α2-γ-Aminobutyric acid (GABA)A receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo
Abstract
Classical benzodiazepines such as diazepam are widely used tranquillisers and hypnotics in various neuropsychiatric diseases including alcohol-related disorders. One of the major drawbacks of benzodiazepine therapy, however, is an exacerbation of the sedative and hypnotic effects associated with alcohol intake, even at low doses. Even though the γ-aminobutyric acid (GABA)A receptor complex is a common target for the actions of both classes of drugs, the molecular mechanisms underlying the enhanced pharmacological properties of the combined use of benzodiazepines and alcohol remain to be identified. The present experiments aimed at clarifying which of the GABAA receptor subtypes mediate the augmented hypnotic-like and sedative effects of combined diazepam and alcohol using the righting reflex and motor activity assays, respectively, in histidine-to-arginine point mutated mice that possess diazepam-insensitive α1-, α2-, α3- or α5-GABAA receptors. The combination of diazepam (2 or 3 mg/kg) and ethanol (3 g/kg) induced loss of righting reflex with a significantly dose-dependent increase of the latency to its full recovery in wild-type, α1(H101R), α3(H126R) and α5(H105R) but not in α2(H101R) mice. A combined treatment with diazepam (1 mg/kg) and ethanol (2.5 g/kg) precipitated motor inhibition similarly in wild-type and α2(H101R) mice. Responsiveness of the α2(H101R) mice to ethanol alone was similar to that of wild-type mice. These results demonstrate that induction of loss of righting reflex by combined diazepam and alcohol is closely dependent on the activation of the α2-GABAA receptors by the benzodiazepine whereas precipitation of sedation involves GABAA receptors other than the α2-GABAA receptors.
Introduction
In clinical practice benzodiazepine full agonists such as diazepam, which are widely used in treatment for transient or chronic states of anxiety and insomnia, are often prescribed without adequate information about the patient's alcohol use history (Graham et al., 1992). Diazepam taken alone at therapeutic doses is relatively safe. Its association with alcohol intake, however, results in a marked augmentation of the sedative and hypnotic effects that can give rise to intoxication, and even fatal poisoning (Chan, 1984; Koski et al., 2002). The molecular and cellular processes that underlie the augmented behavioural sensitivity to the combined use of diazepam and alcohol remain elusive. The interaction between benzodiazepines and ethanol is generally additive rather than synergistic (Chan, 1984; Van Steveninck et al., 1996), thus suggesting the existence of common mechanisms that could mediate the actions of the two drugs in the brain.
At the molecular level, diazepam exerts its effects by modulating allosterically the activity of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) at GABAA receptors containing the α1-, α2-, α3-, or α5-subunit. The sensitivity of GABAA receptors to diazepam is conferred by the presence of a histidine residue at a conserved position in the extracellular portions of the α subunits (α1-H101, α2-H101, α3-H126, α5-H105). Two other GABAA receptor subtypes containing the α4- and α6-subunit, respectively, are insensitive to diazepam because of an arginine residue in the corresponding position. Electrophysiological studies on recombinant receptors have shown that the α1-, α2-, α3- and α5-GABAA receptors can be rendered diazepam-insensitive by replacing this histidine by arginine without altering the GABA sensitivity (Benson et al., 1998). Recently, histidine-to-arginine point mutations have been introduced into the germ line of mice by gene targeting. Four point-mutated mouse lines, which possess diazepam-insensitive α1-, α2-, α3-, or α5-GABAA receptors, respectively, were generated (Rudolph et al., 1999; Löw et al., 2000; McKernan et al., 2000; Crestani et al., 2002b). The analysis of these point-mutated mice provided a great advance in the understanding of the contribution of the respective α1-, α2-, α3- and α5-GABAA receptors in mediating the main pharmacological actions of diazepam and other benzodiazepine site ligands (Rudolph et al., 1999; Crestani et al., 2000, 2002a, b; Löw et al., 2000; McKernan et al., 2000). The GABAA receptors also appear to be important targets for some behavioural effects of ethanol, even though its mechanism of action is more complex involving various neuronal signalling systems (for review see Crews et al., 1996; Grobin et al., 1998; Korpi et al., 1998). The examination of various mutant animal models indicates that the sedative and hypnotic effects of ethanol could result from an enhancement of the GABAA receptor function likely to be through changes in protein kinase activity (Harris et al., 1995; Hodge et al., 1999; Wand et al., 2001; Blednov et al., 2003; Proctor et al., 2003).
To clarify the respective contribution of the α1-, α2-, α3- and α5-GABAA receptor subtypes in mediating precipitation of narcosis and sedation by combined diazepam and alcohol, we examined the effects of diazepam pretreatment on the righting reflex and spontaneous motor activity, respectively, in histidine-to-arginine point-mutated mice following administration of subeffective doses of ethanol. We further studied the behavioural effects of ethanol alone in these mice.
Materials and methods
Animals
The generation of α1(H101R), α2(H101R) and α3(H126R) mice has been described previously (Rudolph et al., 1999; Löw et al., 2000). Briefly, starting from the chimeras, the α1(H101R) mice were crossed first to EIIa-cre mice (third back-cross to 129/SvEv), twice more to 129/SvEv mice and then for 13 generations to 129/SvJ mice; the α2(H101R) and α3(H126R) mice were crossed to EIIa-cre mice (on the 129/SvJ background) and then bred on the 129/SvJ background for 10 (α3(H126R) mice) to 13 (α2(H101R) mice) generations. The α5(H105R) mice were generated from embryonic 129/SvJ stem cells and maintained on this background (Crestani et al., 2002b). All the animals were produced from 20 breeding pairs of homozygous mutants and wild-type mice. Animals from the four wild-type mouse lines were pooled in a single ‘Wild-type’ group and were assumed to represent suitable controls for each of the four point-mutated strains. This appears to be justified because all mutations have been bred with the same pool of 129/SvJ mice. Only male animals were used in this study. Mice were reared in group-housed cages (8–10 mice per cage) in the test room under normal 12 h day–night cycle conditions (light on at 08.00 h). The behavioural tests were performed between 09.00 h and 13.00 h. Food and water were provided ad libitum. At the time of testing, the mice were 7–9-weeks old. Each animal was used only once. All experiments were approved by the Cantonal Veterinary Office in Zürich.
Drugs
Diazepam (gift from F. Hoffmann–La Roche, Basel, Switzerland) was suspended in a 0.3% Tween 80/saline solution, which served as the vehicle. The drug was administered per os in a volume of 4–5 mL/kg body weight. Absolute ethanol was diluted to a 15% solution in distilled water according to the Gay-Lussac table and administered intraperitoneally in doses adjusted by the volumes of injection in proportion to the body weight.
Behavioural procedures
The hypnotic-like properties of combined diazepam + alcohol treatment were evaluated using the righting reflex assay. Mice were first treated with either vehicle or a dose of diazepam (2 or 3 mg/kg) that produces mild sedation only. Thirty minutes later, they were injected with 3 g/kg alcohol. A few seconds later, the mice were gently turned in the supine position and those that were not able to right themselves immediately were considered as having lost the righting reflex. A heat lamp was placed above the mice in order to compensate for hypothermia. The latency to fully recover the reflex after three successive trials was measured with a maximum observation period of 60 min. The sedative action of diazepam + alcohol treatment was assessed by the amount of motor activity recorded for 15 min in individual automated circular arena equipped with four equidistant photocells (Imetronic, Pessac, France) immediately after injection of 2.5 g/kg ethanol in mice pretreated 30 min earlier with vehicle or 1 mg/kg diazepam.
The effects of different doses (2.5–5 g/kg) of alcohol on motor activity and righting reflex were evaluated in similar conditions.
Data analysis
The effects of combined (2 mg/kg diazepam + 3 g/kg ethanol) and (3 mg/kg diazepam + 3 g/kg ethanol) treatments on the righting reflex latencies in the five genotypes were analysed separately using Kruskall–Wallis analysis followed by Mann–Whitney tests whenever appropriate. A latency score of zero was attributed to mice that did not lose the righting reflex after three trials in order to include them in the analysis. Motor activity data were subjected to decimal logarithmic transformation because of the significance of the Levene's test for homogeneity of variances and analysed using two- or three-way analysis of variance (anova) followed by Scheffe's tests for post hoc mean comparisons.
Results
Experiment 1: Effects of combined diazepam and alcohol on the righting reflex in wild type and point-mutated mice
Figure 1 shows a scattergram representation of the effects of combined subeffective doses of diazepam and ethanol on the righting reflex latency in wild-type and histidine-to-arginine point-mutated mice. Alcohol at the dose of 3 g/kg did not affect the righting reflex in mice pretreated with the vehicle. The combination of 2 mg/kg diazepam with the same dose of alcohol induced loss of righting reflex in 29% of wild-type and α3(H126R) mice, 86% of α1(H101R) mice and 100% of α5(H105R) mice (n = 7 per group). None of the α2(H101R) mice (n = 7) treated with 2 mg/kg diazepam lost the righting reflex following alcohol administration. A Kruskall–Wallis analysis revealed genotype differences on the effect of the combination of 2 mg/kg diazepam with 3 g/kg alcohol on the righting reflex latency (H = 13.812, P < 0.007). As in wild-type mice, the median righting reflex latency was zero in α2(H101R) and α3(H126R) mice. The α5(H105R) mice showed a mean duration of loss of righting reflex similar to that of wild type mice whereas the α1(H101R) mice tended to recover the righting reflex less rapidly (P < 0.07 vs. Wild-type, Mann–Whitney test).
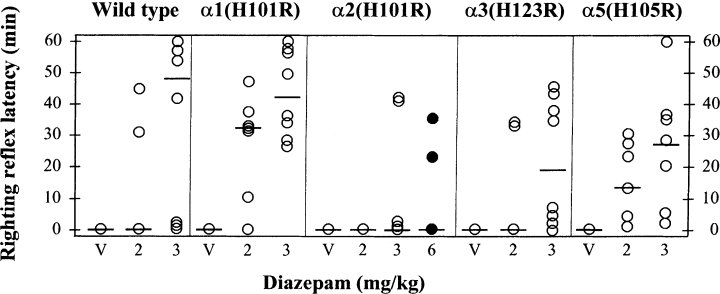
Scattergram representation of the effects of combined diazepam and ethanol on the righting reflex latency in wild type and histidine-to-arginine point-mutated mice. Mice received an intraperitoneal injection of 3 g/kg 15% ethanol solution, 30 min after having been treated with either vehicle or diazepam (2 or 3 mg/kg per os; 6 mg/kg only in the α2(H101R) mice). n = 7–10 mice per group. The solid lines represent the median righting reflex latency values. Note the absence of a substantial effect of a pretreatment with 6 mg/kg diazepam (black circles) on the righting reflex following ethanol administration in α2(H101R) mice. V, vehicle.
A pretreatment with diazepam at the dose of 3 mg/kg resulted in a large proportion of animals having lost the righting reflex following the injection of 3 g/kg alcohol in wild-type (80%, n = 10), α1(H101R) (100%, n = 8), α2(H101R) (50%, n = 10), α3(H126R) (88%, n = 8) and α5(H105R) mice (100%, n = 7). This treatment combination was associated with a similarly prolonged latency to regain the reflex in wild-type, α1(H101R), α3(H126R) and α5(H105R) mice but not in α2(H101R) mice (H = 11.100, P < 0.025). The median righting reflex latency of the α2(H101R) group was zero (P < 0.05 vs. Wild-type). Even though 50% of α2(H101R) mice pretreated with 3 mg/kg diazepam were considered as having lost the righting reflex, 30% of them regained the reflex within 50–120 s following ethanol injection. Even the combination of a pretreatment with diazepam, at an increased dose of 6 mg/kg, which did not induce loss of righting reflex at least during the 30 min preceding ethanol injection, with ethanol failed to affect appreciably the righting reflex in α2(H101R) mice. Indeed, only two out of five animals lost the righting reflex under this combined treatment and again the median latency to recovery was zero (Fig. 1).
Experiment 2: Effects of diazepam + alcohol combination on motor activity in wild-type and α2(H101R) mice
Figure 2 shows the influence of a pretreatment with diazepam (1 mg/kg) on the effects of 2.5 g/kg ethanol on motor activity in wild-type and α2(H101R) mice. A three-way anova with genotype, diazepam and ethanol treatments as the three main factors revealed no significant effect of the genotype (F1,43 = 0.217), ethanol × treatment (F1,43 = 2.512), genotype–ethanol treatment interaction (F1,43 = 0.454), genotype–diazepam interaction (F1,43 = 0.029) and genotype–diazepam–ethanol interaction (F1,43 = 0.002) on the amount of motor activity. However, a significant overall effect of diazepam (F1,43 = 30.309, P < 0.001) and diazepam–ethanol interaction (F1,43 = 7.688, P < 0.001) were observed. Because the genotype factor was not significant, a two-way anova, with diazepam and ethanol treatments as main factors, was further performed and confirmed no overall effect of ethanol treatment (F1,47 = 2.562, not significant) but significant overall effect of diazepam treatment (F1,47 = 32.729, P < 0.001) and diazepam–ethanol interaction (F1,47 = 8.409, P < 0.005). Post hoc mean comparisons showed that ethanol given at the dose of 2.5 g/kg 30 min after a vehicle injection did not affect motor activity as compared to vehicle + water combination in mice of both genotypes. The combination of diazepam at the dose of 1 mg/kg with water induced a slight decrease in the mean amount of motor activity, which did not reach significance as compared with vehicle + water treatment. In contrast, a significant motor depressant effect was observed when the same diazepam pretreatment was followed by 2.5 g/kg ethanol (P < 0.001 vs. diazepam + water and P < 0.05 vs. vehicle + ethanol, Scheffe's tests).
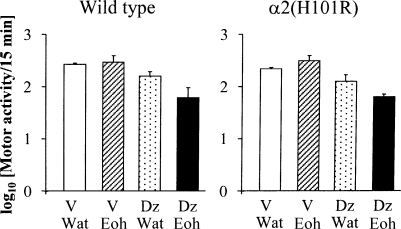
Effects of combined vehicle + water, vehicle + ethanol, diazepam + water or diazepam + ethanol administration on motor activity in wild-type and α2(H101R) mice. Mice were first treated with vehicle or diazepam (1 mg/kg per os). Thirty minutes after, they received an intraperitoneal injection of either water or 2.5 g/kg 15% ethanol solution. They were then placed in individual circular arenas for 15 min. Results are expressed as mean amount of motor activity (log10 ×) ± SEM. n = 5–9 per group. V, vehicle; Wat, water; Eoh, ethanol; Dz, diazepam.
Experiment 3: Effects of alcohol alone on righting reflex and motor activity in wild-type and α2(H101R) mice
Ethanol administered at the doses of 4.5 or 5 g/kg induced loss of righting reflex in all animals and the mean latencies to its full recovery were very similar in wild-type and α2(H101R) mice (Fig. 3A). When administered at the doses of 2.5 and 3 g/kg, alcohol did not affect the mean amount of motor activity as compared to water treatment in both wild-type and α2(H101R) mice (F2,36 = 0.82, not significant; Fig. 3B).
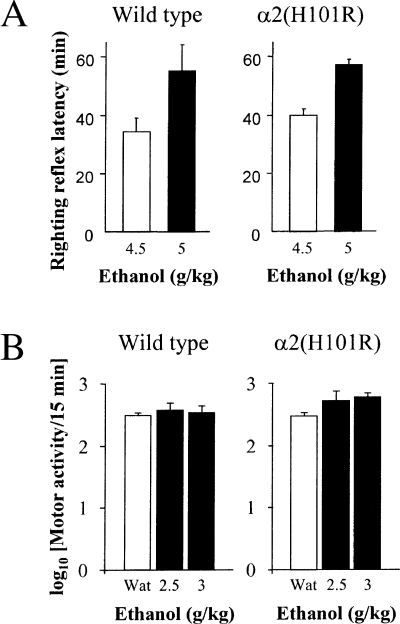
Dose-effects of ethanol on the righting reflex and motor activity in wild-type and α2(H101R) mice. (A) Mean righting reflex latency (min ± SEM) in mice treated intraperitoneally with 4.5 or 5 g/kg 15% ethanol solution; n = 8–14 per group. (B) Mean amount of motor activity (log10 ×) ± SEM) in mice placed in individual circular arena for 15 min immediately after an intraperitoneal injection of water or 2.5 g/kg or 3 g/kg 15% ethanol solution. n = 7 per group. Wat, water.
Discussion
Forensic toxicological studies have underscored the danger of intoxication, fatal poisoning and traffic accidents associated with the occasional use of classical benzodiazepines in patients with alcoholism as well as of alcohol consumption in patients under benzodiazepine therapy. In both humans and animals, diazepam shares common pharmacological properties with alcohol, notably muscle relaxation, sedation and hypnosis. These behavioural effects can be potentiated whenever diazepam is taken, even at therapeutic doses, in combination with moderate alcohol drinking. Even though it has been shown that ethanol elevates plasma and brain diazepam levels (Chan, 1984), the molecular and neural bases of this pharmacological potentiation are unknown. At the molecular level, the GABAA receptor complex is a common target of diazepam and ethanol (Korpi et al., 2002). Diazepam exerts its effects specifically by binding with a similar affinity to the GABAA receptor subtypes containing the α1-, α2-, α3-, or α5-subunit. The interaction of ethanol with GABAA receptors is more complex, likely depending on both subunit composition and post-translational processing (for review see Weiner et al., 1997; Harris, 1999). The present study proposed to clarify the respective contribution of the α1-, α2-, α3- and α5- GABAA receptors in mediating the augmented behavioural action of combined diazepam and ethanol. The recent analysis of histidine-to-arginine point-mutated mice, which have the peculiarity to possess diazepam-insensitive receptors, has permitted the attribution of specific functions to these receptor subtypes with regards to the main pharmacology of diazepam and other benzodiazepine site ligands in vivo (Rudolph et al., 1999; Crestani et al., 2001, 2002a, b; Löw et al., 2000; McKernan et al., 2000). The α1-GABAA receptors have been shown to mediate the sedative and anticonvulsant activity of both diazepam and the sedative/hypnotic drug zolpidem (Crestani et al., 2000). The anxiolytic-like action of diazepam depends selectively on the activation of the α2-GABAA receptors (Löw et al., 2000) whereas its muscle relaxant properties involve, together with the α2-GABAA receptors, the α3- and α5-GABAA-receptor subtypes (Crestani et al., 2001, 2002b).
The α2-GABAA receptor subtype as a specific target for the hypnotic-like action of combined subeffective doses of diazepam and ethanol
To identify the diazepam-sensitive GABAA receptors that mediate the hypnotic action of the combination of a diazepam pretreatment with a subeffective dose of ethanol, we used the righting reflex assay in histidine-to-arginine point-mutated mice. Two drug combinations were studied based on the responsiveness of wild-type mice: a subeffective combination (2 mg/kg diazepam + 3 g/kg ethanol), which induced loss of righting reflex in 30% of wild-type mice and an effective combination (3 mg/kg diazepam + 3 g/kg ethanol), which affected 90% of the animals. The presence or absence of the expected impairing effect of diazepam pretreatment on the righting reflex following ethanol in one of the four point-mutated mouse lines would allow us to exclude or imply the corresponding GABAA-receptor subtype in mediating the benzodiazepine response. Neither diazepam given alone at doses of 2 or 3 mg/kg or up to 6 mg/kg [in α2(H101R) mice only] nor the combination of 3 g/kg ethanol with the vehicle overtly affected the righting reflex in any of the mutant animals. The combined administration of diazepam and ethanol impaired the reflex in α1(H101R), α3(H126R) and α5(H105R) mice to the same extent as in wild type mice. In contrast, in α2(H101R) mice, a pretreatment with diazepam, at least up to 6 mg/kg, followed by a subeffective dose of ethanol failed to impair appreciably the righting reflex in a significant proportion of animals. These results strongly suggest a unique contribution of the α2-GABAA receptor subtype in mediating the potentiating action of diazepam, when combined with ethanol, on the righting reflex.
However, the possibility that the histidine-to-arginine substitution in the α2-subunit gene has modified the sensitivity of α2-GABAA receptors to ethanol cannot be ruled out. Consequently, the failure of α2(H101R) mice to show a substantial righting reflex impairment in response to diazepam + ethanol combinations might be either because of a diminished responsiveness to the behavioural effects of ethanol or the manifestation of acute functional tolerance to ethanol (Erwin et al., 2000). Unfortunately, blood ethanol concentrations, as a measure of ethanol initial sensitivity, could not be determined consistently at the time of loss of the righting reflex because of either the absence or the presence of a very short-lasting impairing effect of the combined drug treatments in the majority of the α2(H101R) mice (Gill & Deitrich, 1998). Indeed, of all α2(H101R) mice (n = 22) pretreated with diazepam (2–6 mg/kg), only four lost the righting reflex for more than 2 min following ethanol injection. Nevertheless, we assessed the capacity of these mutants to lose the righting reflex in response to different higher concentrations of ethanol given alone. As a result, all α2(H101R) mice displayed a dose-dependent and long-lasting loss of righting reflex with a mean duration of the effect very similar to that seen in wild-type mice. This finding indicates that the responsiveness to acute hypnotic doses of ethanol is retained in α2(H101R) mice. Likewise, the development of acute tolerance to the hypnotic effect of ethanol as a possible explanation for the resistance of the α2(H101R) mice to the effect of the combined diazepam + ethanol treatment on the righting reflex is unlikely as, at the dose (3 g/kg) used to assess the potentiating action of diazepam, we showed that ethanol neither induced loss of righting reflex nor impaired motor activity in any of the wild-type and mutant mice. Thus, the resistance of α2(H101R) mice to the effects of diazepam pretreatment on the righting reflex following a subeffective dose of ethanol most likely results from the insensitivity of the point-mutated α2-GABAA receptors to diazepam rather than to ethanol.
The righting reflex ability depends on several reticulo-spinal and vestibulo-spinal functions, including postural support, muscle strength and tone, limb coordination and vestibular input, which are organized in the spinal cord, medulla and mesencephalon in rodents (Bignall, 1974). Consequently, both induction and duration of loss of righting reflex might be closely dependent on the ability of the drug to impair all or part of these functions. As shown earlier (Crestani et al., 2001), the α2(H101R) mice do not show diazepam-induced muscle relaxation as tested on the grasping reflex. This could therefore explain the inability of diazepam + ethanol treatment to significantly impair the righting reflex in these mutants. This explanation, however, is not sufficient because the same diazepam + ethanol combination induced a dose-dependent and long-lasting loss of righting reflex in the α5(H105R) mice, which are as resistant as α2(H101R) mice to the muscle relaxant action of diazepam at doses up to 10 mg/kg (Crestani et al., 2002b). Recently, an early impairment of the righting reflex in the Wobbler mouse, an animal model of motoneuron disease, has been associated with a diffuse pattern of degeneration in notably brainstem motor cranial nerve nuclei, including facial and hypoglossal nuclei, vestibular, spinal and cerebellar nuclei (Bose et al., 1998). Even though all these structures could be involved in righting execution, it is interesting to note that only the two motor cranial nuclei as well as spinal motor neurons are particularly enriched in α2-subunit in comparison to other GABAA receptor α-subunits (Fritschy & Mohler, 1995; Bohlhalter et al., 1996).
Furthermore, under high doses of diazepam or ethanol, or the combination of subeffective doses of both drugs, induction of loss of righting reflex occurs very rapidly and it is prolonged by an artificial sleep state, close to narcosis. It is tempting to propose that the α2-GABAA receptors, which are predominantly expressed in corticolimbic and striatal regions, may mediate the hypnotic-like combined action of the two drugs.
The α2-GABAA-receptor subtype is not the substrate of the augmented sedative action of the combined use of diazepam and ethanol
To test the specificity of the role of the α2-GABAA receptors in mediating the combined action of diazepam and ethanol on the righting reflex, we further studied the property of diazepam + ethanol treatment to precipitate sedation (as defined by a decrease in motor activity) in α2(H101R) mice. The present findings clearly show that the α2(H101R) mice appeared to experience augmented motor depression to the same extent as wild-type mice in response to a combined diazepam + alcohol treatment. This potentiated sedative effect could not be attributed to a simple summation of the sedative properties of the two drugs as both diazepam and ethanol, when given separately at the same doses, did not alter significantly motor activity baselines in the two groups. These results demonstrate that precipitation of sedation by the combined use of diazepam and ethanol is not dependent on the activation of GABAA receptors containing the α2-subunit. The α1-GABAA receptor subtype, which has been shown to mediate the sedative properties of diazepam but not its hypnotic effect, might be a potential candidate (Rudolph et al., 1999; Tobler et al., 2001). Further investigation in the point-mutated mice should help to clarify the molecular substrates involved in this combined drug effect.
sloss Altogether, these results indicate that induction of narcosis, as assessed by the loss of righting reflex, and precipitation of sedation by the combined use of diazepam and ethanol are two distinct behavioural outputs with regards to the neuronal and molecular substrates involved. Induction of loss of righting reflex by the combination of subeffective doses of diazepam and alcohol depends on the interaction of the benzodiazepine with the α2-GABAA receptors whereas precipitation of sedation involves GABAA receptors other than those containing the α2-subunit. Consistent with our findings, the anxioselective SL651498, a pyridoindole derivative, which behaves as a full agonist at recombinant receptors containing the α2- and α3-subunits, and as a partial agonist on the receptors containing the α1- or α5-subunit, has been reported to appreciably potentiate the muscle relaxant action of ethanol in mice (Griebel et al., 2001). Conversely, zolpidem, which displays a highly selective affinity for α1-GABAA receptors, appears to be less potent than diazepam in impairing the righting reflex when associated with alcohol and the drug interaction is additive (Rush, 1998). In keeping with the major contribution of the α2-GABAA receptors in diazepam-induced anxiolysis and muscle relaxation, the present findings could have implications for the search and development of novel therapeutics with a minimal alcohol interaction profile in treatments for anxiety and insomnia.
Acknowledgements
The authors gratefully acknowledge Karin Löw for the generation of the α2(H101R), and Ruth Keist for the generation and maintenance of the α3(H126R) and α5(H105R) mice and Hanns Mohler for his helpful comments.
Abbreviations
-
- anova
-
- analysis of variance
-
- GABA
-
- γ-aminobutyric acid.