthermore, in hypothalamic slices from ovariectomized female guinea pigs, E2 rapidly hyperpolarizes LHRH neurons and reduces the potency of µ-opioid and γ-aminobutyric acid (GABA)B receptor agonists (Kelly et al., 2002; Lagrange et al., 1995). Therefore, it would be expected that, in addition to nuclear effects triggering genomic actions, estrogen will modulate LHRH neurons via rapid alternative pathways, as it has been found in other systems (reviewed in Nadal et al., 2001; Ho & Liao, 2002; Losel et al., 2003).
In contrast to other classical neurotransmitters, there is less evidence supporting a role for acetylcholine (ACh) in the regulation of LHRH secretion from hypothalamic neurons. Based on rather pharmacological approaches, some classical work suggested the potential existence of a cholinergic, atropine-sensitive component in the control of LH secretion in rodents (Everett et al., 1949; Libertum & McCann, 1973). More recently, in rat hypothalamic neurons and GT1-7 cells, ACh has been found to modulate LHRH secretion by acting on both nicotinic and muscarinic receptors (Krsmanovic et al., 1998). As oscillations in intracellular calcium are a critical component of the secretory response of LHRH neurons (Núñez et al., 2000), we decided to characterize the effect of ACh on intracellular Ca2+ signals in GT1-7 cells and its possible acute modulation by 17β-estradiol (E2).
Materials and methods
Cell culture
GT1-7 cells (provided by Dr P. Mellon, University of California) were grown in Dulbecco's modified Eagle's medium, containing 10% fetal bovine serum (FBS, Gibco BRL, NY, USA), 4.5 g/L glucose, 0.58 g/L glutamine, 3.7 g/L NaHCO3, 100 µg/mL gentamicin and 50 µg/mL penicillin. They were cultured at 37 °C under 5% CO2−95% air on glass coverslips until 50–60% confluence. Before any treatment, the cells were washed in phosphate-buffered salt solution (PBS) and cultured for 24 h in serum-free Opti-MEM® (Gibco).
Solutions
The standard extracellular solution contained (in mm): NaCl, 140; KCl, 2.8; CaCl2, 3.0; MgCl2, 2.0; HEPES, 10.0; and glucose, 6.0. Calcium-free solutions were prepared by replacing all CaCl2 from the nominal standard solution and supplementing with 5 mm EGTA. Atropine and nicotine were dissolved in water and added to the solutions immediately before use. Nitrendipine, thapsigargin and ICI812 780 were dissolved in dimethylsulfoxide (DMSO). 17β-estradiol, 17α-estradiol and tamoxifen stock solutions were prepared in ethanol. All these chemicals were purchased from Sigma (Biosigma, Madrid, Spain.)
Measurement of intracellular calcium ([Ca2+]i)
Cells were loaded by incubation with 5 µm Fluo-3 AM (Molecular Probes, Eugene, OR, USA) for 1 h at room temperature in standard extracellular solution containing 1% bovine serum albumin (BSA), and then washed with the same solution for 15 min before the experiments. Calcium signals from individual cells were obtained at 37 °C by confocal microscopy (MRC1024, Bio-Rad, Madrid). Images were collected at 1-s intervals and fluorescence signals were measured as a function of time using the Bio-Rad software package. Results were plotted as the change in fluorescence intensity (ΔF) expressed as a percentage of basal fluorescence intensity (F0) in the absence of stimulus. Calcium signals were evaluated for peak height, area under the curves, slope (25–90%) of the raising phase and half-time (t1/2) to peak using a set of dedicated macros written for IGOR (WaveMetrics, Lake Oswego, OR, USA) in the Department of Pharmacology (University of La Laguna, Tenerife, Spain).
Phosphoinositide hydrolysis
To evaluate the effect of muscarinic receptor activation on phosphoinositide hydrolysis in GT1-7 cells, we measured the total accumulation of labeled inositol phosphates (IP) following stimulation with ACh. Before treatment with agonist, the cells were incubated for 12 h with 20 µm (3 µCi/plate) myo-[3H]inositol (17.1 Ci/mmol, Amersham, UK) in 2 mL of culture medium. Cells were exposed to 100 µm ACh in the presence of 10 mm LiCl to prevent [3H]IP degradation by phosphatase activity. At the end of the incubation periods the cells were washed twice with PBS and the reaction was stopped by adding 1 mL ice-cold methanol. The cells were scraped from the dishes and [3H]IP were extracted as described previously (Sandmann & Wurtman, 1991). Briefly, water-soluble metabolites were separated from phospholipids by extraction in methanol–chloroform water (1 : 1 : 0.5, v/v/v). [3H]IP were isolated by separating the aqueous phase on anion exchange columns (AG 1-X8, formate form, Bio-Rad Laboratories, Madrid, Spain). After loading the samples, free [3H]inositol was washed off with 6 mL water and the [3H]IP fraction was eluted from the columns by adding 5 × 1 mL of 1 mm ammonium formate plus 0.1 m formic acid (Berridge et al., 1982). Radioactivity was measured in aliquots of the fractions by liquid scintillation spectrometry (counting efficiency, 30–40%; Beckman LS380, CA, USA).
Assay for estradiol-BSA-FITC binding
Cells cultured on glass coverslips were washed twice with PBS, pH 7.2, and incubated for 1 h at 37 °C in the presence of 10 µm 17β-estradiol-6-(O-carboxymethyl)oxime-BSA-FITC (E-BSA-FITC, Sigma). In some experiments, cells were preincubated for 1 h in the presence of an excess of either BSA or E2. Cells were washed twice with PBS before being viewed by confocal microscopy (Valverde et al., 1999).
Assay for estradiol-peroxidase binding
Cells grown on glass coverslips were fixed in 4% paraformaldehyde (PFA) for 1 min, and exposed to 4.5 µg/mL estradiol-horseradish peroxidase (E-HRP, Sigma) overnight at 4 °C. Cells were then washed with PBS pH 7.2, and E-HRP binding was developed by using 3,3′-diaminobenzidine tetrahydrochloride (DAB, 0.5 mg/mL) in the presence of 0.3 mg/mL urea hydrogen peroxide in 0.05 m Tris buffer and 0.15 m NaCl for 30 min (Sigma FAST DAB tablet set). To test that the plasma membrane was not permeabilized, cells were alternatively incubated with 2.5 mg/mL dextran-conjugated tetramethylrhodamine (dextran-TMR, Molecular Probes; 40 000 Da) and visualized by laser scanning confocal microscopy as described previously (Nadal et al., 2000).
The DAB-based product of the peroxidase reaction displays maximal light absorption at 488 nm. The absorbed light was monitored by confocal microscopy and was used as an indication of the amount of E-HRP bound to the membrane. A comparative numerical assessment was made by quantifying the percentage of absorbed light in individual cells exposed to the different compounds with respect to E-HRP alone, as described previously (Nadal et al., 2000). To obtain an appropriate background staining, some coverslips were incubated with unconjugated HRP.
Data analysis
Quantitative results are expressed as mean ± SEM. Data from calcium signals are expressed as the percentage of the second response to ACh with respect to the first response. Data were analysed by one-way analysis of variance or Student's t-test where appropriate.
Results
Changes of [Ca2+]i in GT1-7 cells in response to ACh
Treatment of single GT1-7 cells with increasing ACh concentrations (1–100 µm) elicited prominent changes in intracellular calcium signals. ACh induced a dose-dependent rapid increase in [Ca2+]i followed by a slower decline to basal levels (Fig. 1A). Occasionally, transient calcium signals induced by ACh were followed by 3–6 additional transients following an oscillatory pattern of reduced amplitudes. No apparent differences were observed in the magnitude of the response to ACh at concentrations above 100 µm (not shown). In addition, the analyses of cell responsiveness showed that at this concentration more than 90% of monitored cells were responsive to ACh (Fig. 1B). In order to reduce the desensitisation of the response as much as possible, we then determined the optimal exposure time to ACh. At the concentration chosen (100 µm), we found that the minimal time required to achieve a maximal response to ACh in GT1-7 cells was 5 s. Under these conditions, GT1-7 cells were fully recovered after 8–10 min and a second ACh pulse produced a similar response to the first stimulation (Fig. 1C). Longer exposures to ACh, of 60 and 120 s, led to reduced peak amplitudes in response to a second stimulation with ACh (Fig. 1C). Therefore, we have employed a 5-s application of 100 µm ACh as the standard stimulation and a 10-min interstimuli time throughout the present study.
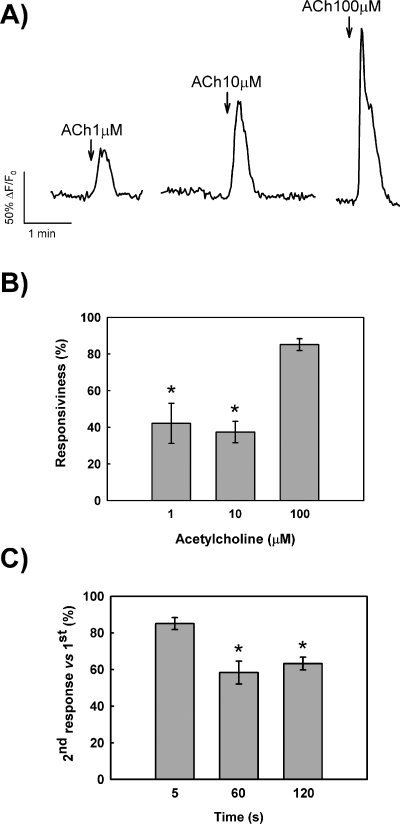
Characterization of ACh-induced Ca2+ transients in GT1-7 cells. (A) Representative traces of single GT1-7 cells exposed to increasing concentrations of ACh. (B) Analyses of responsiveness to different concentrations of ACh expressed as mean (± SEM) percentage of responding cells. *P < 0.001 compared with 100 µm. (C) Effects of the exposure time on the response to ACh (100 µm). Data are expressed as mean (± SEM) percentage of peak amplitude in the second exposure to ACh vs. 100% in the first response. ACh pulses were separated by 10 min. *P < 0.01 compared with 5 s.
To further explore ACh-induced calcium signals in GT1-7 cells we attempted to establish the cellular source for cytosolic [Ca2+]i increase in response to ACh. Acetylcholine-induced calcium transients were not affected by either exposure to the l-type calcium channels blocker nitrendipine (10 µm) or by removal of extracellular calcium (Fig. 2A and B), whereas depletion of intracellular stores with thapsigargin (1 µm), a powerful inhibitor of endoplasmic reticulum Ca2+-ATPase, completely prevented ACh-induced calcium signals (Fig. 2E).
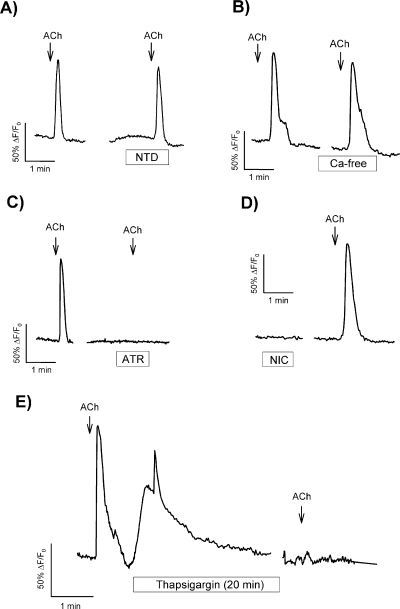
Ca2+ transients in GT1-7 cells are initiated by muscarinic receptor activation and are independent of extracellular calcium. (A) Effect of nitrendipine (NTD, 10 µm) applied for 1 min on ACh-induced calcium signals. (B) Effect of extracellular calcium removal on ACh response. Cells were preincubated with calcium-free solution for 5 min before application of ACh. (C) Effect of preincubation with atropine (ATR, 10 µm) for 1 min on the response to ACh. (D) Exposure to the agonist nicotine (NIC, 100 µm) for 1 min failed to induce any calcium signal in single GT1-7 cells. (E) Response of single GT1-7 cells to ACh after 20 min preincubation with thapsigargin (1 µm). ACh concentration used in all cases was 100 µm. Traces are representative for at least 25 different cells, and each experiment was repeated at least three times.
The effects of ACh described above are likely to be mediated by muscarinic receptors, as ACh-induced calcium transients were blocked completly by the muscarinic receptor antagonist atropine (10 µm). Furthermore, the effects were not reproduced by nicotine (100 µm), an agonist of nicotinic receptors (Fig. 2C and D). These data clearly demonstrate that ACh activates muscarinic receptors in GT1-7 cells that eventually trigger the release of intracellular calcium from thapsigargin-sensitive stores.
Modulation of ACh-induced calcium signals by estradiol
Pretreatment of GT1-7 cells with E2 dramatically changed the magnitude of ACh-induced Ca2+ transients (Fig. 3A). To characterize the effect of E2, we have tested the concentration-dependence of estrogen effects on the response to ACh. In these experiments, 8 min after the first application of ACh, GT1-7 cells were exposed to increasing concentrations of E2 for 2 min, before a second challenge to ACh. The results shown in Fig. 3B indicate that doses as low as 10 nm were effective on reducing signal amplitude, area under the curve and raising slope without altering half-time to peak (t1/2). As 10 nm E2 was found to produce consistent effects in most cells (>90%), this concentration was used to determine the optimal pretreatment time for the modulatory effect of estrogen on cytosolic calcium transients. The results showed that an exposure to E2 as brief as 2 min was sufficient to elicit significant changes on peak amplitude, area under the curve and raising slope of ACh responses (Fig. 3C).
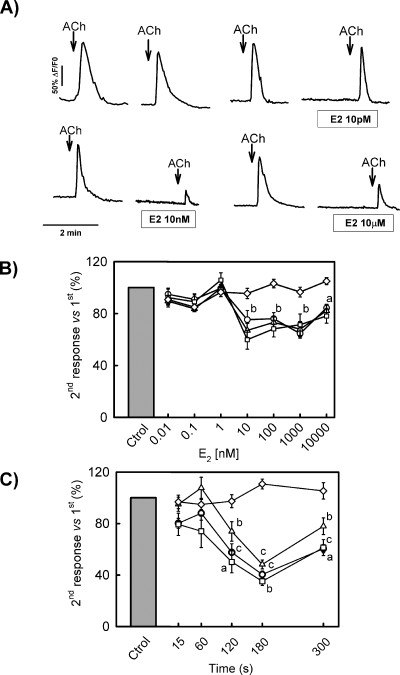
Estrogen modulates ACh-induced Ca2+ transients in GT1-7 cells. (A) Representative recordings of calcium transients induced by ACh (100 µm) in GT1-7 cells exposed to increasing concentrations of 17β-estradiol. (B) Dose–response plot for 17β-estradiol effects on ACh-induced calcium transients. Analysed parameters include peak amplitude (○), signal area (▵), transient slope (□) and t1/2 (◊). (a and b) P < 0.05 and P < 0.01 vs. control, respectively. (C) Effects of 17β-estradiol (10 nm) incubation time on ACh-induced transients. Analysed parameters include peak amplitude (○), signal area (▵), transient slope (□) and t1/2 (◊). (a–c) P < 0.05, P < 0.01 and P < 0.001 vs. control, respectively. Calcium transients were induced by two consecutive 5-s ACh (100 µm) pulses separated by 10 min. Data in B and C are expressed as the percentage of response in the second exposure to ACh vs. 100% in the first response.
Pharmacology of estrogen-induced modulation of calcium signals
The ability of the physiologically inactive steroisomer 17α-estradiol and different synthetic antiestrogens to reproduce the effects of E2 on ACh-induced calcium signals in GT1-7 cells was assessed in another set of experiments. GT1-7 cells were exposed to either 1 µm 17α-estradiol, ICI182 780 or tamoxifen for 2 min before the second application of ACh to the bathing solution. The results shown in Fig. 4 demonstrate that none of these compounds were able to mimic the effects of E2 on ACh-induced calcium signals (P > 0.1, n = 6 in all cases), indicating that the modulatory effect described here was highly specific for E2. Finally, we also tested for the putative antagonistic effect of the pure antiestrogen ICI182 780 on the effect of E2. As can be seen in Fig. 4B, preincubation with ICI182 780 (10 µm) for 20 min did not prevent the modulatory effect of E2 on ACh-induced cytosolic calcium transient. Taken together, these data suggest a unique pharmacology for the putative estrogen receptor involved in the modulation of ACh effects in GT1-7 cells.
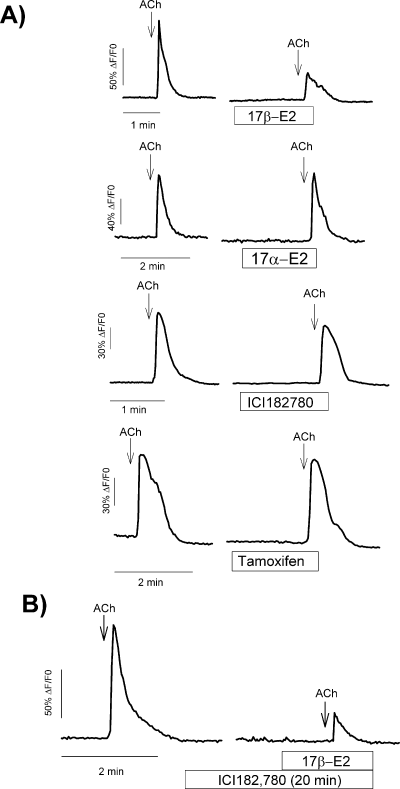
Effects of estrogens and antiestrogens on ACh-induced Ca2+ transients in GT1-7 cells. (A) [Ca2+]i responses to ACh (100 µm for 5 s) in the presence of 17β-estradiol (10 nm), 17α-estradiol (1 µm), ICI182 780 (1 µm) and tamoxifen (1 µm) in four different GT1-7 cells. Cells were preincubated for 2 min with the different compounds before the second application of ACh. ACh pulses were separated by 10 min. (B) Preincubation for 20 min with the estrogen receptor antagonist ICI182 780 (10 µm) failed to prevent the effect of 17β-estradiol (10 nm). Traces are representative for at least 20 different cells, and each experiment was repeated three times.
Estrogen actions are triggered at the plasma membrane
The fast time-course for the effect of E2 on ACh-induced signals suggests that the modulatory process might be initiated at the plasma membrane. Therefore, we used estradiol conjugated to horseradish peroxidase (E-HRP), a membrane impermeant analogue of E2, to investigate whether the rapid action of E2 could be reproduced by a compound that does not cross the plasma membrane. Our results show that a brief 2-min exposure to E-HRP (10 nm) also reduced the response to ACh, thus mimicking the effects of E2 (Fig. 5). The analysis of peak parameters for E2 and E-HRP from 45 different cells gave similar results, i.e. both compounds decreased maximal amplitude, area under the curve and raising slope (Table 1), indicating that both E-HRP and E2 triggered the same modulatory pathway in the generation of ACh-induced Ca2+ signal in GT1-7 cells.
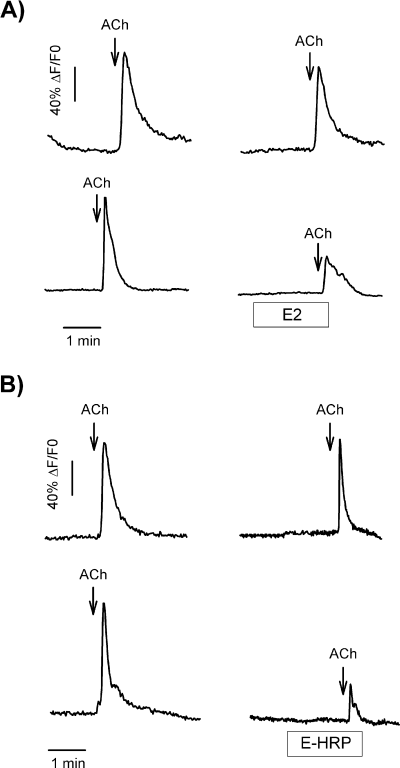
E2 and E-HRP modulate ACh-induced Ca2+ transients in GT1-7 cells. Single GT1-7 cells were exposed to two 5-s pulses of ACh (100 µm), separated by 10 min. Two minutes before the second application of ACh the cells were exposed to 10 nm of either E2 (A, lower panel) or E-HRP (B, lower panel) for 2 min. Control responses are depicted in the upper panels.
| Second response/first response (%) normalised to controls | ||||
|---|---|---|---|---|
| Height | Area | Slope | t 1/2 | |
| E2 | 51.2 ± 5.0*** | 78.7 ± 8.0* | 63.8 ± 8.7** | 95.86 ± 3.9 |
| E-HRP | 60.4 ± 7.0*** | 54.7 ± 7.3*** | 61.7 ± 9.4** | 102.97 ± 6.0 |
- Results are mean ± SEM and correspond to the percentage of the second response to ACh with respect to the first one, as compared with 100% controls exposed to the vehicles (n = 45 cells in both groups). Statistical differences by Student's t-test are: *P = 0.06, **P < 0.02, ***P < 0.001.
Effects of E2 on ACh-induced phosphoinositide hydrolysis
Previous reports have shown that activation of muscarinic (M1) receptors in GT1-7 cells by ACh leads to the stimulation of phosphoinositide hydrolysis which is followed by increased LHRH secretion (Krsmanovic et al., 1998). This finding, together with the observation reported here that calcium transients elicited by ACh were entirely dependent on intracellular sources, raised the question of whether the rapid effects of E2 on ACh-induced calcium signal observed in GT1-7 cells were associated to a reduced generation of inositol triphosphates. To investigate this possibility, we determined the magnitude of phosphoinositide hydrolysis in response to ACh (100 µm) in control and estrogen-treated cells. As shown in Fig. 6A, ACh induced a time-dependent increase of [3H]IPs that was statistically significant after 10 min, with the maximal stimulation being reached 30 min after application of ACh. Preincubation with estradiol (10 nm) for 6 min before the stimulation with ACh caused no significant changes in inositol phosphate production compared to control tissues (Fig. 6B).
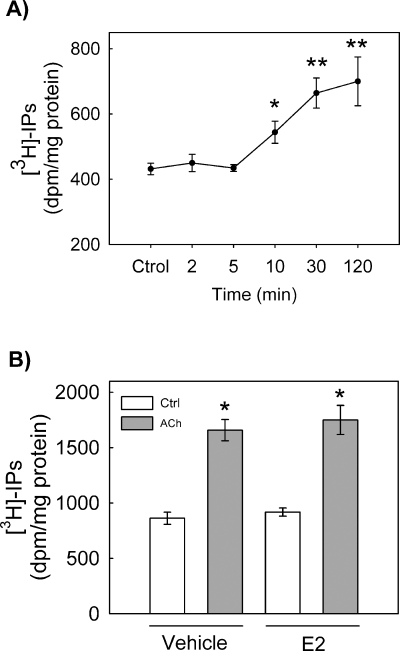
Effects of estrogen on ACh-induced phosphoinositide hydrolysis. (A) Time-course for inositol phosphates accumulation in ACh-treated GT1-7 cells. The stimulatory concentration of ACh was 100 µm. *P < 0.05 and **P < 0.01 vs. control. (B) Effect of preincubation of GT1-7 cells with 17β-estradiol (10 nm) on ACh-induced phosphoinositide hydrolysis. Measurements were taken 30 min after application of ACh. Cells were preincubated with 17β-estradiol was for 6 min before stimulation with ACh. *P < 0.001 vs. control. Data are expressed as mean ± SEM of six different experiments.
Membrane binding site for E2 using E-BSA-FITC
The rapid effects of E2 are indicative of a modulatory action on the generation of ACh-induced Ca2+ signals through an alternative pathway. The fact that these actions are reproduced by the membrane-impermeant molecule E-HRP strongly suggests the presence of estrogen plasma membrane binding sites. We used E2 conjugated to BSA-FITC as a membrane-impermeant fluorescent probe to test this hypothesis. The results illustrated in Fig. 7 reveal that cells exposed to E-BSA-FITC exhibit fluorescent labeling around the plasma membrane, which was unaffected by preincubation with an excess of BSA, but blocked by a 50-fold excess of E2. This suggests the existence of specific membrane binding sites for estrogen in the plasma membrane of GT1-7 cells.
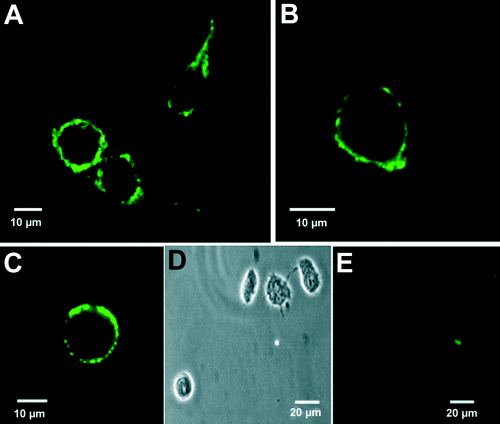
Labeling of GT1-7 cells with E-BSA-FITC. (A and B) Representative fluorescence images of GT1-7 cells treated with E-BSA-FITC (10 µm). Fluorescein labeling is confined to the plasma membrane (B). (C) Fluorescent staining with E-BSA-FITC is unaffected by 100 µm BSA. (D) Transmission image of cells shown in E. (E) E-BSA-FITC labeling is displaced by 500 µm E2.
Pharmacological characteristics of the E2 membrane binding site
The existence of estrogen membrane binding sites in GT1-7 cells was corroborated by a quantitative method using E-HRP (Fig. 8A). The binding of E-HRP was blocked by competition with a 300-fold excess of E2, but not by 17α-estradiol, ICI182 780, progesterone or tamoxifen (Fig. 8B and D). To demonstrate that E-HRP did not enter through membrane pores caused by the fixation procedure, we used dextran-TMR with a molecular weight of 40 000, similar to the 44 000 MW of the E-HRP complex. Cells were impermeable to dextran-TMR after PFA treatment (Fig. 8C). To obtain an appropriate staining background, we used HRP that was not conjugated to E2. This background light absorption was identical to the light measured when E2 was competing with E-HRP, indicating that all the E-HRP membrane labeling was displaced by E2. Therefore, it seems that the effects described up to now are initiated after E2 binding to a membrane binding site with pharmacological properties different to the classical estrogen receptors ERα and ERβ.
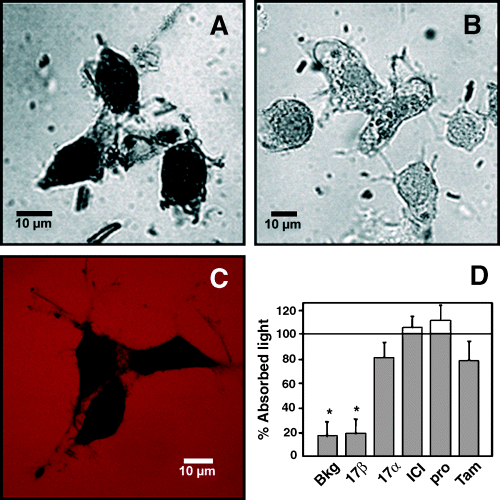
E-HRP binding assay in GT1-7 cells. (A) Binding of E-HRP (100 nm) to nonpermeabilized cells visualized by transmission laser scanning. (B) Binding of E-HRP is blocked by a 300-fold excess of E2. (C) Dextran-TMR (red) does not cross the plasma membrane of PFA-fixed cells (black). (D) Competition of E-HRP binding by 17β-estradiol (17β), 17α-estradiol (17α), ICI182 780 (ICI), progesterone (Pro) or tamoxifen (Tam), all compounds at a final concentration of 30 µm. The quantity of E-HRP bound is expressed as the percentage of absorbed light with respect to cells incubated with E-HRP alone. The lower the percentage of absorbed light, the higher the competition for an E-HRP binding site. Data are mean ± SEM of six different experiments. Number of cells per group: Ctrl, 256; 17β, 279; 17α, 189; ICI, 166; Pro, 248; Tam, 155. *P < 0.01 vs. 100%.
Discussion
The results presented here show that E2 and E-HRP acutely inhibit the rise in [Ca2+]i induced by ACh in GT1-7 cells. They also demonstrate the existence of specific estrogen binding sites in the plasma membrane of these hypothalamic-derived cells that clearly differ from classical estrogen receptors. These observations indicate that E2, at physiological concentrations, might acutely modulate the activity of LHRH-producing cells by acting at the plasma membrane. Moreover, because in both hypothalamic neurons and GT1-7 cells activation of nicotinic and M1 muscarinic receptors increases LHRH release (Krsmanovic et al., 1998), these effects could help to partially explain how estradiol exerts its rapid, negative feedback actions on LHRH and LH secretion in the female (Sarkar & Fink, 1980; Condon et al., 1988).
Despite very early observations on the potential role of ACh in the hypothalamic regulation of pituitary function (Everett et al., 1949; Libertum & McCann, 1973), direct evidence for cholinergic actions at the cellular level has only been provided very recently (Krsmanovic et al., 1998). In this work, ACh induced a dose-dependent rapid increase of [Ca2+]i by the release of calcium from thapsigargin-sensitive stores. The antagonistic effect of atropine and the inability of nicotine to mimic the effect of ACh, and the observation that this neurotransmitter stimulates phosphoinositide hydrolysis in GT1-7 cells, is strong evidence indicating that ACh-induced calcium transients is an IP3-mediated calcium event that may be triggered by activation of muscarinic receptors (Krsmanovic et al., 1998).
Our results also show that E2 modifies the calcium signal induced by ACh in a rapid manner consistent with a nongenomic mode of action, i.e. only a brief 2 min exposure of GT1-7 cells to nanomolar concentrations of estradiol or to the impermeant conjugate E-HRP was sufficient to produce the maximal inhibition of ACh-induced calcium accumulation. This acute effect of estrogen on calcium signals seems to be specific for E2, as it was not reproduced by either 17α-estradiol or by the unrelated synthetic antiestrogens ICI182 780 and tamoxifen. Moreover, our results also demonstrate that the acute effect of estradiol was not affected by preincubation of GT1-7 cells with saturating concentrations of the pure antiestrogen ICI182 780, indicating that classical estrogen receptors were not involved.
The analyses of calcium responses to ACh show that both E2 and E-HRP dramatically changed the kinetic of ACh-induced calcium peaks. Thus, E2 and E2-HRP reduced not only the peak amplitude of calcium signals but also the amount of free intracellular calcium in response to ACh. These effects are suggestive of an estrogen-induced inhibition of the mechanisms involved in cytosolic Ca2+ accumulation by depressing the release mechanism. Alternatively, estrogen could affect the amount of calcium within the intracellular stores by reducing the amount of calcium sequestration into the depleted stores. However, because the second application of ACh to GT1-7 cells gave rise to a calcium transient that was identical to that of the first stimulation, we must conclude that intracellular stores have been presumably refilled to control levels. This leads us to preclude the possibility that the modulatory effect of estradiol on ACh-induced calcium signals could be a result of interference with intracellular store refilling. In this respect, the fact that the transient slope of the calcium signal was reduced significantly argues strongly in favour of oestrogen reducing not only the amount but also the rate of calcium release. Likewise, although a possible stimulation of mitochondrial calcium sequestration induced by estradiol, as reported by Nilsen & Diaz-Brinton (2003) in hippocampal neurons to account for the E2-induced protection against glutamate toxicity, could explain the reduction of ACh-induced signals observed here, this notion is not compatible with the observations in GT1-7 cells that the basal fluorescence remained constant after exposure to estradiol and that the slope of the transients' falling phase in the presence of estradiol were not faster than those from their corresponding controls.
Even though the underlying signaling pathways are presently unknown, this effect does not appear to involve an estrogen-dependent modulation of the ACh-induced phosphoinositide hydrolysis previously described in GT1-7 cells (Krsmanovic et al., 1998), which in turn would alter Ca2+ buffering systems. The reason for this argument is that although an increase of inositol phosphate accumulation could be demonstrated in response to ACh in GT1-7 cells, no change was detected when cells were pre-exposed to estrogen in the range of concentrations used in the present study. This could indicate that the final target of the signaling pathway activated by estradiol at the plasma membrane of GT1-7 cells would be located downstream the muscarinic receptor cascade. In several types of excitable secretory cells, estradiol has been found to directly affect specific calcium currents (Mermelstein et al., 1996) or to modulate intracellular calcium oscillations through G-protein-coupled, cyclic nucleotide-dependent phosphorylation events by acting on nonclassical estrogen membrane receptors (Nadal et al., 2000; Ropero et al., 2002). In this respect, it has been recently reported that, in GT1-7 cells and hypothalamic neurons, estradiol modulates adenylate cyclase activity and cAMP accumulation through an ICI182 780-sensitive membrane receptor that seems to be related immunologically to estrogen receptor α (Navarro et al., 2003). Furthermore, in ovariectomized, estrogen receptor α-knockout mice, estradiol rapidly increased phosphorylation of cAMP response element-binding protein in LHRH neurons (Ábrahám et al., 2003). Therefore, even though the underlying signaling pathways are presently unknown, it may be suggested that estradiol might act rapidly on membrane receptors of GT1-7 cells and activate some second messenger cascade to reduce ACh-induced calcium signals, perhaps by affecting IP3R sensitivity at the endoplasmic reticulum.
The identification of membrane sites mediating rapid estrogen effects on LHRH-producing cells could help to resolve a conceptual conflict that has remained for decades. Even though estrogen is one of the main determinants of the LHRH neuronal system, a series of studies using immunohistochemistry and [3H]estradiol concentration in central LHRH neurons of several species indicated the absence of estrogen receptors within LHRH-secreting neurons (Shivers et al., 1983; Herbison et al., 1993; Sullivan et al., 1995). Therefore, it was thought that both the negative and positive feedback effects of estradiol on LHRH and LH secretion were indirect and exerted through estrogen-responsive interneurons (Herbison, 1998). In the past few years, however, the use of more sensitive analytical methods has allowed the identification of nuclear estrogen receptors in different populations of LHRH-containing neurons of the rodent and immortalized LHRH-producing cells (Butler et al., 1999; Hrabovszky et al., 2001; Martínez-Morales et al., 2001). Furthermore, these neurons also have the capacity to respond directly to estradiol in a pattern compatible with the activation of estrogen receptors coupled to genomic mechanisms (Shen et al., 1998; Martínez-Morales et al., 2001). However, the presence of classical estrogen receptors, and therefore the potential ability of estradiol to modulate gene expression in LHRH neurons, does not explain the acute effect of this hormone to inhibit LHRH and LH secretion during its negative feedback mode of action (Condon et al., 1988; Yamaji et al., 1972; Sarkar & Fink, 1980).
The results presented here are indicative of a rapid estrogen effect on the response of LHRH-producing cells to a classical neurotransmitter. Given the fast time-course of the estrogen effect and the ability of the impermeant conjugate E-HRP to mimic the effect of estrogen, it is probably a nongenomic effect initiated at the plasma membrane; also, this idea is strongly supported by the demonstration of specific oestrogen membrane binding sites. Moreover, the pharmacological profile of E-HRP binding is consistent with that of ACh-induced calcium signal modulation, which, in turn, is clearly different from those of the classical estrogen receptors α and β (Kuiper et al., 1997). This indicates that the binding sites for E2 at the plasma membrane of GT1-7 cells could well correspond to a proper estrogen membrane receptor as has been demonstrated for other cell types (Ropero et al., 1999, 2002; Nadal et al., 2000; Doolan & Harvey, 2003). Finally, the relatively low local E2 concentrations required suggests that these effects could be related to the estrogen negative feedback regulation of the hypothalamic–pituitary system. Pharmacological dissection of the different signaling pathways activated by E2 will give us additional clues to their potential modulatory mechanisms. This information is particularly relevant as it reinforces the possibility of a direct modulatory action of estrogen on LHRH-secreting neurons, in addition to those mediated by intracellular estrogen receptors. Moreover, it would provide a clue to explain the early observations for acute reduction of LHRH and LH secretion in different animal models following single E2 injections Yamaji et al., 1972; Sarkar & Fink, 1980; Condon et al., 1988). The ability of estradiol to rapidly modulate the physiology of LHRH neurons through interactions with specific receptor sites at the plasma membrane adds a new dimension to the mechanisms participating in the central control of mammalian reproduction.
Acknowledgements
Supported by grants 1FD97-1065-C03, SAF2001-3614-C03-01/02, and BFI2002-01469 from Ministerio de Ciencia y Tecnologia (Spain), Astra-Zeneca, and Lilly. We are grateful to Raquel Marín for her excellent help in the confocal imaging experiments and to Juan Ramón Martínez for his technical support in phosphoinositide assays. We also wish to thank Miguel Brioso and Ricardo Borges who kindly provided us with their IGOR-based macros for calcium signal analyses.
Abbreviations
-
- ACh
-
- acetylcholine
-
- BSA
-
- bovine serum albumin
-
- DAB
-
- 3,3′-diaminobenzidine tetrahydrochloride
-
- E2
-
- 17β-estradiol
-
- E-BSA-FITC
-
-
- estradiol-BSA-fluorescein
-
- isothiocyanate
-
- FBS
-
- fetal bovine serum
-
- HRP
-
- horseradish peroxidase
-
- LHRH
-
- luteinizing hormone-releasing hormone
-
- PBS
-
- phosphate-buffered saline
-
- PFA
-
- paraformaldehyde.