Direct evidence for the up-regulation of spinal µ-opioid receptor function after repeated stimulation of κ-opioid receptors in the mouse
Abstract
The present study was designed to investigate the possible change in spinal µ-opioid receptor function after repeated administration of a selective κ-opioid receptor agonist (1S-trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl]-benzeneacetamide hydrochloride [(−)U-50,488H] in the ICR mouse. A single s.c. or i.t. injection of (−)U-50,488H produced a dose-dependent antinociception. Repeated s.c. or i.t. administration of (−)U-50,488H resulted in the development of tolerance to (−)U-50,488H-induced antinociception. Under these conditions, we demonstrated here that repeated s.c. injection of (−)U-50,488H significantly enhanced the antinociceptive effect induced by the i.t. administration of a selective µ-opioid receptor agonist [d-Ala2,N-Me-Phe4,Gly5-ol] enkephalin (DAMGO). Using the guanosine-5′-o-(3-[35S]thio) triphosphate ([35S]GTPγS) binding assay, we found that (−)U-50,488H was able to produce a dose-dependent increase in [35S]GTPγS binding to membranes of the mouse spinal cord. Repeated administration of (−)U-50,488H caused a significant reduction in the (−)U-50,488H-stimulated [35S]GTPγS binding in this region, whereas repeated treatment with (−)U-50,488H exhibited an increase in the DAMGO-stimulated [35S]GTPγS binding in membranes of the spinal cord. Using a receptor binding assay, repeated treatment with (−)U-50,488H significantly increased the density of [3H]DAMGO binding sites in membranes of the mouse spinal cord. In contrast, the expression of µ-opioid receptor was not affected after repeated treatment with (−)U-50,488H. These results suggest that repeated stimulation of κ-opioid receptors leads to the up-regulation of µ-opioid receptor functions in the spinal cord, which may be associated with an increase in the number of functional µ-opioid receptors in the mouse spinal cord.
Introduction
The concept of existence of multiple opioid receptors is well established. Three different receptor types, µ-, δ- and κ-opioid receptors have been cloned and shown to belong to the G-protein-coupled receptors (GPCRs) family (Evans et al., 1992; Kieffer et al., 1992; Chen et al., 1993; Fukuda et al., 1993; Meng et al., 1993; Wang et al., 1993; Yasuda et al., 1993). Activation of these receptors by agonists affects various physiological functions, including pain perception, locomotion, motivation, rewarding effect, immunomodulation, neuroendocrine secretion and regulation of intestinal motility (Pasternak, 1988; Mansour et al., 1988; Cahill et al., 2001).
The κ-opioid receptor participates in the control of chemical, mechanical and thermal pain (von Voightlander et al., 1983; Bhargava et al., 1989), mainly at the spinal level (Millan, 1990). Numerous molecular studies have reported that the stimulation of κ-opioid receptors by agonist, such as (1S-trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl]-benzene acetamide hydrochloride [(−)U-50,488H] enhances the G-protein-coupled receptor kinases (GRK)-dependent phosphorylation of the receptor (Appleyard et al., 1999; Li et al., 1999). Subsequent binding of arrestin-2 to phosphorylated receptor, in turn, initiates the internalization process by binding to clathrin and the receptor-arrestin-2 complex is then sequestered in clathrin-coated pits (Claing et al., 2002). By the action of dynamin, the clathrin-coated pits are pinched off to become clathrin-coated vesicles. After prolonged U-50,488H treatment, the receptors are trafficked to lysosomes for degradation (Li et al., 2000; von Zastrow, 2001; Tanowitz & von Zastrow, 2002), resulting in the down-regulation of κ-opioid receptor functions (Bhargava et al., 1989; Narita et al., 2003).
Although κ-opioid receptor can transduce its effect independently, a growing body of evidence has been accumulating for the existence of a cellular or molecular interaction between κ-opioid receptor and other opioid receptor types. It is of interest to note that coadministration of U-50,488H with morphine completely suppressed the development of tolerance to morphine-induced antinociception (Narita et al., 1993). In our recent study, we found that repeated s.c. administration with (−)U-50,488H produced an enhancement of µ-opioid receptor functions in the mouse thalamus and periaqueductal grey (Narita et al., 2003).
In the present study, we focused on the interaction between κ- and µ-opioid receptors in the mouse spinal cord. Here we report a substantial enhancement of spinal µ-opioid receptor function after repeated treatment with (−)U-50,488H in the mouse.
Materials and methods
The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals Hoshi University, as adopted by the Committee on Animal Research of Hoshi University. Every effort was made to minimize the numbers of animal used in the following experiments.
Animals
Male ICR mice were obtained from Tokyo Laboratory Animals Science Co. Ltd, Tokyo, Japan, weighing 20–25 g at the beginning of experiments. Animals were housed in groups of eight in a temperature-controlled room. They were maintained on a 12-h light : 12-h -dark cycle (light on 08:00 am to 20:00 pm) and were allowed to adapt to this environment for a period of 1 week before the experiments. Food and water were available ad libitum.
Intrathecal injection
Intrathecal (i.t.) administration was performed as described previously (Hylden & Wilcox, 1980). Briefly, the injection was made with a 30-gauge needle (Becton Dickinson and Company, Franklin Lakes, NJ) attached to a 25-µL Hamilton microsyringe (Hamilton Company, Reno, NV). Solution was injected in a volume of 4 µL per mouse.
Assessment of antinociceptive response
Mice were repeatedly s.c. or i.t. injected with (−)U-50,488H or saline once a day for seven consecutive days. Two hours after the last injection, all of mice were treated with agonist for the antinociceptive assay. The antinociceptive response was evaluated by the tail-flick test (Tail Flick Analgesia Meter Model MK 300B, Muromachi Kikai Co. Ltd, Tokyo, Japan). To prevent tissue damage, we established a 10-s cut off time. The tail-flick latency was measured both before and after the challenge with agonist. Antinociceptive response was calculated as a percentage of maximum possible effect (percentage of antinociception) according to the following formula: percentage antinociception = 100 × (test latency − predrug latency)/(cut off time − predrug latency).
Guanosine-5′-o-(3-[35S]thio)triphosphate ([35S]GTPγS) binding assay
Mice were repeatedly injected s.c. with (−)U-50,488H at 20 mg/kg or saline once a day for seven consecutive days. The animals were decapitated with a sharp guillotine 2 h after the last injection. For the membrane preparation, the spinal cords were rapidly excised at 4 °C. The tissue was homogenized using a Potter-Elvejham tissue grinder with a Teflon pestle in 20 volumes (w/v) of ice-cold Tris-HCl buffer containing 50 mm Tris-HCl (pH 7.4), 5 mm MgCl2 and 1 mm EGTA. The homogenate was centrifuged at 4 °C for 10 min at 48 000 × g. The pellet was resuspended in [35S]GTPγS binding assay buffer containing 50 mm Tris-HCl (pH 7.4), 5 mm MgCl2, 1 mm EGTA and 100 mm NaCl and centrifuged at 4 °C for 10 min at 48 000 × g. The final pellet was resuspended in [35S]GTPγS binding assay buffer and stored at −70 °C until use.
The membrane homogenate (3–8 µg protein/assay) was incubated at 25 °C for 2 h in 1 mL of assay buffer with various concentrations of the agonist, 30 µm guanosine-5′-diphosphate (GDP) and 50 pm[35S]GTPγS (specific activity, 1000 Ci/mmol; Amersham, Arlington Heights, IL). The reaction was terminated by the filtration using a Brandel cell harvester (Model M-24, Brandel, MD) and Whatman GF/B glass filters presoaked in 50 mm Tris-HCl (pH 7.4) and 5 mm MgCl2 at 4 °C for 2 h. Filters were then washed three times with 5 mL of an ice-cold Tris-HCl buffer (pH 7.4), transferred to scintillation-counting vials containing 0.5 mL of a tissue solubilizer (Soluene-350, Packard Instrument Company, Meriden, CT) and 4 mL of a scintillation cocktail (Hionic Fluor, Packard Instrument Company), equilibrated for 12 h and the radioactivity in the samples determined with a liquid scintillation analyser. Non-specific binding was measured in the presence of 10 µm unlabelled GTPγS. The binding of [35S]GTPγS to the membrane was calculated as a percentage of basal [35S]GTPγS binding measured in the presence of GDP and absence of agonist. The data are expressed as the mean ± SEM for percentage stimulation. Similar results were obtained from at least three independent sets of experiments.
The µ-opioid receptor binding assay
The membrane homogenate (130–170 µg protein/assay) was incubated at 25 °C for 2 h in 50 mm Tris-HCl buffer (pH 7.4) with 0.2–20 nm[tyrosyl-3,5–3H(N)]-DAMGO (specific activity, 55.3 Ci/mmol; NEN, Boston, MA) in a total volume of 1 mL. The reaction was terminated using a Brandel cell harvester (Model M-24, Brandel, MD) and filtration through Whatman GF/B glass filters presoaked in 50 mm Tris-HCl (pH 7.4), 0.01% (w/v) polyethylenimine at 4 °C for 2 h. Filter were washed three times with 5 mL of Tris-HCl buffer (pH 7.4, 4 °C), transferred to scintillation counting vials containing 0.5 mL of tissue solubilizer (Soluene-350, Packard Instrument Company) and 4 mL of a scintillation cocktail (Hionic Fluor, Packard Instrument Company). After 12 h, the radioactivity in the samples was determined with a liquid scintillation analyser (Model 1600CA, Packard Instrument Company). The values from Scatchard plot analyses represent the mean ± SEM of three independent experiments.
RNA preparation and quantitative analysis by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA in the spinal cord was extracted using SV Total RNA Isolation system (Promega, Madison, WI) following the manufacturer's instructions. The purified total RNA was quantified spectrophotometrically at A260. To prepare the first strand of cDNA, 1 µg of RNA was incubated in 100 µL of buffer containing 10 mm dithiothreitol, 2.5 mm MgCl2, dNTP mixture, 200 units of reverse transcriptase II (Life Technologies, Inc.) and 0.1 mm oligo(dT)12−18 (Life Technologies, Inc.). The µ-opioid receptor gene was amplified in a 50 µL PCR solution containing 0.8 mm MgCl2, dNTP mixture and DNA polymerase with synthesized primers: a sense primer of µ-opioid receptor, which is at position 299–320 (5′-AGACTGCCACCAACATCTACAT-3′) of the receptor and an antisense primer at position 623–643 (5′-TGGACCCCTGCCTGTATTTTG-3′), which was designed according to GenBankTM sequence accession number U26915. Samples were heated to 95 °C for 2 min, 55 °C for 2 min, and 72 °C for 3 min and cycled 35 times through 95 °C for 1 min 55 °C for 2 min and 72 °C for 3 min. The final incubation was 72 °C for 7 min. The resulting 344-base pair PCR product amplified with the above primers was subcloned into pGEM-T vector (Invitrogen Corp., San Diego, CA) by the T–A cloning method. The DNA sequencing for the inserted region confirmed that the amplified nucleotides corresponded to those of murine µ-opioid receptor cDNA (Fukuda et al., 1993). The mixture was run on 1% agarose gel electrophoresis with the indicated markers and primers for the internal standard glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The agarose gel was stained with ethidium bromide and photographed with UV trans-illumination. The intensity of the bands was analysed and semiquantified by computer-assisted densitometry using the NIH image. The values represent the mean ± SEM of three independent experiments.
Drugs
(1S-trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzene acetamide hydrochloride [(−)U-50,488H] and [d-Ala2,N-Me-Phe4,Gly5-ol] enkephalin (DAMGO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). β-Funaltrexamine hydrochloride (β-FNA) was obtained from Tocris Cookson Ltd. (Ballwin, MO). All drugs were dissolved in saline for in vivo experiments or assay buffer for in vitro experiments.
Statistical analysis
Antinociceptive response represents as the mean ± SEM of percentage antinociception. The ED50 value was calculated by GraphPad Prism Programs version 3.0 (GraphPad Software Inc., CA). The statistical significance of differences between the groups was assessed with a two-way anova followed by the Bonferroni–Dunn test.
Results
Development of tolerance to (−)U-50,488H-induced antinociception in mice
Groups of mice were repeatedly injected s.c. (Fig. 1A) or i.t. (Fig. 1B) with saline or different doses of (−)U-50,488H once a day for seven consecutive days. Repeated s.c. or i.t. administration of (−)U-50,488H with all doses used in the present study produced a time-dependent decline in the antinociception of (–)U50,488H, indicating the development of antinociception tolerance to (−)U-50,488H.
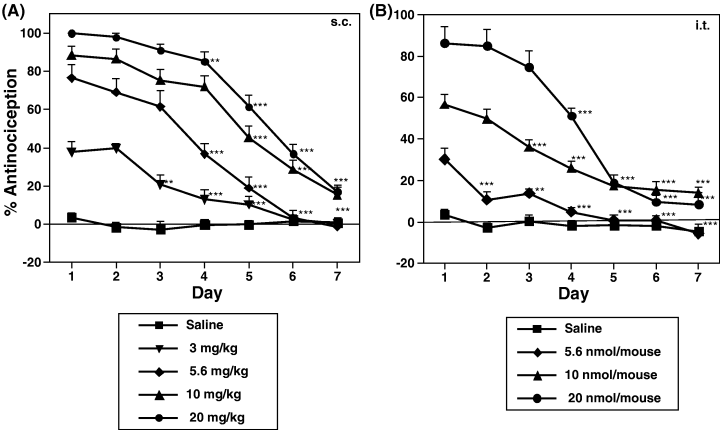
Development of tolerance to (−)U-50,488H-induced antinociceptive effect in mice. Groups of mice were repeatedly injected s.c. or i.t. with saline or (−)U-50,488H at 3–20 mg/kg (A) or 5.6–20 nmol/mouse (B), respectively, once a day for seven consecutive days. Each value represents the mean ± SEM of 9–12 mice. The statistical significance of differences between days was assessed with the Bonferroni/Dunn test. **P < 0.01, ***P < 0.001, compared to each group on day 1.
Effect of repeated administration of (−)U-50,488H on the (−)U-50,488H-stimulated [35S]GTPγS binding to membranes of the mouse spinal cord
(−)U-50,488H caused a concentration-dependent increase in [35S]GTPγS binding to membranes of the mouse spinal cord. Repeated administration of (−)U-50,488H at 20 mg/kg produced a significant reduction in the (−)U-50,488H-stimulated [35S]GTPγS binding in the spinal cord (Fig. 2), indicating the down-regulation of κ-opioid receptor function to activate G-protein in the spinal cord following repeated treatment with (−)U-50,488H.
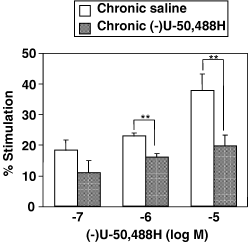
Effect of repeated administration of (−)U-50,488H on the (−)U-50,488H-induced [35S]GTPγS binding to membranes of the spinal cord. Groups of mice were repeatedly treated with saline or (−)U-50,488H (20 mg/kg, s.c.). Two hours after the last injection, membranes of the mouse spinal cord were prepared and incubated with 50 pm[35S]GTPγS and 30 µm GDP with various concentrations of DAMGO at 25 °C. The values are expressed as the percentage of basal [35S]GTPγS binding and represent the mean ± SEM of three independent sets of experiment. The statistical significance of differences between the groups was assessed with two-way anova followed Bonferroni–Dunn test. **P < 0.01 vs. saline-treated group.
Enhancement of spinal antinociceptive effect induced by a selective µ-opioid receptor agonist DAMGO following repeated administration of (−)U-50,488H
The next study was to investigate the effects of repeated s.c. or i.t. treatment with (−)U-50,488H on DAMGO-induced antinociception. Groups of mice were repeatedly injected with s.c. saline, s.c. (−)U-50,488H (20 mg/kg), i.t. saline or i.t. (−)U-50,488H (20 nmol/mouse) once a day for seven consecutive days. Two hours after the last injection, groups of mice were then challenged i.t. with DAMGO (0.5–20.0 pmol/mouse). The spinal antinociceptive effect reached its peak at 10 min after i.t. injection of DAMGO. Either repeated s.c. (Fig. 3A) or i.t. (Fig. 4A) injection of (−)U-50,488H significantly enhanced the spinal antinociceptive effect induced by i.t.-administered DAMGO relative to repeated treatment with saline. Furthermore, no alteration in baseline latencies between saline- and (−)U-50,488H-treated groups (3.28 ± 0.24 s, n = 12 and 3.10 ± 0.17 s, n = 12 for s.c. injection; 3.23 ± 0.17 s, n = 12 and 3.01 ± 0.25 s, n = 12 for i.t. injection, respectively) was observed 2 h after repeated treatment. As shown in 3, 4, the dose–response line four- and two-fold left shift after repeated s.c. and i.t. treatment with (−)U-50,488H, respectively. ED50 values for DAMGO-induced antinociception in mice treated repeatedly with (−)U-50,488H were significantly reduced from 14.7 (12.8–17.3) pmol for s.c. (−)U-50,488H-treated mice and 8.0 (6.6–10.1) pmol for i.t. (−)U-50,488H-treated mice to 3.3 (2.8–4.1) and 3.2 (2.6–4.0) pmol, respectively (P < 0.01 vs. saline-pretreated group).
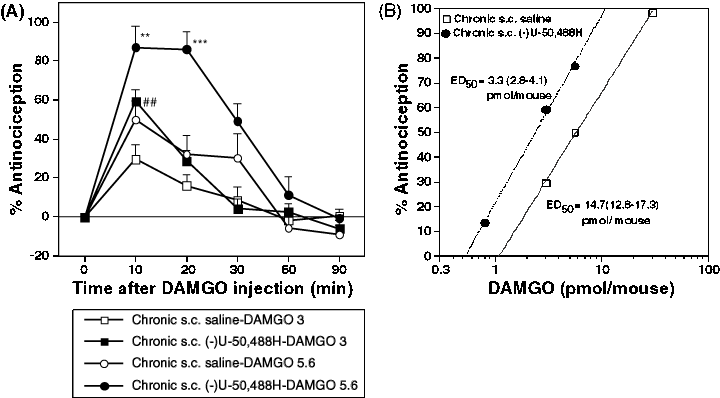
Effect of repeated s.c. administration of (−)U-50,488H on the DAMGO-induced antinociception in mice. Groups of mice were injected s.c. with saline or (−)U-50,488H (20 mg/kg) once a day for seven consecutive days. Two hours after the last injection with saline or (−)U-50,488H, mice were treated i.t. with DAMGO. (A) Time courses of antinociceptive response induced by i.t.-administered DAMGO at 3 pmol/mouse (▪, □) or 5.5 pmol/mouse (●, ○) after repeated injection with (−)U-50,488H (▪, ●) or saline (□, ○). (B) Dose–response lines for antinociceptive effect induced by i.t.-administered DAMGO in saline- or (−)U-50,488H-treated mice. Each value represents the mean ± SEM of 8–12 mice. The statistical significance of differences between the groups was assessed with two-way anova followed the Bonferroni–Dunn test. *P < 0.05, ***P < 0.001, compared to saline-treated group challenged with DAMGO at 5.6 pmol; ##P < 0.01, compared to saline-treated group challenged with DAMGO at 3 pmol.
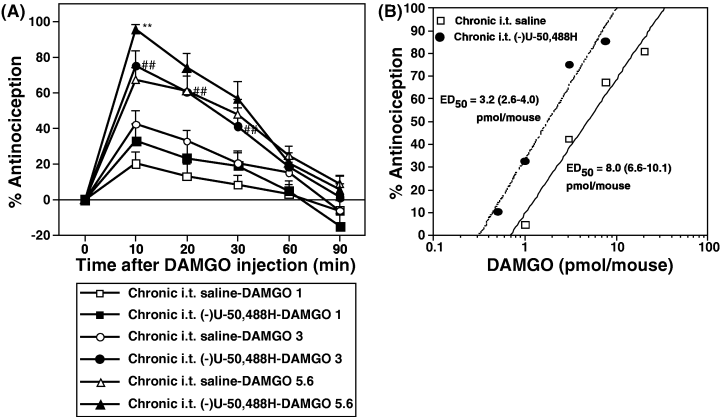
Effect of repeated i.t. administration of (−)U-50,488H on the DAMGO-induced antinociception in mice. Groups of mice were injected i.t. with saline or (−)U-50,488H (20 pmol/mouse) once a day for seven consecutive days. Two hours after the last injection of saline or (−)U-50,488H, mice were treated i.t. with DAMGO. (A) Time courses of antinociceptive response induced by i.t-administered DAMGO at 1 pmol/mouse (▪, □), 3 pmol/mouse (●, ○) or 5.6 pmol/mouse (▴, ▵) after repeated injection with (−)U-50,488H (▪, ●, ▴) or saline (□, ○, ▵). (B) Dose–response lines for antinociceptive effect induced by i.t.-administered DAMGO in saline- or (−)U-50,488H-treated mice. Each value represents the mean ± SEM of 8–12 mice. The statistical significance of differences between the groups was assessed with two-way anova followed the Bonferroni–Dunn test. ##P < 0.01, compared to saline-treated group challenged with DAMGO at 3 pmol; **P < 0.01, compared to saline-treated group challenged with DAMGO at 5.6 pmol.
Effect of a selective µ-opioid receptor antagonist on the antinociception induced by DAMGO
To investigate whether the increased DAMGO-induced antinociception after repeated administration of (−)U-50,488H could enhance the functional µ-opioid receptors, we demonstrated the effect of pretreatment with β-FNA on the DAMGO-induced antinociception in repeatedly saline- or (−)U-50,488H-treated mice. As shown in Fig. 5, the DAMGO-induced antinociception after repeated treatment with either saline or (−)U-50,488H at 20 mg/kg was completely blocked by pretreatment with a selective µ-opioid receptor antagonist β-FNA (P < 0.001 vs. DAMGO alone).
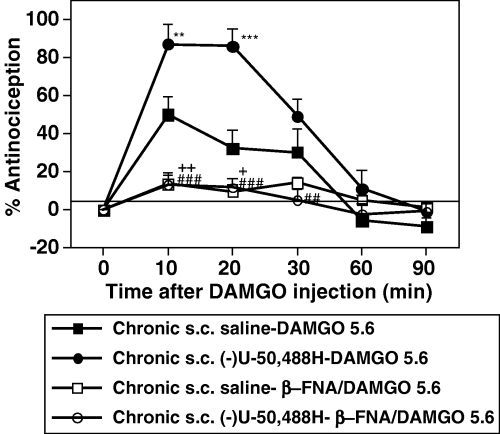
Effect of pretreatment with β-FNA on the DAMGO-induced antinociception in saline- and (−)U-50,488H-treated mice. Groups of saline- or (−)U-50,488H-treated mice were pretreated with β-FNA (40 mg/kg, s.c.) at 24 h before DAMGO injection. Each value represents the mean ± SEM of 8–12 mice. The statistical significance of differences between the groups was assessed with two-way anova followed Bonferroni–Dunn test. **P < 0.01, ***P < 0.001, compared to saline-treated mice; +P < 0.05, ++/##P < 0.01, ###P < 0.001, compared to each group with DAMGO alone.
Effect of repeated administration of (−)U-50,488H on the DAMGO-stimulated [35S]GTPγS binding to membranes of the mouse spinal cord
Repeated administration of (−)U-50,488H had no significant effect on the basal [35S]GTPγS binding to membranes of the spinal cord (data not shown). In membranes of the spinal cord obtained from mice repeatedly treated with saline, DAMGO was able to increase the binding of [35S]GTPγS in a concentration-dependent manner. The stimulatory effect of DAMGO on [35S]GTPγS binding to the spinal cord was significantly increased by repeated treatment with (−)U-50,488H compared to repeated treatment with saline (P < 0.001; Fig. 6A). This robust increase in [35S]GTPγS binding was completely attenuated by coincubation with β-FNA (Fig. 6B).
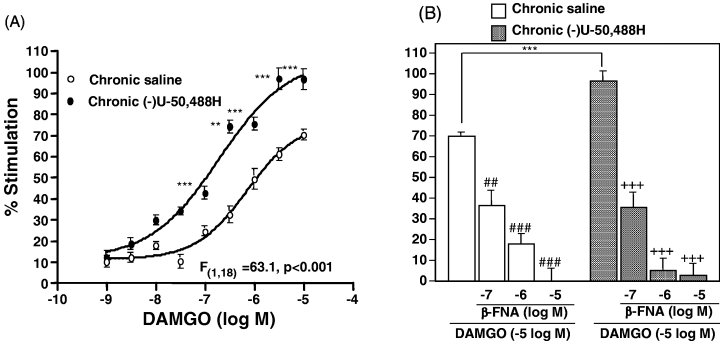
(A) Effect of repeated s.c. administration of (−)U-50,488H on the µ-opioid receptor agonist-stimulated [35S]GTPγS binding to membranes of the spinal cord. Groups of mice were repeatedly treated with saline or (−)U-50,488H (20 mg/kg, s.c.). Two hours after the last injection, membranes of the mouse spinal cord were prepared and incubated with 50 pm[35S]GTPγS and 30 µm GDP with various concentrations of DAMGO at 25 °C. (B) Effect of coincubation with β-FNA on the DAMGO-stimulated [35S]GTPγS binding to membranes of the spinal cord. The values were expressed as the percentage of basal [35S]GTPγS binding and represent the mean ± SEM of three independent sets of experiment. The statistical significance of differences between the groups was assessed with two-way anova followed the Bonferroni–Dunn test. **P < 0.01, ***P < 0.001 vs. saline-treated group; ##P < 0.01, +++/###P < 0.001 vs. compared to each group with DAMGO alone.
Effect of repeated administration of (−)U-50,488H on the number of µ-opioid receptors in membranes of the mouse spinal cord
To evaluate whether the increased functional responses to µ-opioid receptor by repeated injection of (−)U-50,488H could result from an increase in the number of µ-opioid receptors in membranes of the spinal cord, we performed the saturation binding analysis using [3H]DAMGO. Saturation binding studies with [3H]DAMGO at different concentrations revealed a single high affinity binding site that represents the µ-opioid receptor. The maximal binding of [3H]DAMGO in spinal cord membranes prepared from repeated treatment with (−)U-50,488H was significantly increased as compared to repeated treatment with saline (from 60.74 ± 0.53 to 74.45 ± 2.56 fmol/mg protein). Furthermore, the affinity (Kd value) of [3H]DAMGO in the spinal cord was significantly increased by repeated treatment with (−)U-50,488H (from 0.70 ± 0.01 to 1.04 ± 0.08 nm) (Table 1).
| Treatment | B max (fmol/mg protein) | K d (nm) |
|---|---|---|
| Chronic saline | 60.74 ± 0.53 | 0.70 ± 0.01 |
| Chronic (−)U-50,488H | 74.45 ± 2.56** | 1.04 ± 0.08** |
- ** P < 0.01 vs. saline-treated mice.
Effect of repeated administration of (−)U-50,488H on the expression of µ-opioid receptors in the mouse spinal cord
We next investigated the change in the expression of µ-opioid receptor mRNA in membranes of the spinal cord following repeated (−)U-50,488H treatment. RT-PCR assay revealed that repeated treatment with (−)U-50,488H failed to alter the expression of µ-opioid receptor mRNA (Fig. 7).
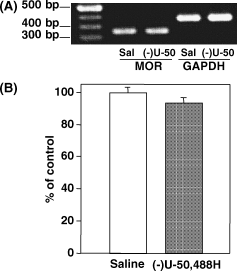
Effect of repeated s.c. administration of (−)U-50,488H on the expression of µ-opioid receptor (MOR) mRNA in the mouse spinal cord. The expression of µ-opioid receptor mRNA in the spinal cord was semiquantified using RT-PCR. (A) Representative RT-PCR for the µ-opioid receptor mRNA in the spinal cord obtained from saline (sal)-treated or (−)U-50,488H [(−)U-50]-treated mice. The mixture was run on 1% agarose gel electrophoresis with the indicated markers and primers of the internal standard GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Three independent experiments were performed in this study. (B) Semiquantification of the intensity of the bands was conducted by using an NIH image. The value for (−)U-50,488H-treated mice was expressed as percentage of the value in saline-treated mice. Each column represents the mean ± SEM of three samples.
Discussion
First of all, we confirmed that repeated either s.c. or i.t. injection of a selective κ-opioid receptor agonist (−)U-50,488H showed a time-dependent decline in the antinociceptive effect, indicating the development of antinociceptive tolerance to (−)U-50,488H. It has been well characterized that κ-opioid receptors are members of the superfamily of G-protein-coupled receptors (GPCRs)(Li et al., 1993; Meng et al., 1993; Yasuda et al., 1993). The activation of GPCRs by specific ligands triggers signal propagation via G-proteins, which subsequently regulate the activities of downstream effector molecules (Li et al., 2002). Using the [35S]GTPγS binding assay, we demonstrated that repeated administration of (−)U-50,488H produced a significant reduction in the (−)U-50,488H-stimulated [35S]GTPγS binding in the mouse spinal cord. These results suggest that repeated (−)U-50,488H treatment causes the down-regulation of κ-opioid receptor function to activate G-proteins in the mouse spinal cord.
The κ-opioid receptor has been shown to possess the phosphorylation sites regulated by various enzymes including G-protein-coupled receptor kinase and protein kinases (Appleyard et al., 1999; Narita et al., 2001a, 2001b). Taken together, the present data support the idea that repeated activation of κ-opioid receptors may lead to the phosphorylation of κ-opioid receptors and in turn cause the uncoupling of κ-opioid receptors from G-proteins and/or the internalization of κ-opioid receptors from the plasma membrane in the mouse spinal cord. This phenomenon could cause a drastic reduction in the function of the κ-opioidergic system in the spinal cord.
A major finding of the present study was that repeated administration of (−)U-50,488H affected the µ-opioid receptor agonist-induced antinociceptive effect. We found here that the antinociceptive effect induced by i.t.-administered DAMGO was significantly increased by more than two-fold following repeated either s.c. or i.t. treatment with (−)U-50,488H. Indeed, we have previously reported that repeated s.c. administration with (−)U-50,488H also produced an enhancement of µ-opioid receptor functions in the mouse thalamus and periaqueductal grey (Narita et al., 2003). The DAMGO-induced antinociception was completely blocked by a selective µ-opioid receptor antagonist β-FNA. We also demonstrated that repeated s.c. injection with (−)U-50,488H produced an enhancement of the increased [35S]GTPγS binding induced by DAMGO in membrane fractions of the spinal cord. Like the antinociception, the stimulation of [35S]GTPγS binding was abolished by β-FNA, indicating a specific involvement of µ-opioid receptors in this event. Thus, it is most likely that the potentiation of the µ-opioid receptor agonist-induced spinal antinociception in (−)U-50,488H-treated mice may result, at least in part, from an enhancement of the µ-opioid receptor function to activate G-protein in the spinal cord.
The mechanisms underlying interactions between spinal κ- and µ-opioid receptors after repeated treatment with the selective κ-opioid receptor agonist remain unclear. We previously reported that the concomitant activation of κ-opioid receptors by repeated treatment with U-50,488H suppressed the development of tolerance to the µ-opioid receptor agonist-induced antinociceptive effect (Suzuki et al., 1992). Furthermore, coadministration of morphine with U-50,488H produced an additive effect on antinociception (Narita et al., 1993). The anatomical approach has indicated that the µ- and κ-opioid receptors and their mRNAs are colocalized in many brain regions including the spinal cord (Mansour et al., 1995). Moreover, the recent electrophysiological study indicates that 53% of spinally projecting rostral ventromedial medulla neurons respond to µ-opioid receptor agonist, 14% respond to κ-opioid receptor agonist and 23% respond to both µ- and κ-opioid receptor agonists (Marinelli et al., 2002). These findings indicate that possible colocalization of κ- and µ-opioid receptors results in a functional interaction between them.
In the present study, using receptor binding assay we found that repeated treatment with (−)U-50,488H produced a substantial increase in the Bmax of [3H]DAMGO binding sites (from 60.74 ± 0.53 to 74.45 ± 2.56 fmol/mg of protein) even in the spinal cord membranes. These data suggest that repeated stimulation of κ-opioid receptor by (−)U-50,488H causes an up-regulation of µ-opioid receptors in the spinal cord. However, it should be noted that repeated (−)U-50,488H treatment produced a 1.48-fold increase in Kd values for [3H]DAMGO binding under the present condition, indicating a significant decrease in the µ-ligand affinity for its increased receptors. Although the specific reason for the decrease in the affinity is unclear, a hypothesis could be advanced that repeated stimulation of κ-opioid receptors leads to an increase in both functional and nonfunctional (G-protein-uncoupled) µ-opioid receptors on membranes of the mouse spinal cord. To determine further whether repeated treatment with (−)U-50,488H could increase the functional and G-protein-coupled µ-opioid receptors on the plasma membrane, we performed the [35S]GTPγS binding assay, which can only detect the membrane-associated and functional receptors. As a result, the increased DAMGO-stimulated [35S]GTPγS binding was clearly observed by chronic (−)U-50,488H treatment. It is therefore likely that functional µ-opioid receptors located on the membrane surface can be increased by repeated stimulation of κ-opioid receptors. Under these conditions, the expression of µ-opioid receptor mRNA was not changed in the spinal cord of (−)U-50,488H-treated mice. Taken together, these findings suggest that repeated stimulation of κ-opioid receptors markedly increases the availability of µ-opioid receptors at the membrane surface without affecting the levels of newly synthesized µ-opioid receptors in the mouse spinal cord.
We tried to propose the possible mechanisms underlying the up-regulation of µ-opioidergic functions after repeated administration of (−)U-50,488H. We cannot exclude the possibility that repeated stimulation of κ-opioid receptor by agonist results in the facilitation of µ-opioid receptor recycling. Following the desensitization, receptors are often endocytosed into an intracellular compartment, from which they can be recycled to membrane, leading to receptor resensitization or targeted for degradation (Lefkowitz, 1998; Nestler, 2001; von Zastrow, 2001; Tanowitz & von Zastrow, 2002). It is therefore possible that repeated stimulation of κ-opioid receptor may affect the process of µ-opioid receptor internalization by protecting its phosphorylation or facilitating its dephosphorylation. The other possible mechanism is the direct molecular interaction between µ- and κ-opioid receptors in the spinal cord. Although the hypothesis for the possible heterodimerization seems to be often advanced at this time, several groups have reported the dimerization of opioid receptors (Jordan & Devi, 1999; Jordan et al., 2001; He et al., 2002). Thus, further research is required to prove this point.
In conclusion, the present data provide direct evidence that repeated stimulation of κ-opioid receptors leads to the up-regulation of µ-opioid receptor function in the mouse spinal cord, which is associated with the increased level of functional µ-opioid receptors on the membrane surface without affecting the expression of µ-opioid receptor mRNA.
Acknowledgements
This work was supported in part by grants from the Ministry of Health and Welfare, and the Ministry of Education, Science, Sports and Culture of Japan to T. Suzuki.
Abbreviations
-
- DAMGO
-
- [d-Ala2,n-Me-Phe4,Gly5-ol] enkephalin
-
- β-FNA
-
- β-funaltrexamine
-
- [35S]GTPγS
-
- guanosine-5′-o-(3-[35S]thio) triphosphate
-
- (−)U-50,488H
-
- (1S-trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide hydrochloride.