Adrenoreceptor-mediated modulation of the spinal locomotor pattern during swimming in Xenopus laevis tadpoles
Abstract
This study focused on the contribution of different adrenoreceptor subtypes to the modulation of fictive swimming activity in a relatively simple, yet intact, lower vertebrate system, the immobilized Xenopus laevis tadpole and explored their possible role in mediating the noradrenergic modulation of spinal motor networks. In Xenopus embryos, near the time of hatching, activation of α1 adrenoreceptors increased the duration of episodes of fictive swimming, whilst in larvae, 24 h after hatching, they were decreased. Activation of α2 adrenoreceptors, however, markedly reduced episode duration at both developmental stages. Cycle periods in both stages were increased by the activation of α1 and/or α2 receptor subclasses, whereas β adrenoreceptors were not apparently involved in the modulation of cycle periods or the duration of swim episodes. However, both β and α1 receptor activation decreased the intersegmental delay in the head-to-tail propagation of swimming activity, while α2 receptors did not influence these rostro-caudal delays. Activation of neither α, nor β, receptor subclasses had any consistent effect on the duration of ventral motor bursts. Our findings suggest that noradrenergic modulation of the swim-pattern generator in Xenopus tadpoles is mediated through the activation of α and β adrenoreceptors. In addition, activation of particular receptor subclasses might enable the selective modulation of either the segmental rhythm generating networks, the intersegmental coordination of those networks or control at both levels simultaneously.
Introduction
The rhythmic motor patterns generated by neuronal networks during vertebrate locomotion are characterized by a high degree of intrinsic variability, which provides the animal with the behavioural flexibility to respond to different environmental requirements (Kiehn et al., 1997; Sillar et al., 1997). Such variability can be achieved by afferent inputs, but can also be due to the intrinsic influences of centrally acting neuromodulators that affect the spinal motor circuitry itself. (for reviews, see Harris-Warrick, 1991; Kiehn & Katz, 1999; Pearson, 2000).
In vertebrates, biogenic amines such as serotonin (5-HT) and noradrenaline (NA) play important roles in initiating, sustaining and modulating motor output. Much has been documented on the role of 5-HT in a range of vertebrates (e.g. cat, Barbeau & Rossignol, 1991; rabbit, Viala & Buser, 1969; rat, Cazalets et al., 1992; Kiehn & Kjaerulff, 1996; turtle, Sillar et al., 1992a), but less is known of another biogenic neuromodulator, NA. In higher vertebrates, such as the cat (Chau et al., 1998; lamprey, Harris-Warrick & Cohen, 1985; Hounsgaard & Kiehn, 1989; McDearmid et al., 1997; tadpole, McDearmid, 1998; Kiehn et al., 1992 and rat Kiehn et al., 1999; Sqalli-Houssaini & Cazalets, 2000), NA is able to initiate, sustain and modulate rhythmic motor activity in spinal cord preparations.
The effects of 5-HT on initiating and modulating vertebrate motor patterns are mediated at a range of 5-HT receptor subtypes (e.g. Wallis, 1994; Wedderburn & Sillar, 1994; Wikstroem et al., 1995). The metabotrophic α1, α2, β1 and β2 adrenoreceptor subclasses have been previously defined as putative receptors for catecholamines, based upon both their physiological actions and pharmacological specificity (e.g. Hirst & Neild, 1980; Lefkowitz & Caron, 1985; Summers & McMartin, 1993). In vertebrates, there is evidence that activation of adrenergic receptors can modulate motor output (Forssberg & Grillner, 1973; Barbeau & Rossignol, 1991; Kiehn et al., 1992; Sqalli-Houssaini & Cazalets, 2000).
Most previous work on NA modulation has been performed either on the spinal cord in vitro or on acutely spinalized preparations in which locomotor activity was triggered pharmacologically or by tonic electrical stimulation. The present study utilizes a relatively simple and essentially intact lower vertebrate, the Xenopus tadpole, to study NA modulation of a well-characterized (Roberts & Clarke, 1982; Roberts, 2000) spinal network that generates self-sustaining rhythmic activity. Immobilized embryonic and larval Xenopus tadpoles produce a ‘fictive’ locomotor pattern, which, like real swimming, involves rhythmic activity that alternates between left and right sides and progresses rostro-caudally with a brief delay between successive muscle segments (Kahn & Roberts, 1982). Previous work in the Xenopus tadpole system has shown that noradrenaline reversibly slows and weakens fictive locomotion (McDearmid et al., 1997) and decreases rostro-caudal delays (McDearmid, 1998).
The present study investigates the specific adrenoreceptor subtypes through which the modulatory effects of NA are most likely mediated. We have used both general and specific adrenoreceptor agonists and antagonists to investigate the role that pharmacologically distinct adrenoreceptors might play in the modulation of locomotion in Xenopus tadpoles.
Materials and methods
Animals
All experiments were performed on prefeeding embryos (stage 37/8) and larvae (stage 42, staged according to Nieuwkoop & Faber, 1956) of the South African clawed frog, Xenopus laevis. This work was performed in accordance with the UK 1986 Animals (Scientific Procedures) Act. Experimental animals were obtained by induced breeding, following the injection of human chorionic gonadotropin (HCG, 1000 U/mL, Sigma, UK) into adult pairs of a laboratory colony. Eggs were kept in de-chlorinated tap water at 17–23 °C.
Preparation
The tadpoles were immobilized using the neuromuscular blocker α-Bungarotoxin (12.5 µm) and transferred into a preparation bath (volume ∼2 mL) containing continuously re-circulating frog ringer solution (basic composition in mM: NaCl, 115; KCl, 2.5; NaHCO3, 2.5; HEPES, 10; MgCl2, 1; CaCl2, 2; pH 7.4). The preparation is shown schematically in Fig. 1A. For a detailed description of the preparation procedure, see Reith & Sillar, (1997). Episodes of fictive swimming activity were elicited by a short current pulse (0.5–1 ms) on the tail skin via a separate glass suction electrode (using a Digitimer DS2 isolated stimulator, Digitimer Ltd, Welwyn Garden City, UK).
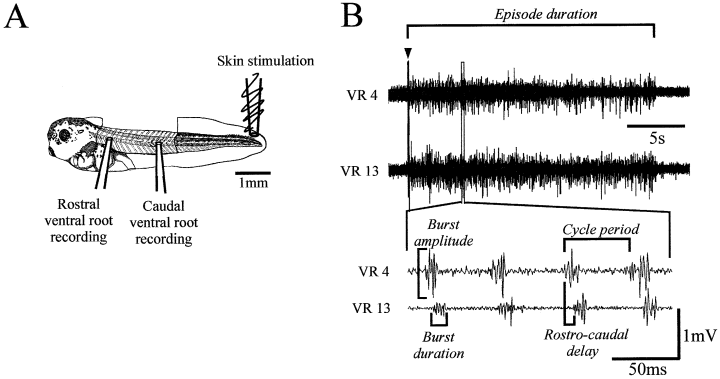
The Xenopus tadpole preparation. (A) Standard preparation (shown with a stage 42 larva) for synchronous ipsilateral ventral root recordings. Suction electrodes are placed over the myotomal clefts after removal of the flank skin. Swimming activity is elicited by applying a brief (0.5–1 ms) current pulse to the tail skin. (B) Evaluated parameters of the swimming pattern demonstrated by original ipsilateral ventral root recordings from the 4th (VR 4) and the 13th (VR13) ventral roots (arrowhead indicates the stimulation artefact).
Pharmacological agents
All drugs were purchased from Sigma (UK), except for propranolol (Tocris Cookson, Bristol, UK) and benoxathian (RBI, UK). Drugs were bath-applied to the perfusate by adding known concentrations to the stock bottle (100 mL) to achieve the desired final concentration.
Parameters of fictive swimming
The effects of NA and drugs acting on adrenoreceptors were investigated using the following parameters of fictive swimming: (i) the episode duration (in s), measured from the first to the last motor burst observed during an episode; (ii) the burst duration (in ms), measured as the duration of each discrete motor burst; (iii) the burst amplitude (in mV), measured as the peak-to-peak interval (iv) the cycle period (in ms), measured as the time interval from the start of one burst to the start of the next and (v) the rostro-caudal (RC-) delay between two ipsilateral ventral roots (in ms), measured as the time interval between the start of a rostral burst and the start of the corresponding caudal burst (Fig. 1B). When investigating burst amplitudes, the coefficient of variation (CV) was used as an additional monitor of the drug effects.
Data evaluation and statistical analysis
Electrophysiological data were stored on videotape and analysed with the Spike 2 data analysis package (Cambridge Electronics, UK) and the Dataview signal analysis program (W.J. Heitler, University of St Andrews, UK). Data analysis of motor activity was performed on 30 cycles per episode of three separate episodes, 1 s after their start (to minimize possible contamination by effects from the initiating sensory stimulus). Data are presented as means and standard errors of the mean (SEM). Statistical procedures were computer aided (KaleidaGraph for Windows, StatView and MS Excel). Analysis of linear correlation and regression was performed using the Least Square Difference (LSD) method. r indicates the linear coefficient of correlation. Statistics were considered to be significant at P < 0.05. N gives the number of animals; n indicates the number of samples acquired from one particular animal. Comparisons of two sets of data were performed using the Student's t-test. A modified t-test (mt-test, see Dixon & Massey, 1969) was used in cases of unequal variances. Non-parametric comparisons were performed using the Mann– Whitney U-test. Comparison of regression coefficients and intercepts as well as of CV were tested and judged in terms of the standard normal distribution by a two way t-test (e.g. Lohrding, 1975; Sachs, 1984).
Results
Unless stated otherwise, the data are from experiments on stage 42 larvae, but are equally valid for stage 37–8 embryos. Developmental comparisons between stages 37–8 and stage 42 are outlined only when a significant difference was present.
Effects of general α-and β-adrenoreceptor antagonists on the NA-mediated modulation of the spinal locomotor rhythm
The role of adrenoreceptors in modulating Xenopus swimming was first addressed using a general α-antagonist, phentolamine (PHENT) after prior application of noradrenaline (NA). In eight preparations, bath application of 4 µm NA significantly decreased episode duration to, on average, 64.4 ± 8.8% of the control value (100%; U-test, P < 0.05, N = 8). After subsequent addition of PHENT (40–100 µm), episode durations were not significantly affected (64.4 ± 8.8% to 70.1 ± 9.7%, U-test, P > 0.05, N = 8; Fig. 2A).
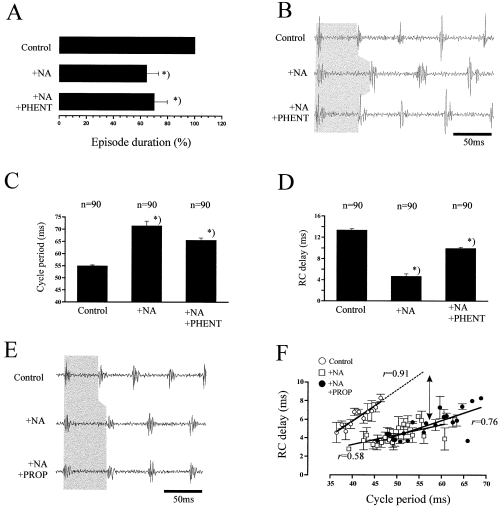
Effects of phentolamine (PHENT) and propranolol (PROP) on the noradrenergic modulation of swimming in Xenopus. (A) Noradrenaline (NA) significantly reduces the duration of swimming episodes. PHENT does not reverse these effects. (B) Effects of NA and PHENT on swimming frequency (as indicated by the grey shaded area). (C) Changes in average cycle period and (D) RC-delay under control, NA and PHENT. Data in A–D are from the same animal. (E) PROP did not reverse the NA-mediated effects on cycle period, as indicated by the grey shaded area. (F) RC-delay is significantly correlated to the cycle period in all three conditions tested (P < 0.01). Arrow indicates a systematic difference in RC-delays during control and under NA. Data were pooled from different observations at a given cycle period. Data in E and F are from the same animal. Asterisks (*) indicate values different from the control (P < 0.01) and from each other (P < 0.05)
Under NA (4 µm), cycle periods were increased in all the animals tested; on average to 143.2 ± 8.0% of their length in control (t-test, P < 0.001, N = 8; changes in individual preparations ranging from 105 to 166%). Subsequent application of PHENT reduced this increase to 117.7 ± 6.8% (P < 0.001, N = 8; Fig. 2B and C). In six of the eight animals tested, rostro-caudal (RC-) delays were significantly decreased under NA to 71.8 ± 8.5% of their control values (mt-test, P < 0.05, N = 6; Fig. 2D). Subsequent addition of PHENT (40–100 µm) reversed these effects (P < 0.05; Fig. 2D). In the remaining preparations, however, RC-delays increased after application of NA (P < 0.05, N = 2). NA did not strongly influence the burst duration in embryos or larvae (data not shown; for a detailed study, see McDearmid, 1998). NA had no consistent effects on the relative amplitude of the ventral root bursts (average control, 0.633 ± 0.087 mV, N = 8; data pooled from both ventral roots, amplitudes not significantly different between rostral and caudal or between embryos and larvae, mt-test, P > 0.05). In two animals, relative amplitude decreased in both roots by 31.6 ± 16.0% and increased in one animal by 15.5% (mt-test, P < 0.05). In four animals, only one ventral root showed a 19.7% decrease in amplitude rostrally (N = 2) and a 16.2% decrease caudally (N = 2). NA did not change the CV of burst amplitudes in 9/16 recordings (two way t-test, P > 0.05, individual CV ranging from 0.21 to 0.61). In the remaining recordings, variability was either decreased (N = 5) or increased (N = 2, P < 0.05).
In four different experiments, the β receptor antagonist propranolol (PROP, 40–45 µm) was applied subsequent to 4 µm NA. In each animal, NA increased cycle periods (on average, from 51.3 ± 7.2 ms in control to 64.0 ± 11.2 ms after 4 µm NA, t-test, P < 0.05), however, in none of the animals did cycle periods decrease after the subsequent application of PROP (66.6 ± 10.2 ms, t-test, P > 0.05, N = 4; Fig. 2E). Episode durations under PROP were not different from those under NA alone (U-test, P > 0.05, N = 4; data not shown). Furthermore, PROP did not reverse the NA-mediated decrease in RC-delays (t-test, P > 0.05, N = 4; Fig. 2F).
Modulation of the locomotor rhythm by α1-adrenoreceptor agonists
In five embryos and five larvae, the specific α1-agonist phenylephrine (PHEE, 50–100 µm) was bath-applied. In embryos, episode duration under PHEE increased to, on average, 175 ± 10.7% of the control value (average duration, 16.8 ± 5.7 s; U-test, P < 0.01, N = 5; Fig. 3A) and was reversed by either washing, or the application of PHENT (on average, to 103 ± 18.6%, P < 0.01, N = 5). During larval swimming, episode duration strongly decreased to 45.6 ± 10.7% of the control (P < 0.01, N = 5; average duration, 14.5 ± 5.7 s) but recovered to 76.8 ± 19.9% after washing (P < 0.05, N = 2) or addition of PHENT (P < 0.05, N = 3; Fig. 3A).
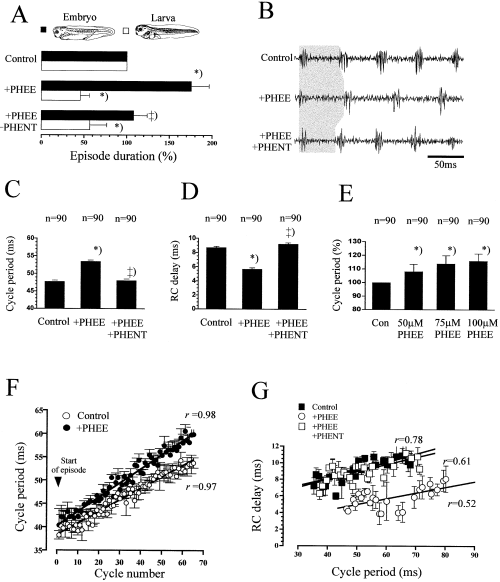
Effects of phenylephrine (PHEE) on the locomotor rhythm. (A) PHEE significantly increases embryonic episode duration and shortens larval episodes. In embryos, subsequent addition of PHENT reversed the effects of PHEE. (B) Effects of PHEE and PHENT on the swimming frequency (as indicated by the grey shaded area). Average change in cycle period (C) and RC-delay (D) after application of PHEE and PHENT. (E) Dose-dependent effects of PHEE on cycle period (data confirmed in five animals). (F) Time course of cycle period during the first 65 continuous cycles of a larval episode. Data points pooled from three episodes. (G) The effects of PHEE and PHENT do not depend on the changes in cycle duration. Data points pooled from different observations at a given cycle period. Data in (B–E) are from the same animal. ++) not different from control (P > 0.05); *) different from control (P < 0.01).
In both stages, cycle periods increased under PHEE in all animals (t-test, P < 0.001, N = 10; Fig. 3B–C and F). On average, cycle periods increased by 12 ± 1.5% (N = 10; effect in different preparations ranged from 6–18%). Furthermore, PHEE caused an increase in cycle periods from the very beginning of an episode (as indicated by the different intercepts of the regression lines, two way t-test, P < 0.05; Fig. 3F). The average response to PHEE depended on the applied concentration; however, the minimum concentration for eliciting a response was different between the preparations (40–60 µm; Fig. 3E). In 7/10 preparations, the RC-delay was decreased after the application of PHEE (mt-test, P < 0.001; Fig. 3D) by, on average, 18.6 ± 3.2% (N = 7; changes in the different preparations ranging from 7–27%). For a given cycle period, the RC-delay was decreased under PHEE, compared to the control (intercepts significantly different, two-way t-test, P < 0.05; Fig. 3G). In these animals, swimming therefore occurred with a ‘systematically’ decreased RC-delay. In the remaining experiments, RC-delays did not differ from the controls (mt test, P > 0.05, N = 3). However, in none of the animals investigated, were the RC-delays increased after the application of PHEE.
In both embryos and larvae, PHEE did not change the burst duration in 7/10 preparations (on average, in embryos, 5.1 ± 0.6 ms, N = 4; in larvae, 10.2 ± 4.7 ms, mt test, P > 0.05, N = 3; for each animal, data from rostral and caudal recordings were pooled). In the remaining preparations, mean burst duration was either increased rostrally by 21% (P < 0.05) or decreased caudally by 19% (P < 0.05, N = 2). In 6/10 animals, PHEE did not change the relative burst amplitude (mt-test, P > 0.05; average control, 0.523 ± 0.086 mV, N = 10), in four animals, the relative changes were inconsistent between the two roots (i.e. 12.5% increase in one and 9.3% decrease in the other, N = 2) or restricted to a change in only one root (N = 2). Furthermore, PHEE did not change burst amplitude variability (i.e. CV) in 75% of the recordings (two-way t-test, P > 0.05; individual CV ranging from 0.23–0.67). In the remaining recordings, the CV was significantly decreased (P < 0.05).
The effects of PHEE on cycle duration and/or RC-delays were reversed (i) by washing out (P < 0.05, N = 4; data not shown); (ii) by the application of PHENT (50–100 µm; t-test or mt-test, P < 0.05, N = 4, Fig. 3B–D) and (iii) by benoxathian, a specific α1-antagonist (P < 0.05, N = 2; 50 µm) that was tested in two of the preparations (data not shown). In four different preparations, the effects of PHEE were investigated after prior application of PHENT. In none of the experiments was a significant increase in cycle period observed (t-test, P > 0.05, N = 4; average cycle period 57.42 ± 4.3 ms under PHENT and 57.40 ± 4.0 ms after additional application of PHEE, pooled from all four animals). PHENT did not prevent the reduction of episode duration by PHEE (P < 0.05, N = 4). The observed effects on RC-delays after prior application of PHENT were not consistent (increase in one, decrease in two, no change in one).
Modulation of the locomotor rhythm by α2-adrenoreceptor agonists
In nine preparations, the specific α2-receptor agonist, clonidine (CLON), was bath-applied at concentrations of 35–200 µm. In all animals, episode duration decreased under CLON to 34.2 ± 8.2% of the control value (U-test, P < 0.05, N = 9). This effect was not reversed either by washing (P > 0.05, N = 7) or by PHENT (P > 0.05, N = 2; Fig. 4A). Cycle periods increased in all the animals tested (t-test, P < 0.001, N = 9) on average, to 120.0 ± 5.01% of the control value after addition of CLON. This effect was reversed by washing out (P < 0.01, N = 7) or by PHENT (50–100 µm; N = 2, P < 0.05) (Fig. 4B–C and F). The average response to CLON depended on the applied concentration; the minimum concentration for eliciting a response was different between the preparations (35–50 µm; Fig. 4E). CLON had no consistent effects on RC-delays; in three preparations, RC-delay did not change after CLON (mt-test, P > 0.05, N = 3), in another three, RC-delays increased to 118.8 ± 3.4% of the control value (P < 0.01, N = 3) and in the remaining three animals, RC-delays under CLON decreased to 88.2 ± 1.8% of the control value (P < 0.05, N = 3; Fig. 4D). For a given cycle period, the RC-delay did not differ under CLON (intercepts not different, P > 0.05; Fig. 4G).
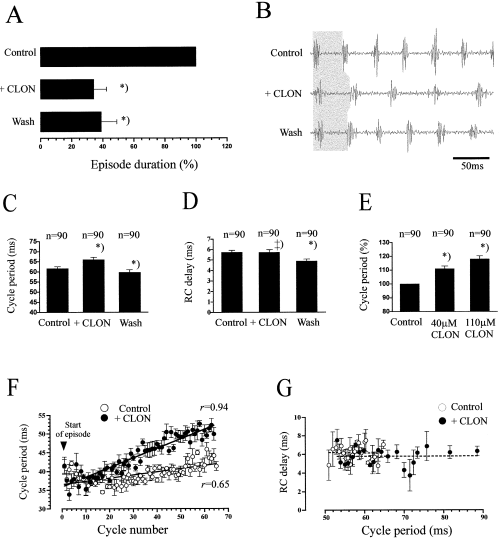
Effects of clonidine (CLON) on the locomotor rhythm. (A) CLON significantly reduces episode duration. No significant reversal was produced by washing. (B) Effects of CLON on swimming frequency (as indicated by grey shaded region). Average change in cycle period (C) and RC-delay (D) after application of CLON. (E) Dose-dependent effects of CLON on cycle period (data confirmed in five animals). (F) Time course of cycle periods over the first 65 cycles of swim episode. Data points pooled from three episodes under each condition; intercepts of the linear regression lines differ significantly at P < 0.05. (G) The increase in cycle period under CLON does not influence the RC-delay (dashed lines indicate no significant correlation, P > 0.05). Data shown in (A–C) and (D–G) are from different animals. *) different from control (P < 0.01) but not different from each other (P > 0.05).
CLON had no consistent effects on burst durations. In two preparations, burst durations did not significantly change after application of CLON (mt-test, P > 0.05, N = 2). In three preparations, burst durations decreased under CLON (on average to 88.7 ± 2.9% of the control value, P < 0.01, N = 3), whilst in the remaining four preparations, burst durations increased in both caudal and rostral roots (on average to 145.0 ± 24.1%, P < 0.05, N = 2) or in only one root (to 110.4 ± 1.6%, P < 0.05, N = 2). In 5/9 animals, CLON decreased the relative burst amplitude of both roots (mt-test, P < 0.05; average control, 0.694 ± 0.069 mV; average decrease, 17.4 ± 6.0%, N = 5), in a further three, only the caudal amplitude decreased and in one preparation, only the rostral amplitude changed (mt-test, P < 0.05). In 5/9 animals, PHENT (N = 2) or washing out (N = 3) reversed the CLON effects on burst amplitudes in both roots (mt-test, P < 0.05; average after PHENT, 95.5 ± 8.92%; after washing, 97.2 ± 22.9% of control). CLON did not change amplitude variability (CV) in 13/18 the recordings (two-way t-test, P > 0.05; individual CV ranging from 0.21 to 0.39). In the remaining five recordings, the CV was significantly decreased (P < 0.05).
Effects of β-adrenoreceptor agonists on the locomotor rhythm
In five embryos and five larvae, the effects of β receptors were investigated using the unspecific β receptor agonist, isoproterenol (ISOP, 50–100 µm). In six preparations, ISOP did not influence episode duration (U-test, P > 0.05, N = 6), in four animals episode durations were either increased (P < 0.05, N = 1) or decreased (P < 0.05, N = 3; data not shown).
ISOP did not influence cycle periods in 60% of the preparations (t-test, P > 0.05, N = 6; Fig. 5A) whereas, cycle period was either increased (P < 0.05, N = 1) or decreased (P < 0.05, N = 3) in the remaining animals. In the majority of animals, burst duration was not changed under ISOP (mt-test, P > 0.05; average burst duration in controls, 10.42 ± 1.1 ms, and ISOP, 10.45 ± 0.9 ms; N = 9; data from rostral and caudal recording pooled). In five preparations, ISOP did not influence relative burst amplitude of either root (mt-test, P > 0.05; average control, 0.619 ± 0.087 mV; N = 10). In the remaining preparations, burst amplitude in both roots was either increased (12.3%; N = 1) or decreased (9.6%; N = 1) compared to the controls, three animals exhibited significantly different effects (P < 0.05) between rostral and caudal burst amplitudes. In nine animals, the CV of the burst amplitude was not different to the control after ISOP (two-way t-test, P > 0.05; CV ranging from 0.16–0.7).
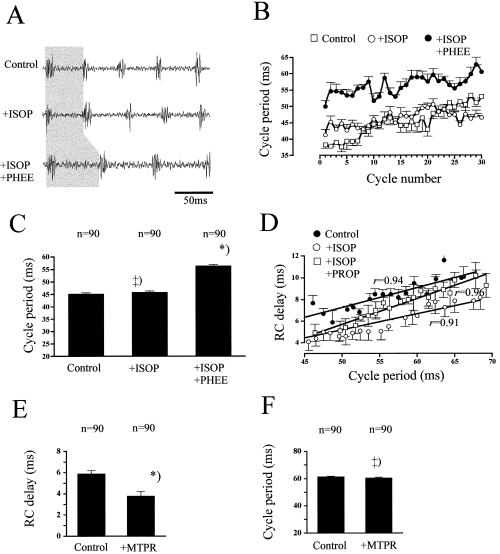
Effects of isoproterenol (ISOP) and metaproterenol (MTPR) on the larval locomotor rhythm. (A) Effects on swimming frequency after application of ISOP and PHEE (indicated by the grey shaded area). (B) Time course of cycle period during 30 continuous cycles. Data points pooled from three episodes. (C) Average cycle period after the application of ISOP and of PHEE. (D) Relationship between RC-delay and cycle period under control, ISOP and additionally, PROP. Data points at a given cycle number pooled from three episodes. Data in (A–C) stem from the same larval preparation (D) and (E–F) are from two further animals. MTPR did not influence cycle period (F), however, the RC-delays were significantly decreased (E; P < 0.05). ++) not different (P > 0.05); *) different at P < 0.001.
Activation of β receptors did not prevent the effects of α receptor activation. After the prior application of ISOP, PHEE (N = 4, Fig. 5A–C) and CLON (N = 2, not shown) caused a significant increase in cycle periods in all the preparations (t-test, P < 0.05; N = 6). ISOP decreased RC-delay in 60% of the animals (mt-test, P < 0.05; average decrease, 18.2 ± 4.7%; N = 6; Fig. 5D), whereas the RC-delays did not change in the remaining four preparations (P > 0.05). In none of the animals was the RC-delay increased under ISOP. In five different experiments, PROP (35–50 µm) was applied subsequent to the addition of ISOP. In 4/5 animals, RC-delay recovered from 65.8 ± 3.6% to 89.5 ± 2.8% of the control value (mt-test, P < 0.01; N = 4) under PROP. ISOP did not interfere with the general relationship between cycle period and RC-delays (regression coefficients were not significantly different, two-way t-test, P > 0.05; Fig. 5D).
The specific β2-receptor agonist metaproterenol (MTPR; 50–150 µm) did not influence episode duration in embryos or larvae (U-test, P > 0.05; average, 23.7 ± 9.0 s; N = 5; data not shown). In all preparations, the RC-delay decreased under MTPR (mt-test, P < 0.001; N = 5; average reduction, 19.5 ± 4.7%; Fig. 5E). MTPR did not influence cycle periods (P > 0.05; N = 5; Fig. 5F). MTPR had no significant effect on the burst duration (t-test, P > 0.05; N = 5; controls, 10.24 ± 0.98 ms; after MTPR, 10.46 ± 1.1 ms; data of both ventral root recordings pooled). MTPR had no consistent effect on burst amplitude: average control; 0.637 ± 0.17 mV; N = 5; no change in both roots in 2/5 preparations, 10.9% decrease in both roots of one animal, increase in either rostral (8.9%) or caudal root (13.3%) in two animals. Furthermore, MTPR did not change the CV of the burst amplitudes in 4/5 preparations (two way t-test, P > 0.05).
Discussion
Noradrenaline and drugs acting on adrenoreceptors have been found to play an important role in the initiation and modulation of spinal locomotor patterns in higher vertebrates (cats, e.g. Forssberg & Grillner, 1973; Barbeau et al. 1987; Barbeau & Rossignol, 1991; Kiehn et al., 1992; Rossignol et al., 1998; Marcoux & Rossignol, 2000; rats, e.g. Kiehn et al., 1999; Sqalli-Houssaini & Cazalets, 2000). Furthermore, the different adrenoreceptor subtypes have been found to perform various specific functions in mediating these locomotor effects (Sakitama, 1993; Chau et al., 1998; Sqalli-Houssaini & Cazalets, 2000).
The present study has investigated the potential involvement of adrenoreceptors in the modulation of spinal motor patterns in a lower vertebrate locomotor system, the immobilized Xenopus tadpole. We have used this essentially intact locomotor system because it is able to generate a self-sustaining, ‘fictive’ swimming rhythm without reliance on persistent drug application. This locomotor rhythm is characterized by a periodic activation of motoneurons innervating each body segment, with the left and right sides activated in strict alternation. Ipsilateral motorneuron pools are activated successively from rostral–caudal with a characteristic intersegmental rostro-caudal (RC-) delay (for other spinal locomotor networks generating ‘undulatory’ movement, see, e.g. Williams & Sigvardt, 1994; Grillner et al., 1993, 1995). Unlike higher vertebrate systems (reviewed in Pearson, 2000), tadpole locomotor rhythm generation is little affected by sensory feedback (Kahn & Roberts, 1982).
In postembryonic tadpoles (as in other aquatic animals, e.g. Lighthill, 1969; Grillner & Kashin, 1976), cycle periods and RC-delays are positively correlated (Tunstall & Sillar, 1993), so producing a constant phase-lag and presumably enabling the maintenance of a constant undulatory body shape regardless of the speed of locomotion (Von Seckendorff-Hoff & Wassersug, 1986). Unusually, however, co-ordination of embryonic swimming is characterized by a constant latency in the successive activation of the body segments irrespective of the actual swimming frequency (Tunstall & Sillar, 1993).
In larval tadpoles, two parallel aminergic neuromodulatory systems control locomotion in an antagonistic manner: noradrenergic pathways decrease swimming frequency, whereas serotonergic (5-HT) pathways increase locomotor intensity (reviewed in McLean et al. 2000). Noradrenergic neurons are present in the brain stem and spinal cord of both embryos and larvae (reviewed in Smeets & Gonzalez, 2000). However, the ingrowth of serotonergic raphe spinal neurons occurs largely during the transition from embryonic to the early larval stage (van Mier et al., 1986; Woolston et al., 1994) and plays a significant role in reconfiguring the networks from the relatively stereotyped embryonic swim pattern towards a much more flexible larval pattern (Sillar et al., 1992a, b, 1995, 1998). Furthermore, as shown in this study, NA and adrenoreceptor agonists have comparable effects on the embryonic and larval locomotor network. Collectively, this anatomical and pharmacological evidence suggests that noradrenergic neuromodulatory pathways are established and available for utilization by these early stages.
The present study shows that, largely independent of the developmental stage, specific adrenoreceptor subclasses exert distinct effects on particular facets of the fictive swimming rhythm: (i) α2 receptor activation mainly modulates swimming frequency (Fig. 4) but, apparently, is not involved in the intersegmental coordination of swimming. (ii) β receptors are involved in the intersegmental coordination of the locomotor rhythm but do not affect cycle periods (Fig. 5). (iii) α1 receptors influence both rhythm generation and intersegmental coordination (Fig. 3). In tadpoles, the effects of NA on cycle periods are reversed by α receptor antagonists but not by β antagonists (Fig. 2). This suggests the participation of α receptors in mediating the effects of NA on swimming frequency. However, we cannot exclude a role for the β receptors in mediating the NA modulation of intersegmental coordination (Fig. 5).
Selective activation of α receptors, or application of NA reduced swimming frequency and, in general, was clearly dependent on the applied drug concentration (3, 4, not shown for NA, but see McDearmid, 1998). However, the minimum drug concentration needed to elicit an effect as well as the precise magnitude of the effect at a given concentration varied considerably between the preparations. Since the spinal cord of the Xenopus preparation was not deliberately exposed, the observed variations in drug effect and threshold concentration between the preparations may be due to drug access problems.
In general, none of the receptor subclasses was able to increase ongoing swimming intensity at any of the concentrations applied. Consistent with this finding, α2 receptors are known to slow ongoing locomotion in other locomotor systems (e.g. cat, Chau et al., 1998; rat, Sqalli-Houssaini & Cazalets, 2000). However, in tadpoles, there is no evidence so far either for excitatory NA effects on the pattern mediated by α1 receptors, or for a role of β receptors in decreasing locomotor activity. Both of these effects have been reported in rats (Sqalli-Houssaini & Cazalets, 2000). The activation of adrenoreceptors systematically influenced cycle period and/or RC-delay, but did not affect the functional relationship between these two parameters (see 3-5). At neither developmental stage did α1 and β receptor agonists have consistent influences on the motor burst duration, the burst amplitude or on the variability of the latter parameter (as measured by the coefficient of variation). This suggests that adrenoreceptor activation may not have strong direct effects on motorneurons during the modulation of swimming. This fits with previous findings in tadpoles that: (i) the receptors mediating the effects of NA are presumed to be located presynaptically (McDearmid et al., 1997); and (ii) that, burst amplitude and durations are strongly influenced by the excitatory drive onto motorneurons and its interaction with serotonin-dependent nonlinear membrane properties (e.g. in Rana, Sillar & Simmers, 1994; Xenopus, Scrymgeour-Wedderburn et al., 1997; Reith & Sillar, 1999; for other systems, Hounsgaard et al., 1988; McLean et al., 1996). Activation of α2 receptors by clonidine, however, decreased motor burst amplitudes during swimming (data not illustated). Consistently, in higher vertebrates (e.g. cat), where the α2 receptors are presumed to be postsynaptically located (Chau et al., 1998) (i) activation of α2 receptors affects locomotor burst duration, and (ii) the application of NA modulates properties of motorneurons (Connell et al., 1989; Elliot & Wallis, 1992; Sqalli-Houssaini & Cazalets, 2000). It remains to be determined whether postsynaptic adrenoreceptors are present on motorneurons of the Xenopus tadpole and contribute to the overall effect of NA.
In only one parameter of the locomotor pattern, the swim episode duration, were developmental differences in response to the activation of α1 receptors observed between embryos and larvae (Fig. 3). Swimming in embryos can be terminated by the activation of mid–hindbrain reticulospinal (mhr) GABAergic neurons by primary afferents innervating the rostral cement gland (′the stopping response’, Boothby & Roberts, 1992). This pathway begins to functionally degenerate during the developmental transition between both stages, although the GABAergic mhr neurons appear to remain (Reith, 1995). Termination of swimming in larvae, however, often occurs spontaneously, coincident with a barrage of GABAergic IPSP's, presumably due to an increase in the potency of inhibitory transmission from the mhr neurons (Reith & Sillar, 1997, 1999). A plausible explanation for the differences in episode duration in response to α1 receptor activation at the two stages, therefore, is the developmental change in this particular inhibitory pathway which does not affect parts of the network controlling the other locomotor parameters (Fig. 3).
The cellular and synaptic mechanisms underlying the NA-mediated modulation of tadpole swimming have recently been investigated, but are by no means fully understood. The NA-mediated increase in the midcycle inhibitory component of the swimming rhythm reduces swimming frequency via presynaptic enhancement of glycine release from the terminals of commissural interneurons (McDearmid et al., 1997). Furthermore, other inhibitory pathways involving GABA participate in the termination of swimming (described above, for review, see McLean et al. 2000). The similar effects on episode duration after the application of either NA (Fig. 2, see also McDearmid, 1998), GABAA receptor agonists (Reith & Sillar, 1997, 1999) and the effects of NA receptor agonists (this paper) raise the possibility that all of these pharmacological agonists share common targets – namely the inhibitory pathways affecting tadpole swimming. However, these hypotheses now need to be addressed by further experimentation.
Acknowledgements
We thank Dr Jonathan R. McDearmid for his contribution to the initial parts of this work and his valuable advice. This work was supported by a grant of the Wellcome Trust to K.T.S. S.D.M. is supported by a BBSRC studentship.
Abbreviations
-
- 5-HT
-
- 5-hydroxytryptamine (serotonin)
-
- CLON
-
- clonidine
-
- CV
-
- coefficient of variation
-
- GABA
-
- gamma-aminobutyric acid
-
- IPSP
-
- inhibitory post synaptic potential;-ISOP, isoproterenol
-
- mhr
-
- mid-hindbrain reticulospinal
-
- MTPR
-
- metaproterenol
-
- NA
-
- noradrenaline
-
- PHEE
-
- phenylephrine
-
- PHENT
-
- phentolamine
-
- PROP
-
- propranolol
-
- RC-delay
-
- rostro-caudal delay
-
- VR
-
- ventral root.




