Expression of RGS2, RGS4 and RGS7 in the developing postnatal brain
Abstract
The abundant expression of RGS (regulator of G-protein signalling) proteins in neurons, together with their modulatory function on G-protein-dependent neurotransmission, provides the basis for cellular adaptation to sensory inputs. To identify the molecular mechanism involved in the sensory experience-induced neural development, we performed a systematic survey of the localization of mRNAs encoding three subtypes of the RGSs (RGS2, RGS4 and RGS7) in developing rat brains by in situ hybridization through postnatal day 2 (P2), P10 and P18 to adult. The most dramatic changes of expression patterns were observed in the discrete neuronal cell layers of the cerebral neocortex (for RGS2 and 4), the hippocampus (for RGS2, 4 and 7), the thalamus (for RGS4) and the cerebellum (for RGS2 and 7). In the neocortex, RGS2 mRNA was enriched in the superficial cortical plate at P2, in contrast to RGS4, which was enriched in more mature neurons of the deeper layer V and VI. In the hippocampus, the neuronal cell layer-specific expression pattern of RGS2 developed from P2 to P18. RGS4 expression was temporarily confined to the CA pyramidal cell layer and not detectable in the dentate gyrus at P10 and P18. Similarly, a high level of expression of RGS7 was observed in the CA area, but not in the dentate gyrus at P2 and P10. In the cerebellum, the maturation of laminar expression patterns for the three RGSs correlated with neuronal maturation and synaptogenesis at P18. The most characteristic temporal pattern among the three RGSs was observed for RGS4 mRNA, which was highly enriched in the thalamocortical regions. The peaks of RGS4 expression were seen in the following regions with distinct onset and duration: the neocortex (from P2 onward), the hippocampus (P10 and P18) and the thalamus (from P18 onward). The divergent temporal and spatial expression of RGS subtypes and their dynamic control in the cortex, the hippocampus and the thalamus suggest that the RGS family could play multiple distinct roles in experience-dependent brain development.
Introduction
Regulator of G-protein signalling (RGS) proteins are GTPase-activating-proteins for several G-protein subunit members and serve as signalling modulators of G-protein-coupled receptors (Berman et al., 1996; Koelle & Horvitz, 1996; Siderovski et al., 1996; Dohlman & Thorner, 1997). Activation of G-protein-coupled receptors catalyses the exchange of Gα-bound GDP for GTP to cause the dissociation of Gα from the Gβγ dimer and initiate downstream signal propagation; RGS proteins terminate signalling by accelerating the intrinsic Gα-GTPase activity and recycling the G-protein complex back to its inactive GDP-bound heterotrimeric configuration (Chen et al., 1996; Druey et al., 1996; Hunt et al., 1996; Watson et al., 1996). Several studies further indicated that RGS proteins may function as essential regulators of G-protein gated inward rectifier K+ channels, some voltage-sensitive Ca channels, and the G-protein-dependent electrophysiological excitability of neurons (Doupnik et al., 1997; Chuang et al., 1998; Zhou et al., 2000; Sondek & Siderovski, 2001). Overall, these findings suggest an important role for the RGS proteins in regulating the duration of G-protein-mediated signalling and a possible modulatory function in neuronal activation or desensitization processes, which provides the basis for cellular adaptation to external inputs.
The brain has an intrinsic capacity to modify neuronal interactions and this plasticity is essential for the normal development and function of the brain (Goodman & Shatz, 1993). In many experimental models, plasticity is known to be dependent on synaptic activity and consequent transmitter activation of postsynaptic receptors. Whereas the initial events including G-protein activation and calcium entry induced by transmitter stimulation are well characterized, mechanisms underlying long term structural and regulatory changes remains conjectural, but are believed to involve the activation of specific genes (Steward et al., 2001). Several mammalian members of the RGS family demonstrate regional specificity and heterogenous expression within the rat brain (Gold et al., 1997). In the previous study, we have demonstrated that mRNA encoding RGS2 is rapidly induced in brain neurons in response to plasticity-inducing synaptic stimuli (Ingi et al., 1998). Current understanding of the physiological role of the neuronal types relies heavily on a study of RGS2 knockout mice (Oliveira-Dos-Santos et al., 2000). The morphological and electrophysiological analysis of rgs2-deficient mice demonstrated a significant decrease in the spines' density of hippocampal CA1 neurons, which correlated with significantly reduced electrical activity in these cells. These findings, as well as the regulatory function of RGS protein on G-protein signalling, raises the possibility that multiple RGSs may play a role in the formation, modulation and re-organization of neural circuits.
Early in development, internally generated spontaneous activity sculpts neural circuits on the basis of genetic information at the initial configuration of connections necessary for function (Katz & Shatz, 1996). With maturation of the sense organs after birth, the developing brain relies less on spontaneous activity and increasingly on sensory experience (Katz & Shatz, 1996). The dynamic shift from spontaneous activity to experience-dependent synaptic activity and the consequent expression of specific genes endow the brain with an ongoing ability to accommodate dynamically changing inputs during development and throughout life. The predicted roles of RGS subtypes in this process depend in part on the temporal onset and cellular localization of mRNA expression. Therefore, we have used in situ hybridization analysis to determine the distribution of the major neuronal RGS transcripts; RGS2, RGS4 and RGS7 during postnatal brain development. Here we report that the expression of the RGS2, 4 and 7 genes is dynamically regulated with distinct spatiotemporal patterns, and suggest that this G-protein signal regulation could play multiple roles in the development of the central nervous system.
Materials and methods
Animals and materials
Adult male rats (Sprague Dawley or Fischer-344) were used in the studies of RGS regulation. Developmental studies used male and female pups of postnatal day (P)2, P10, P18, and adults. Rats were anaesthetized with diethyl ether and decapitated. Materials were purchased from the following suppliers: animals (Asazuma Animal Inc., Niigata, Japan); pBluescript vector (Stratagene, La Jolla, CA, USA); pGEM vector (Promega, Madison, WI, USA); [35S]UTP (ICN Biochemicals, Costa Mesa, CA, USA); MAXIscript (Ambion, Austin, TX, USA); and Cresyl fast violet (Chroma, Muenster, Germany). All other reagents were from Sigma (St.Louis, MO, USA) and Wako Pure Chemical (Osaka, Japan), unless specifically noted. Experiments were performed in accordance with the Guidelines for Animal Experimentation of Niigata University.
In situ hybridization
In situ hybridization was performed as described previously (Ingi et al., 2001). Freshly dissected brain tissue was rapidly frozen in plastic moulds placed on dry ice/ethanol slurry. Frozen 10 µm thick sections were mounted on gelatin-coated glass slides and stored desiccated at −20 °C. Prior to hybridization, sections were fixed with freshly prepared, depolymerized with 4% paraformaldehyde, acetylated with 0.5% acetic anhydride in 0.1 m triethanolamine dissolved in 0.9% NaCl adjusted to pH 8.0, and delipidated. Hybridization was performed in a moist chamber under unsealed coverslips at 56 ° using 1 × 106 c.p.m. of probe for each slide in 100 µL of hybridization buffer containing 50% formamide, 10 mm dithiothreitol, 600 mm NaCl, 1 × Denhardts (containing Ficoll, polyvinylpyrrolidone, and bovine serum albumin each at 0.02% wt/vol), 1 mm EDTA, 0.2 mg/mL of yeast tRNA, and 10% dextran. After overnight incubation, slides were washed in 2 × SSC (1 × SSC contains 150 mm NaCl and 15 mm sodium citrate adjusted to pH 7.0) at 37 °, treated with 10 µg/mL of RNase A (Worthington Biochem., Lakewood, NJ, USA) in 2 × SSC at 37 ° and washed three times in 2 × SSC. After washing, sections were dehydrated by sequential immersion in 30%, 70% and 95% ethanol, then air-dried and exposed to BioMax MR film (Eastman Kodak, Rochester, NY, USA) at room temperature for 15–30 days. For Nissl staining, adjacent sections were stained with cresyl fast violet.
Preparation of riboprobes
35S-labelled riboprobes were prepared by using [35S]UTP and T7 or SP6 RNA polymerase to synthesize RNA sense and antisense transcripts of full-length RGS cDNA sequences cloned in pGEM or pBluescript, essentially as described in the MAXIscript. Riboprobes were purified using Sephadex G50 columns (Amersham Pharmacia Biotech, Uppsala, Sweden).
Results
Using subtype-specific 35S-labelled cRNA probe, we examined the regional and cellular mRNA distribution of the RGS2, RGS4 and RGS7 genes in coronal sections from P2, P10, P18 and adult rat brains. To ensure specificity of in situ hybridization, wash conditions described above were based on empirically determined melt conditions (Ingi et al., 2001). The labelling patterns produced by the three RGS antisense cRNAs were highly specific for each RGS subtype (Fig. 1A, C and E). Furthermore, in order to verify the specificity of staining in these sections, we performed control experiments using sense cRNA probes. Adjacent sections were probed with three 35S-labelled sense cRNAs for each RGS transcript (Fig. 1B, D and F). The three sense cRNAs showed no specific labelling signal. These results indicate that the in situ signal detected in brain sections results from specific hybridization and indicates the presence of RGS mRNAs in these sections.

In situ hybridization of rat brain sections with antisense and sense cRNA probes of RGS 2, 4 and 7. Bright-field photomicrographs of film autoradiograms illustrating the labelling patterns of coronal brain sections for antisense (A, C and E) and sense (B, D and F) cRNA probes of RGS2 (A and B), RGS4 (C and D) and RGS7 (E and F). The specificity of hybridization for the antisense cRNA probes was verified by control experiments using sense cRNA probes for each transcript. Identical hybridization patterns were detected in independent experiments (n = 2). Neo, neocortex; Pu, caudate putamen.
Cerebral cortex
Spatiotemporal patterns of RGS2 and RGS4 mRNA expression changed dramatically during cerebral neocortical development. In the P2 and P10 neocortex, the highest signal intensities for RGS2 were temporarily present in the superficial layers (2, 3). This localization corresponds to the cortical plate at P2 (Fig. 2J). The level of RGS2 expression in the superficial layers of P10 frontal and cingulate/retrosplenial cortices was relatively low (Fig. 2B), which is characteristic of early postnatal cortices. In the P18 and adult cortices, RGS2 expression in the neocortices exhibited a moderate and diffuse distribution through all layers from II to VI (2, 3). In the brain regions shown in Fig. 2, the adult patterns of the three RGSs mRNA expression were identical to those of P18 (data not shown).
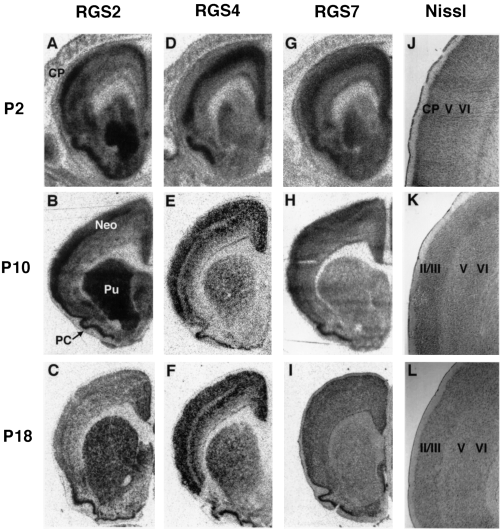
Postnatal expression of RGS 2, 4 and 7 mRNAs in coronal planes of brain sections through the rostral striatum. Bright-field photomicrographs of film autoradiograms illustrating the distribution of RGS2 (A–C), RGS4 (D–F) and RGS7 (G–I) mRNAs in coronal sections of P2 (A, D and G), P10 (B, E and H) and P18 (C, F and I) rat brains. Illustrations of adjacent Nissl-stained sections of P2 (J), P10 (K)and P18 (L)brains enhanced identification of the neocortical structure. At later developmental stages, no differences were seen between adult and P18. Identical hybridization patterns were detected in independent experiments (n = 2–6). Neo, neocortex; CP, cortical plate; PC, pyriform cortex; Pu, caudate putamen.
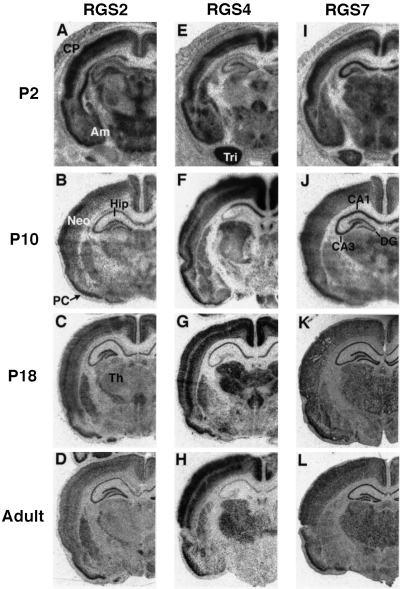
Postnatal expression of RGS 2, 4 and 7 mRNAs in coronal planes of brain sections through the septal hippocampus. Bright-field photomicrographs of film autoradiograms illustrating the distribution of RGS2 (A–D), RGS4 (E–H) and RGS7 (I–L) mRNAs in coronal sections of P2 (A, E and I), P10 (B, F and J), P18 (C, G and K), and adult (D, H and L) rat brains. Identical hybridization patterns were detected in independent experiments (n = 2–6). Neo, neocortex; CP, cortical plate; PC, pyriform cortex; Hip, hippocampus; CA1, CA3, hippocampal fields; DG, dentate gyrus; Am, amygdala; Th, thalamus; Tri, trigeminal nerve.
The highest levels of RGS4 mRNA expression in the P2 neocortex were restricted to the intermediate layers, which corresponds to layers V and VI of the cortex (Fig. 2D and J). Between P2 and P10, RGS4 expression in the neocortex changed from the intermediate layers (2, 3) to a pattern characteristic of the adult brain, with striking layer-specific expression in layers II–III and V–VI (2, 3). The high level expression of RGS4 persisted during the later development and adult period (2, 3). The expression pattern of RGS7 was rather homogenous throughout postnatal development of the neocortex (2, 3), but staining in the deep layer was slightly weaker, particularly at P2 (2, 3). These results demonstrate that the expression of RGS2, RGS4 and RGS7 mRNA is regulated at early postnatal stages of neocortical development, and are summarized with other major changes in Table 1.
| Region | RGS2 | RGS4 | RGS7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2 | P10 | P18 | Adult | P2 | P10 | P18 | Adult | P2 | P10 | P18 | Adult | |
| Neocortex | ||||||||||||
| Layer II/III | ++++ | ++++ | ++ | ++ | + | ++++ | ++++ | ++++ | ++++ | +++ | +++ | +++ |
| Layer IV | ++ | ++ | ++ | ++ | + | + | + | + | ++++ | ++ | ++ | ++ |
| Layer V/VI | ++ | ++ | ++ | ++ | ++++ | ++++ | ++++ | ++++ | +++ | ++ | ++ | ++ |
| Hippocampus | ||||||||||||
| CA1/2 | ++ | ++ | ++ | ++ | ++ | ++++ | ++++ | 0 | ++++ | ++++ | ++++ | ++++ |
| CA3 | ++ | ++ | ++ | ++ | ++ | +++ | + | 0 | ++++ | ++++ | ++++ | ++++ |
| Dentate gyrus | ++ | ++ | ++ | ++ | ++ | 0 | 0 | 0 | + | ++ | ++++ | ++++ |
| Thalamus | + | + | + | + | + | ++ | ++++ | ++++ | +++ | +++ | +++ | +++ |
| Cerebellum | ||||||||||||
| Purkinje cells | 0 | 0 | 0 | 0 | +++ | +++ | +++ | +++ | 0 | 0 | 0 | 0 |
| Granule cells | 0 | +++ | +++ | +++ | 0 | ++ | ++ | ++ | 0 | ++ | +++ | +++ |
- Regional signal intensities of the hybridization sections were evaluated on autoradiographic films (n = 2–6). The signal intensities are as follows: ++++, intense; +++, strong; ++, moderate; +, faint; 0, not detectable.
In contrast to the dynamic changes in neocortex, the transcripts for the three RGS subtypes were constantly and abundantly expressed in the superficial layers of the pyriform cortex, throughout all postnatal developmental stages from P2 to adult (2, 3).
Basal nuclei
The RGS2 and RGS4 mRNAs were expressed abundantly in the caudate putamen throughout postnatal development (Fig. 2A–F), although RGS4 expression level at P2 was relatively low (Fig. 2D). In contrast, RGS7 mRNA level in the caudate putamens were constantly low throughout postnatal development (Fig. 2G–I).
Hippocampus
The neuronal cell layer-specific expression pattern of RGS2 developed from P2 to P18. At P2, the pattern of RGS2 expression was relatively diffuse (Fig. 3A). However, the RGS2 mRNA was localized prominently to the pyramidal cell layer of the hippocampus and the granule cell layer of the dentate gyrus through P18 to adult (Fig. 3C and D). These findings support previous data indicating a role for RGS2 in hippocampal neuronal plasticity and development (Ingi et al., 1998; Oliveira-Dos-Santos et al., 2000). Throughout the development, RGS2 was expressed uniformly in most subregions of the hippocampus.
In contrast, subregional changes of RGS4 and RGS7 mRNAs expression occurred during postnatal hippocampus development. At P2, RGS4 mRNA was expressed slightly in neuronal cell populations of the hippocampus and the dentate gyrus (Fig. 3E). In the P10 and P18 hippocampus, RGS4 was expressed selectively and abundantly in the pyramidal cell layer, with the ranking of signal intensity being CA1 = CA2 > CA3, but was not detectable in the dentate granule cell layer (Fig. 3F and G). No signals for RGS4 were found in the adult hippocampus and dentate gyrus (Fig. 3H). At P2, RGS7 mRNA was expressed abundantly in the pyramidal cell layer, with the ranking of signal intensity being CA1 = CA2 = CA3, but was weakly detectable in the dentate gyrus (Fig. 3I). Subsequently, RGS7 expression in the dentate gyrus increased through to P10, and from P18 onward a high level expression of RGS7 persisted in both the hippocampus and the dentate gyrus (Fig. 3J–L). The results are summarized in Table 1.
Amygdala
At P2, overall labelling in the amygdala regions was dense and relatively homogenous for all three RGS subtypes (Fig. 3A, E and I). From P10 onward, labelling patterns for the three RGS subtypes were differentiated and differed across subregions of specific nuclei. In adult amygdala, overall labelling was less dense for the three subtypes (Fig. 3D, H and L).
Thalamus
Throughout postnatal development, the level of expression of RGS2 in the thalamus was constantly low (Fig. 3A–D), whereas the level of RGS7 was constantly moderate or strong (Fig. 3I–L). In contrast, RGS4 expression increased dramatically between the early and the late stages of development. The RGS4 expression levels at P2 and P10 were low (Fig. 3E and F), whereas a high level expression persisted from P18 onward (Fig. 3G and H). The results are summarized in Table 1.
Hypothalamus
Relatively high amounts of RGS2, RGS4 and RGS7 mRNA were distributed rather heterogeneously throughout the hypothalamic region at P2 (Fig. 3A, E and I). However, expression of the three RGS subtypes in this region decreased to low or undetectable levels after P10.
Trigeminal nerve
At P2, an extremely high level of expression of RGS4 was observed in the trigeminal nerve (Fig. 3E). The RGS7 expression level was relatively high in the same region but no signal for RGS2 was detected (Fig. 3A and I).
Cerebellum
The laminar expression patterns of the three RGSs were first seen at P2 (for RGS4; Fig. 4D) and P10 (for RGS2 and RGS7; Fig. 4B and H) stages of cerebellar cortex development, and mature patterns for the three RGSs were established at P18 (Fig. 4C, F and I). Throughout the developmental process, RGS2 and RGS7 mRNA expression was restricted to the granule cell layers of the cortex, whereas the highest level of RGS4 mRNA expression was observed in Purkinje cell layers, and a more modest expression present in the granule cell layers. Strikingly, RGS2 mRNA was found in both the external and internal granule cell layers of the P10 cortex (Fig. 4B). The results are summarized in Table 1.
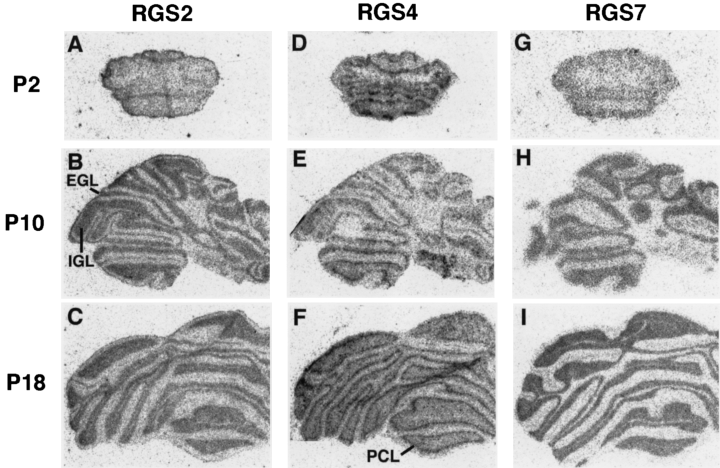
Postnatal expression of RGS 2, 4 and 7 mRNAs in coronal planes through the cerebellum. Bright-field photomicrographs of film autoradiograms illustrating the distribution of RGS2 (A–C), RGS4 (D–F) and RGS7 (G–I) mRNAs in coronal sections of P2 (A, D and G), P10 (B, E and H), and P18 (C, F and I) rat cerebellums. At later developmental stages, no differences were seen between adult and P18. The same hybridization patterns were detected in two independent experiments (n = 2). IGL, EGL, internal and external granule cell layers; PCL, Purkinje cell layer.
Discussion
The major finding of this study is the distinct spatiotemporal pattern and specificity of the mRNAs for the three subtypes of RGS proteins in postnatal brain development. The most dramatic changes of expression pattern were observed in the discrete neuronal cell layers of the cerebral neocortex (for RGS2 and RGS4), the hippocampus (for RGS2, RGS4 and RGS7), the thalamus (for RGS4) and the cerebellum (for RGS2 and RGS7). In contrast to the dynamic changes in these regions, few changes were observed in the pyriform cortex and basal nuclei. The periods where maximum change occurred were distinct but overlapping for each RGS transcript in the brain region and were as follows: (i) the first and second weeks of postnatal development (for the three RGSs in the neocortex and the cerebellum); (ii) the second and third weeks (for RGS4 in the thalamus, and RGS7 in the dentate gyrus); (iii) and the whole four weeks (for RGS4 in the hippocampus). One of the most characteristic patterns of expression was observed for RGS4 mRNA, which was highly enriched in the thalamocortical regions. The dense patterns of RGS4 expression were established at different developmental stages of P2 trigeminal nerve, P2–P10 neocortex, P10–P18 hippocampus, and P18 thalamus. The high level expression in the neocortex and the thalamus was maintained during later development and the adult period. Whereas RGS4 expression in the hippocampus was maximal at P10 and P18, it decreased to an undetectable level in the adult period. Thus, peaks of RGS4 mRNA expression were seen with different onsets and duration. The thalamus receives inputs on different sensory modalities, including somatic sensation, audition and vision, and relays them to the cortices. The cortex and hippocampus are involved in higher functions including cognition, memory and learning, as well as sensory perception. During postnatal development of these networks, synaptic activity elicited by sensory experience is required for the proper formation of connections and coordinated functions (Katz & Shatz, 1996). The sequential regulation of RGS4 in this pathway, in response to postnatal sensory inputs, suggests a role in the formation of multiple level processing functions for enviromental information.
Several RGS subtypes and the dynamic regulation of their RNA have been reported in rat brain. The recent study of RGS2 knockout mice offers the key to understanding the physiological and functional role of this neuronal type (Oliveira-Dos-Santos et al., 2000). rgs2–/– mice display an increased anxiety response and decreased male aggression in the absence of cognitive or motor deficit. rgs2–/– mice did not exhibit gross developmental defects in the brain, whereas hippocampal CA1 neurons of rgs2–/– mice showed a significant decrease in the density of apical and basilar spines compared with the rgs2+/– control mice. The morphological deficit in spine numbers of rgs2–/– CA1 neurons correlates with the significantly reduced electrical input/output relationship in these cells.
In neocortical development, RGS2 mRNA was enriched in the cortical plate at P2. This contrasts with RGS4, which was enriched in more mature neurons of the deeper layers V and VI. The cortical plate comprises immature postmitotic and postmigrational neurons with a simple bipolar phenotype that lacks a well-defined dendritic arbor (Bayer & Altman, 1991). Between P2 and P10, neurons in the cortical plates undergo maturational development of dendritic structure, and the II–VI cellular layers characteristic of the adult cortex are formed (Bayer & Altman, 1991). At P10, a bilaminar distribution of RGS4 expression predominated throughout the cortex layer, whereas the high level of RGS2 expression remained restricted to the superficial layer and decreased from P10 onwards. In summary, our data indicate that RGS2 expression is transiently maximal in immature cortical neurons and RGS4 expression is abundant in mature neurons, and a high level persists in adult brains. This result agrees with the previous data that dendritic morphology and branching were comparable among cortical neurons of rgs2+/– and rgs2–/– mice (Oliveira-Dos-Santos et al., 2000). In cortical development, RGS2 is likely to play an important role for immature neurons, rather than in the synaptic formation process characteristic of mature neurons.
In the hippocampus, RGS2 mRNA was diffusely distributed at P2 and subsequently increased and exhibited a neuronal cell layer-specific pattern at P18. RGS2 continued to be expressed at moderately high levels in the adult hippocampus. This pattern of developmental expression parallels that of several other immediate-early genes (Worley et al., 1990; Kaufmann et al., 1994), and suggest a role for RGS2 in activity-dependent hippocampal development. These results agree with the data that morphological deficit in spine numbers of rgs2–/– CA1 neurons correlates with the significantly reduced electrical input/output relationship in these cells (Oliveira-Dos-Santos et al., 2000). RGS4 expression was strikingly confined to the CA pyramidal cell layer of the hippocampus and not detectable in the dentate gyrus during the second and third weeks. Similarly, a high level of expression of RGS7 was observed in the CA area during the first and second weeks. There is a ‘rhinal-to-dentate’ gradient of neurogenesis and cell differentiation in the hippocampus (Bayer, 1980). The entorhinal cortex starts to differentiate first; followed by the subiculum, then the CA3 field of Ammon's horn and, finally, the dentate gyrus. The CA1 region is an exception to this gradient; CA1 forms significantly later than adjacent CA3 cells. The gradient pattern of RGS4 and RGS7 mRNA expression could be linked to the level of neuronal maturation attained.
In the cerebellum, the maturation of laminar expression patterns correlated with neuronal maturation and synaptogenesis. The laminar expression of RGS2 and RGS7 in granular cell layers reached adult patterns at P18 when a large number of synapses were established between mossy fibres and granule cell dendrites (Altman, 1972). Identical patterns of RGS2 and RGS7 expression suggest that in granule cells the two RGSs could have coordinated functions. Also, the highest level of RGS4 mRNA, reaching a maximal intensity at P18, was observed in the Purkinje cell layers. At P18, parallel fibres are known to form synapses on Purkinje cell dendrites (Jacobson, 1991).
Thus, we observed distinct but overlapping patterns of RGS subtype-specific mRNA expression throughout postnatal development. In several brain regions, dynamic regulation of mRNA expression correlates with the onset and principal sites of certain synapse establishment. This suggests that specific RGSs are likely to play multiple distinct roles in the developing and adult brains, including the axonal growth or the formation and consolidation of synapses.
Acknowledgements
We thank K. Takahashi for excellent technical assistance. This work was supported in part by research grants from the Japan Society for the Promotion of Science, by the Inamori Foundation, and by the Kouwa Life Science Foundation.
Abbreviations
-
- P
-
- postnatal day
-
- RGS
-
- regulator of G-protein signalling.




