P2Y receptor-mediated inhibition of voltage-activated Ca2+ currents in PC12 cells
Abstract
To search for inhibitory nucleotide receptors in the sympathoadrenal cell lineage of the rat, voltage-activated Ca2+ currents were recorded in PC12 cells after differentiation with nerve growth factor. ADP and ATP, but not uridine nucleotides, reduced Ca2+ current amplitudes and slowed activation kinetics. This effect was mediated by GTP binding proteins, as it was abolished by intracellular GDPβS and after treatment with pertussis toxin. Furthermore, depolarizations preceding the activation of Ca2+ currents abolished the ADP-induced slowing of activation kinetics and attenuated its inhibitory action on current amplitudes. The modulatory effect of ADP was neither altered in the presence of adenosine receptor antagonists, nor mimicked by agonists at these receptors. In addition, the action of ADP was antagonized by reactive blue 2, but not by suramin or PPADS. Nucleotides tested for their inhibitory action on Ca2+ currents displayed the following rank order of potency: 2-methylthio-ADP ≥ 2-methylthio-ATP >> ADPβS > ADP = ATP. When P2X receptors were blocked, the P2X agonists ATP and 2-methylthio-ATP still reduced Ca2+ currents. The P2Y1 receptor antagonists adenosine-2’-phosphate-5’-phosphate and adenosine-3’-phosphate-5’-phosphate did not alter the inhibitory action of ADP, whereas the Sp-isomer of adenosine-5’-O-(1-thiotriphosphate) and 2’- and 3’-O-(4-benzoylbenzoyl)-ATP showed significant antagonistic activity. These results demonstrate that PC12 cells express an as yet unidentified P2Y receptor with pharmacological characteristics similar to those of P2Y1. As receptor-dependent modulation of Ca2+ channels is a key event in presynaptic inhibition, this receptor may correspond to previously described presynaptic nucleotide receptors mediating autoinhibition of sympathetic transmitter release.
Introduction
Transmembrane Ca2+ entry via voltage-activated Ca2+ channels (VACCs) is of major physiological importance since several neuronal functions, such as cellular migration, gene expression, and neurotransmitter release, depend on this event (Liu et al., 1996). A multitude of neurotransmitters modulate VACCs via appropriate G-protein-coupled receptors and thereby influence neuronal signalling. The modulation of VACCs has been investigated most frequently in sensory and sympathetic neurons (Hille, 1994). There, receptor-dependent activation of G-proteins leads to an inhibition of Ca2+ currents either through a direct interaction of G-protein βγ subunits with VACCs (Zamponi & Snutch, 1998; Kammermeier et al. 2000) or via the generation of diffusible second messengers (Hille, 1994) and activation of protein kinases (Boehm et al., 1996).
Apart from noradrenaline, ATP is a transmitter in postganglionic sympathetic neurons (von Kügelgen & Starke, 1991). While noradrenaline, acting at α2-adrenoceptors, has long been known to cause presynaptic autoinhibtion in sympathetic neurons (Starke, 1987), an analogous mechanism has been described for ATP more recently, but the receptors involved have remained elusive (Fuder & Muth, 1993; von Kügelgen et al., 1993). Moreover, the investigation of inhibitory presynaptic receptors for ATP in sympathetic neurons is hampered by the fact that the nucleotide may also elicit presynaptic facilitation by activating ATP-gated ion channels (Boehm, 1999a). Closure of VACCs via α2-adrenoceptors is the only mechanism underlying the noradrenergic autoinhibition of transmitter release (Boehm & Huck 1996), and, in general, neurotransmitter receptors that cause inhibition of VACCs also mediate presynaptic inhibition (Miller, 1998; Boehm, 1999b). We therefore decided to search for nucleotide receptors inhibiting VACCs in the symapthoadrenal cell lineage. Previous experiments revealed that uridine nucleotides did not to affect VACCs in sympathetic neurons, although they did inhibit M-type K+ channels (Boehm, 1998). ADP, in contrast, did reduce voltage-activated Ca2+ currents (ICa), albeit by < 15% (Filippov et al. 2000). In the present study, we have used the rat phaeochromocytoma cell line PC12 to investigate whether these cells possess nucleotide receptors that modulate ICa. PC12 cells are ontogenetically related to sympathetic neurons, develop a neuronal phenotype upon treatment with nerve growth factor (NGF; Greene & Tischler, 1976), and express various types of neuronal VACCs (Liu et al., 1996).
Like many other neurotransmitters, ATP exerts its actions via two principal classes of receptors, ionotropic P2X receptors and G-protein-coupled P2Y receptors (Fredholm et al., 1994). Currently, four different types of P2Y receptors (P2Y1, −2, −4, and −6) have been described in the rat. P2Y1 receptors are activated by adenine nucleotides, P2Y2 and −4 receptors by ATP and UTP, and P2Y6 receptors by uridine nucleotides only. Upon heterologous expression, all these receptors couple to phospholipase C (Ralevic & Burnstock, 1998), and when expressed in neuronal cells, P2Y1, −2, and −6 receptors mediate an inhibition of VACCs (Filippov et al. 2000; references therein). Previously, NG108-15 (neuroblastoma × glioma hybrid cell line) cells were found to possess an UTP- and ATP-sensitive nucleotide receptor that inhibits ICa (Filippov & Brown, 1996). The present results demonstrate that PC12 cells express a P2Y1-like receptor that inhibits ICa.
Materials and methods
Materials
Na-UTP, Na-UDP, Na2-ATP, Na-ADP, Li3-ADPβS, Li-GTP, Li3-GDPβS, Li4-GTPγS, reactive blue 2 (basilene blue E3G), adenosine-2‘-phosphate-5’-phosphate (A2P5P) and adenosine-3’-phosphate-5’-phosphate (A3P5P), 2‘-& 3‘-O-(4-benzoylbenzoyl)-ATP, creatine phosphokinase type III and creatine phosphate were purchased from Sigma (Vienna, Austria); 2-methylthio-ADP and -ATP, pyridoxal-phosphate-6-azophenyl-2’,4’-disulphonic acid tetrasodium (PPADS), N6-cyclopentyladenosine, 2-p-(2-carboxyethyl)phenethylamino-5’-N-ethylcarboxamidoadenosine (CGS 21680), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), and xanthine amine congener (XAC) from RBI (Natick, MA, USA); the Sp-isomer of adenosine-5’-O-(1-thiotriphosphate) (ATPαS) from Roche Diagnostics (Vienna, Austria); tetrodotoxin from Latoxan (Rosans, France). Suramin was a gift of Bayer, Austria.
Cell culture
PC12 cells were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK), plated onto collagen-coated (Biomedical Technologies Inc., Stoughton, MA, USA) culture dishes (NUNC, Roskilde, Denmark) and were kept in OptiMEM (Life Technologies, Vienna, Austria) supplemented with 0.2 mm l-glutamine (HyClone, Aalst, Belgium), 25.000 IU/L penicillin and 25 mg/L streptomycin (Sigma, Vienna, Austria), 5% fetal calf serum and 10% horse serum (both Life Technologies, Vienna, Austria). Once per week, cell cultures were split, and the medium was exchanged twice weekly. Prior to the recording of ICa, PC12 cells were detached from culture dishes by incubation in Ca2+-free buffer and replated at low density. In order to induce neuronal differentiation, PC12 cells were exposed to recombinant human β-nerve growth factor (NGF, 50 ng/mL, R & D Systems Inc., Wiesbaden, Germany) for 5–6 days.
Electrophysiology
Whole-cell currents were recorded at room temperature (20–24 °C) from PC12 cells 24–48 h after replating at low density, according to pulished procedures (Boehm et al., 1996), using an Axopatch 200B amplifier and the Pclamp 6.0 hard- and software (Axon Instruments, Foster City, CA, USA). Currents were low-pass filtered at 5 kHz, digitized at 1–5 kHz, and stored on an IBM compatible computer. Traces were analysed off-line by the Clampfit program (Axon). Patch electrodes were pulled (Flaming-Brown puller, Sutter Instruments, Novato, CA, USA) from borosilicate glass capillaries (Science Products, Frankfurt/Main, Germany) and filled with a solution consisting of (mM): CsCl, 130; tetraethylammonium chloride, 20; CaCl2, 0.24; glucose, 10; HEPES, 10; EGTA, 5; Mg-ATP, 2; and Li-GTP, 2; adjusted to pH 7.3 with KOH, to yield tip resistancies of 2–3 MΩ. Where indicated, 2 mm GTP was replaced by either 2 mm GDPβS or by 0.2 mm GTPγS. The external bathing solution consisted of (mM): NaCl, 120; tetraethylammonium chloride, 20; KCl, 3; MgCl2, 2; CaCl2, 5; glucose, 20; HEPES, 10, adjusted to pH 7.3 with KOH. Drugs were applied via a DAD-12 drug application device (Adams & List, Westbury, NY, USA) which permits a complete exchange of solutions surrounding the cells under investigation within less than 100 ms (see, e.g. Boehm, 1999b). To evaluate the effects of adenosine and nucleotide receptor antagonists (e.g. suramin, PPADS, reactive blue 2, A2P5P, A3P5P, ATPαS, benzoyl-ATP), neurons were first exposed to these agents for at least 30 s, and then ADP or other nucleotides were applied together with antagonist. Afterwards, nucleotides were tested in the absence of antagonists.
Unless indicated otherwise, whole-cell Ca2+ currents were elicited by 30 ms depolarizations from a holding potential of −80 mV to 0 mV at a frequency of 2–4 per min. To investigate the voltage-dependence of the inhibition of ICa by ADP, a 50-ms depolarization to 0 mV was preceded by a 50-ms predepolarization to +50 mV and a subsequent 10 ms repolarization to −80 mV (Fig. 2C). Currents through P2X receptors were evoked by the direct application of nucleotides at a membrane potential of −80 mV at a frequency of 2 min. In order to enable complete equilibration of the cytoplasm with the internal solution of the pipette containing diverse drugs (Pusch & Neher, 1988), current recordings were started more than 5 min after the establishment of the whole-cell configuration, unless indicated otherwise.
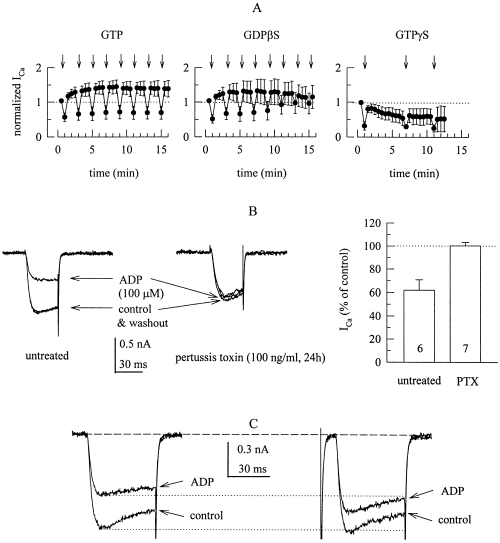
The inhibition of voltage-activated Ca2+ currents by ADP is mediated by G-proteins. (A) Ca2+ currents were determined as shown in B with pipette solutions containing 2 mm GTP, 2 mm GDPβS, or 0.2 mm GTPγS, and the time course of normalized amplitudes is shown. Recordings were started one min after formation of the whole-cell configuration, and 100 µm ADP was applied as indicated by arrows. Current amplitudes were expressed as fraction of the first current amplitude measured; n = 4. (B) Ca2+ currents were evoked by 30 ms depolarizations from −80 to 0 mV before (control), during and after (washout) the application of 100 µm ADP in an untreated (left) and a pertussis toxin-treated (right) PC12 cell. The graph summarizes the inhibitory action of 100 µm ADP in 6 untreated vs 7 pertussis toxin-treated (PTX) PC12 cells by showing current amplitudes in the presence of ADP as percentage of the amplitudes in its absence (percentage of control). (C) Ca2+ currents were evoked by 50 ms depolarizations from −80 to 0 mV before (control) and during the application of 100 µm ADP. In the right hand traces, this depolarization to 0 mV was preceded by a 50-ms depolarization to +50 mV (not shown) followed by a 10-ms repolarization to −80 mV (which elicits the tail current that is lacking in the left hand traces).
Calculations
ICa was quantified by measuring peak current amplitudes during the depolarization to 0 mV. To account for time-dependent changes in Ca2+ current amplitudes (see Fig. 2A), drug effects were evaluated by evoking currents in the presence of test drugs (B) and by comparing them to control currents recorded before (A) and after (washout, C) the application of the drugs, according to the equations: 200 × B/(A + C) = percentage of control current, or 100 − [200 × B/(A + C)] = percentage inhibition (see, e.g. Boehm et al., 1996). Currents through P2X receptors were also quantified by determination of peak current amplitudes.
All data represent arithmetic means ± SEM; n represent numbers of single neurons. Concentration–response curves were fitted to experimentally obtained data by the ALLFIT programme (DeLean et al., 1978) which provides estimates ( ± SEM) for half maximal concentrations, as well as minimal and maximal effects, and determines the qualities of fitted results. Differences between single concentration–response curves were determined by simultaneous fitting with shared parameters and subsequent calculation of the F-statistic on the resulting ‘extra sum of squares’ (DeLean et al., 1978). Significance of differences between single data points was evaluted by the Mann–Whitney test.
Results
ADP inhibits ICa in untreated as well as NGF-treated PC12 cells
Depolarization of PC12 cells, whether treated with NGF or not, from −80 mV to 0 mV elicited ICa with rapid activation kinetics, the time constants of activation were: 2.2 ± 0.2 ms in untreated (n = 7) and 2.5 ± 0.3 ms in NGF-treated (n = 5) PC12 cells. As reported previously (Bouron et al., 1999), whole cell Ica were significantly larger in NGF-treated cells than in untreated cells, but this rise in current amplitudes was accompanied by an increase in cell capacitance, so that current densities in NGF-treated cells were not significantly larger than in untreated cells (Fig. 1A and C). In the presence of 100 µm ADP, ICa amplitudes were reduced by 30% in untreated and by 60% in NGF-treated PC12 cells, and this effect was entirely reversible upon removal of the nucleotide (Fig. 1A and C). Apart from reducing current amplitudes, ADP markedly slowed activation kinteics which resulted in a biexponential activation time course, the fast components of activation had time constants of: 1.6 ± 0.2 ms in untreated (n = 7) and of 2.4 ± 0.7 in NGF-treated (n = 5) cells, whereas the second slow components had time constants of 51.2 ± 32.8 and 71.4 ± 65.8 ms, respectively.
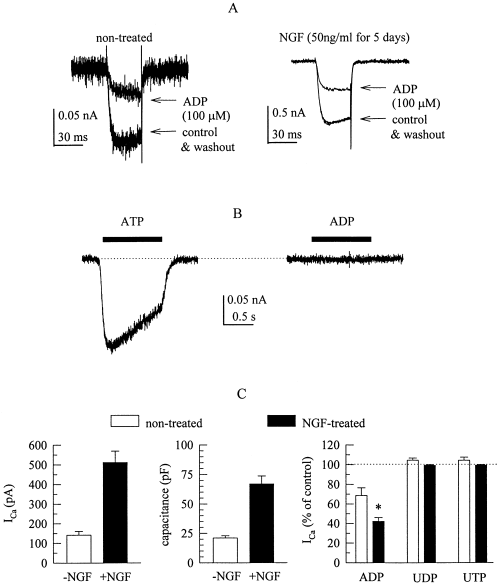
Adenine, but not uridine, nucleotides reduce voltage-activated Ca2+ currents in differentiated as well as nondifferentiated PC12 cells. Where indicated, PC12 cells had been differentiated by exposure to 50 ng/mL NGF for 5 days prior to the recording of Ca2+ currents. (A) Ca2+ currents were evoked by 30 ms depolarizations from −80 to 0 mV before (control), during and after (washout) the application of 100 µm ADP in a nontreated (left) and a NGF-treated (right) PC12 cell. (B) ATP, but not ADP (both at 100 µm), rapidly elicits inward currents in a nondifferentiated PC12 cell clamped at a holding potential of −80 mV. Nucleotides were present as indicated by the black bars. (C) Summary of the characteristics of Ca2+ currents (recorded as shown in A) in differentiated (n = 5; black bars) as well as nondifferentiated (n = 7; white bars) PC12 cells. The graphs show mean Ca2+ current amplitudes (left), cell capacitance (middle), and Ca2+ current amplitudes in the presence of ADP, UDP, and UTP (all at 100 µm, right), expressed as percentage of current amplitudes in the absence of nucleotides (percentage of control).
In addition to 100 µm ADP, PC12 cells were exposed to the same concentration of ATP, UDP and UTP. In the presence of such high concentrations of ATP, Ca2+ currents could no longer be measured, since ATP acts at P2X receptors and thereby elicits rapidly activating inward currents at negative membrane potentials, an effect that is not seen in the presence of ADP (Fig. 1B). Subsequent to the application of 100 µm ATP, ICa amplitudes were irreversibly diminished (not shown), most likely due to transmembrane Ca2+ entry caused by the opening of the ATP-gated ion channels. UDP and UTP, both at 100 µm, failed to alter ICa (Fig. 1C).
The inhibitory action of ADP is mediated by GTP binding proteins
Receptor-dependent inhibition of VACCs most commonly involves GTP binding proteins (e.g. Hille, 1994; Zamponi & Snutch, 1998). To test for a role of G-proteins, electrodes were filled with solutions containing 2 mm GTP, 2 mm GDPβS, or 0.2 mm GTPγS, and ICa recordings were started one minute after formation of the whole-cell configuration. With GTP and GDPβS, ICa displayed significant ‘run-up’, whereas accelerated ‘run-down’ was observed with GTPγS. With GTP in the electrode, the inhibitory action of 100 µm ADP was constant for more than 15 min, whereas the effect was lost during this period of time when GTP was replaced by GDPβS. With GTPγS, the rapid run-down occluded the inhibition by ADP.
To obtain evidence as to which types of G-proteins might be involved in the inhibitory action of ADP, PC12 cells were treated with pertussis toxin (100 ng/mL for 24 h) and ICa was recorded in the absence and presence of 100 µm ADP; in these cells, ADP failed to alter ICa. In sister cultures, not treated with pertussis toxin, ICa was reduced in the presence of ADP by about 40% (Fig. 2B).
Upon activation, G-proteins may inhibit VACCs through a direct interaction of βγ subunits with the ion channels, an effect that is voltage-dependent and, thus, attenuated by depolarizing prepulses (Zamponi & Snutch, 1998; Kammermeier et al. 2000). Accordingly, predepolarization of PC12 cells to +50 mV prior to the activation of ICa attenuated the inhibitory action of ADP and prevented the agonist-induced slowing of activation kinetics (Fig. 2C).
The inhibitory action of ADP does not involve adenosine receptors
Adenine nucleotides are rapidly degraded by a cascade of nucleotidases to generate adenosine as the final product. Therefore, we tested adenosine receptor agonists and antagonists to find out whether the nucleoside might contribute to the action of ADP. However, in cells which clearly showed an inhibition of ICa by ADP, neither the A1 adenosine receptor agonist cyclopentyladenosine (1 µm, Fig. 3C) nor the A2 receptor agonist CGS 21680 (5 µm; Fig. 3A and B) caused significant alterations in ICa amplitudes. Furthermore, the inhibitory action of 100 µm ADP was not altered in the presence of the A1 receptor antagonist DPCPX (3 µm) or the nonselective adenosine receptor antagonist XAC (1 µm; Fig. 3D).
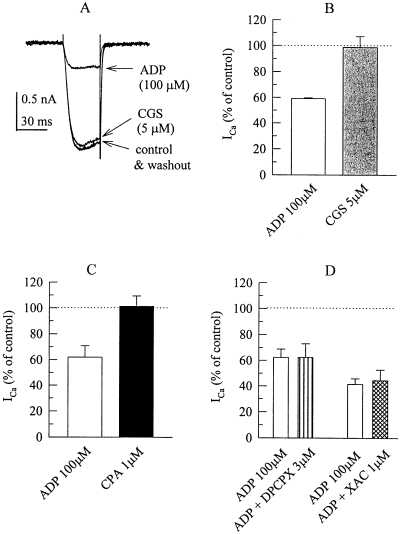
The inhibition of voltage-activated Ca2+ currents by ADP does not involve adenosine receptors. (A) Ca2+ currents were evoked by 30 ms depolarizations to 0 mV before (control), during, and after (washout) the application of 100 µm ADP and 5 µm CGS 21680. (B) Summary of the effects of ADP and CGS 21680 on Ca2+ current amplitudes (shown as percentage of control) as determined in a set of 5 PC12 cells. (C) Summary of the effects of ADP and cyclopentyladenosine on Ca2+ current amplitudes (shown as percentage of control) as determined in a set of 6 PC12 cells. (D) Summary of the effects of ADP applied either alone or together with DPCPX or XAC on Ca2+ current amplitudes (shown as percentage of control) in two different sets of 5 PC12 cells each.
Effects of P2 receptor antagonists on the inhibition of ICa by ADP
The results shown above suggest that it was the nucleotide itself and not the degradation product, adenosine, that caused the inhibition of ICa. To prove that ADP acted via a nucleotide receptor, we investigated the effects of several nonselective P2 receptor antagonists on the inhibition of ICa by ADP. Suramin and PPADS, both at concentrations of up to 100 µm, did not alter the reduction of ICa amplitudes by 100 µm ADP (Fig. 4B). However, reactive blue 2 at 30 µm virtually abolished the inhibitory action of this concentration of ADP (Fig. 4A and B). In addition, reactive blue 2 per se enhanced ICa amplitudes (Fig. 4A) by 16.5 ± 5.4% (n = 5; P < 0.05), whereas suramin and PPADS had no such effect (not shown).
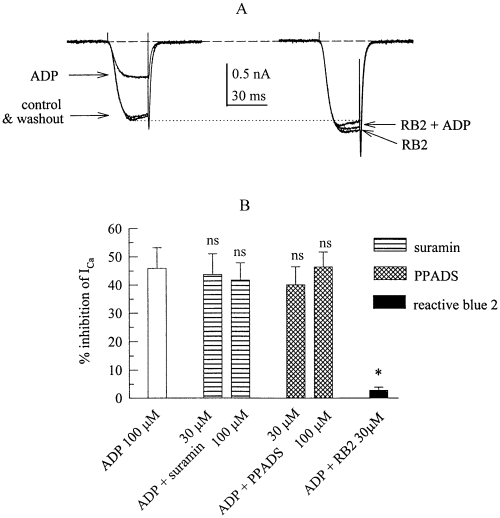
The inhibition of voltage-activated Ca2+ currents by ADP is antagonzied by a P2 purinoceptor antagonist. (A) Ca2+ currents were evoked by 30 ms depolarizations to 0 mV before (control), during, and after (washout) the application of 100 µm ADP (left hand traces) and then in the presence of 30 µm reactive blue 2 (RB2) applied either alone or together with 100 µm ADP. (B) Summary of the inhibitory effects of ADP (shown as percentage inhibition) applied either alone or together with suramin (30 or 100 µm), PPADS (30 or 100 µm), or reactive blue 2 (RB2, 30 µm) on Ca2+ current amplitudes in s set of 5 PC12 cells. n.s., not significantly different from the inhibition caused by 100 µm ADP applied alone; *, significantly different from the inhibition caused by 100 µm ADP applied alone at P < 0.05.
Agonist pharmacology of the P2 receptor mediating the inhibition of ICa by ADP
The data presented so far indicates that the action of ADP on ICa was mediated by a G-protein coupled nucleotide receptor, i.e. a P2Y purinoceptor. To further characterize this receptor, the following nucleotides were applied at concentrations between 1 nm and 300 µm: ATP, ADP, ADPβS, 2-methylthio-ATP and 2-methylthio-ADP. To account for differences in the inhibitory actions of nucleotides in between different cells, the effects of these nucleotides were compared with the effect of 100 µm ADP in the very same cell. Concentration–response curves for the reduction of ICa amplitudes obtained in this manner revealed that the action of ADP was half maximal at 34.1 ± 9.1 µm. ATP could be tested at concentrations of up to 30 µm only (higher concentrations produced large P2X receptor-mediated inward currents, see 1, 6); at these concentrations, ATP inhibited ICa to about the same extent as ADP. Assuming that ATP might cause the same maximal inhibition as ADP (see Fig. 6C), the action of ATP was calculated to be half maximal at 25.0 ± 7.5 µm (Fig. 5A). ADPβS caused the same extent of maximal inhibition as ADP, but was significantly more potent than ADP with half maximal inhibition of ICa at 8.8 ± 1.5 µm (P < 0.05 vs ADP). The most potent agonists were 2-methylthio-ADP and -ATP, the inhibition being half-maximal at 38.4 ± 14.4 nm and 104.2 ± 47.4 nm, respectively; these half maximal concentrations were not significantly different from each other (P > 0.1).
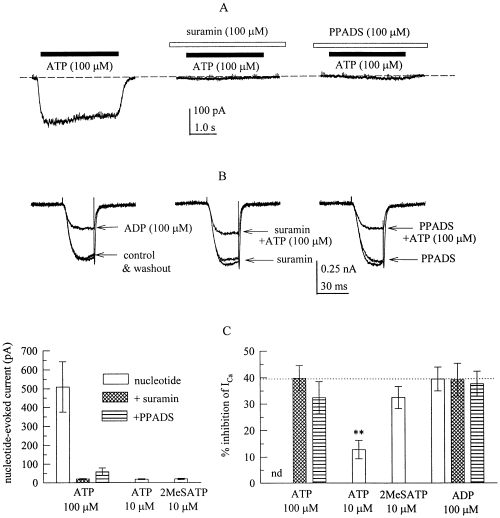
The inhibition of voltage-activated Ca2+ currents by ATP and 2-methylthio-ATP does not involve P2X receptors. ADP, ATP and 2-methylthio-ATP were applied either alone or in the continuous presence of suramin or PPADS, and nucleotide-evoked currents or Ca2+ currents were determined as described in the legend to Fig. 1. A shows inward currents elicited by 100 µm ATP in the absence or presence of 100 µm suramin or PPDAS in a PC12 cell clamped at a holding potential of −80 mV. Nucleotides were present as indicated by the black bars, antagonsits as shown by the white bars. (B) Ca2+ currents were evoked by 30 ms depolarizations to 0 mV before (control), during, and after (washout) the application of 100 µm ADP or ATP either in the absence of antagonists or with 100 µm suramin or PPDAS being present before, together with, and after the application of 100 µm ATP. (C) Summary of the amplitudes of nucleotide-evoked inward currents (left graph) and the amount of inhibition of Ca2+ currents (right graph) induced by ADP (100 µm), ATP (10 or 100 µm), or 2-methylthio-ATP (2MeSATP, 10 µm) in either the absence or presence of 100 µm suramin or PPDAS. **P < 0.01 vs the inhibition of ICa by 100 µm ADP; n = 9–15.
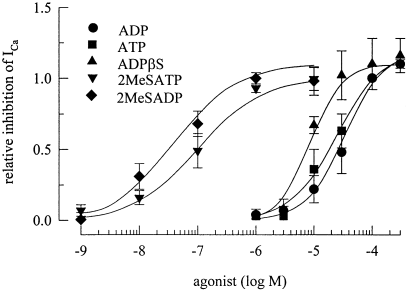
Agonist pharmacology of the receptor mediating the inhibition of voltage-activated Ca2+ currents. Ca2+ currents were determined as described above, and ADP, ATP, ADPβS, 2-methylthio-ADP, or 2-methylthio-ATP were applied. To account for differences in the amount of inhibition observed in different cells, the inhibitory action of each agonist concentration was normalized to the effect of 100 µm ADP in the very same cell, and the graph shows the concentration-dependences of this relative inhibition of ICa amplitudes. n = 5–10. Lines were fitted to the experimentally obtained data by the ALLFIT programme (see Methods).
P2X rexceptors do not contribute to the inhibition of ICa
At least two of the agonists used above, namely ATP and 2-methylthio-ATP, may activate not only P2Y, but also P2X receptors (Boehm, 1999a; Ralevic & Burnstock, 1998), and several P2X receptor subunits are expressed in PC12 cells (Arslan et al. 2000). To exclude that activation of P2X receptors might contribute to the inhibition of Ica by these agonists, 100 µm ATP was applied in the absence and presence of the P2 antagonists suramin or PPADS (both at 100 µm), respectively; these antagonists did not attenuate the inibition of ICa by ADP (see Fig. 4) but they are known to block ATP-evoked currents through P2X receptors (e.g. Boehm, 1999a). ATP applied alone elicited inward currents with amplitudes in the range of 0.1–1 nA, which were reduced by suramin or PPADS by ≥ 90% (Fig. 6A and C). Nevertheless, in the presence of these antagonists, ATP (100 µm) did reduce ICa to the same extent as ADP (100 µm), and the action of ADP in the presence of suramin or PPADS was not different from its inhibitory effect when applied alone (Fig. 6B and C). To corroborate that activation of P2X receptors was not required for the inhibition of ICa, 10 µm ATP or 2-methylthio-ATP were used. At these concentrations, the adenine triphosphates did not cause significant inward currents, but clearly reduced ICa (Fig. 6C) to about the same relative extent as indicated in the concentration–response in Fig. 5. These results prove that P2X receptor activation was not a prerequisite for the inhibition of ICa.
The receptor mediating inhibition of ICa is P2Y1-like but not P2Y1
The agonist pharmacology described in Fig. 5 is compatible with those of cloned P2Y1 receptors (e.g. Leon et al., 1997; Ralevic & Burnstock, 1998; Filippov et al. 2000). Therefore, ADP (100 µm) was applied in presence of 100 µm of the selective P2Y1 receptor antagonists A2P5P and A3P5P (Boyer et al., 1996) which, however, failed to alter the inhibitory action of the nucleotide (Fig. 7A). In contrast, the inhibition of ICa by 100 µm ADP was significantly attenuated by 100 µm benzoyl-ATP (Fig. 7A) which blocks not only P2Y1, but also P2Y1-like, receptors (Vigne et al., 1999). A2P5P, A3P5P, and benzoyl-ATP per se did not alter ICa amplitudes. ATPαS has been shown to block P2Y1-like (Hechler et al., 1998a), but not P2Y1 receptors, at which it rather appears to be an agonist (Vöhringer et al. 2000). At 100 µm, this nucleotide elicited inward currents (presumably by activation of P2X receptors), and subsequently reduced ICa in an irreversible manner, as did ATP (see above). At 30 µm, however, ATPαS had no such effect, but attenuated the inhibitory action of 30 µm ADP on ICa (Fig. 7A).
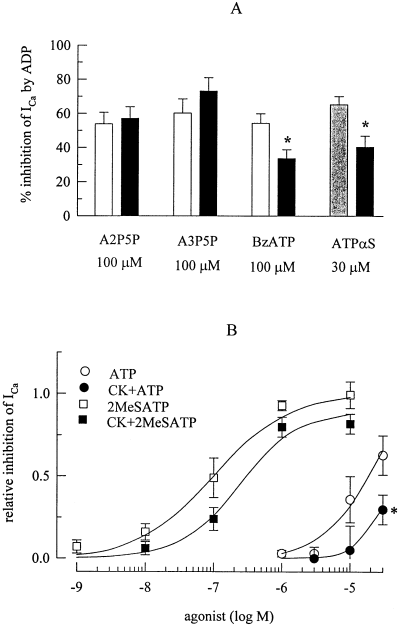
The inhibition of voltage-activated Ca2+ channels is not mediated by P2Y1, but by P2Y1-like receptors. Ca2+ currents were determined as described above. (A) Summary of the inhibitory effects (shown as percentage inhibition) of 100 µm (white bars) or 30 µm (shaded bar) ADP applied either alone or together with A2P5P, A3P5P, benzoyl-ATP (BzATP), or ATPαS on ICa amplitudes in sets of 9–12 PC12 cells each. (B) Summary of the inhibitory effects of ATP and 2-methylthio-ATP applied either alone or after a 90-min treatment with, and in the presence of creatine phosphokinase (20 U/mL) and 10 mm creatine phosphate. To account for differences in the amount of inhibition observed in different cells, the inhibitory action of each agonist concentration was normalized to the effect of 100 µm ADP in the very same neuron, and the graph shows the concentration-dependences of this relative inhibition of Ca2+ current amplitudes (see Fig. 5). n = 5–11. Lines were fitted to the experimentally obtained data by the ALLFIT programme (see Methods). *P < 0.05 vs the effect of the same concentration of ATP in the absence of creatine phosphokinase and creatine phosphate.
ATP preparations may contain amounts of ADP sufficient to activate P2Y receptors. To find out whether the actions of ATP and 2-methylthio-ATP were caused by respective diphosphate contaminations, ATP and 2-methylthio-ATP containing solutions were treated with an ATP regenerating system provided by creatine phosphokinase (20 U/mL) and 10 mm creatine phosphate for at least 90 min, which has been shown to virtually eliminate diphosphate contaminations (Hechler et al., 1998b). In addition, ADP may be generated from ATP by ectonucleotidases. For this reason, ATP and 2-methylthio-ATP were applied to PC12 cells together with creatine phosphokinase (20 U/mL) and 10 mm creatine phosphate. This procedure did not significantly alter the maximal inhibition achieved by 2-methylthio-ATP, but raised its half maximal concentration to 250.2 ± 78.8 nm (P < 0.05 vs 2-methylthio-ATP in the absence of creatine phosphokinase and creatine phosphate; Fig. 7B). The fitted concentration–response curves for ATP in the absence and presence of the ATP regenerating system, respectively, were not significantly different from each other, most likely because concentrations of up to 30 µm only could be applied (see Fig. 6). Nevertheless, at this concentration, ATP produced significantly less inhibition of ICa in the presence than in the absence of creatine phosphokinase and creatine phosphate (Fig. 7B).
Discussion
Our results demonstrate that adenine, but not uridine, nucleotides inhibit ICa in PC12 cells. Previously, ATP and other nucleotides have been found to diminish ICa in rat (Lim et al., 1997) and bovine (Gandia et al., 1993) adrenal chromaffin cells, in frog sympathetic neurons (Elmslie, 1992), in neuroblastoma × glioma hybrid cells (Filippov & Brown, 1996), and in ferret cardiac myocytes (Qu et al., 1993). In contrast, ATP has also been reported to augment ICa in rat hippocampal neurons (Dave & Mogul, 1996) and cardiac cells (Scamps & Vassort, 1994). Thus, the modulation of VACCs by extracellular nucleotides is a known phenomenon, but the receptors involved in this effect remained largely unknown. When heterologously expressed in sympathetic neurons, rat P2Y1, −2, -and −6 receptors have recently been found to cause inhibition of neuronal Ca2+ channels (Filippov et al. 2000; references therein). This indicates that most, if not all, known P2Y receptor subtypes possess the capacity to mediate an inhibition of neuronal VACCs, at least in heterologous expression systems.
P2Y, but not P2X or P1, receptors cause inhibition of VACCs in PC12 cells
The superfamily of receptors for purines and pyrimidines comprises representatives of two principal classes of neurotransmitter receptors, i.e. ligand-gated ion channels and G-protein-coupled receptors (Fredholm et al., 1994; Ralevic & Burnstock, 1998). Several of the results presented above show that the nucleotide-induced inhibition of ICa was mediated by G-proteins, and not by ATP-gated ion channels, i.e. P2X receptors. (i) Under conditions that prevented the activation of ionotropic purine receptors, the nucleotides used did reduce ICa. (ii) Intracellular application of the transphosphorylation-resistant GDP analogue GDPβS, which traps G-proteins in an inactive conformation, abolished the inhibitory effect of ADP on ICa; this occurred in a time-dependent manner which reflects, on one hand, the time that is needed for dialysis of the cytoplasm with the GDPβS-containing soution (Pusch & Neher, 1988), and on the other hand, the time that is required to exchange GDPβS for GDP within the nucleotide binding pocket of the G-protein α-subunits (Nanoff et al., 1994). (iii) Intracellular application of GTPγS, which traps G-proteins in an active conformation, slowly reduced ICa amplitudes and thereby increasingly occluded the inhibitory effect of ADP. (iv) Treatment of the cells with pertussis toxin which prevents the receptor-dependent activation of Gi and Go proteins entirely abolished the inhibition of ICa by ADP. (v) Depolarizing prepulses attenuated the inhibitory action of ADP on ICa. This voltage-dependent type of ICa inhibition has been shown to be mediated by G-protein βγ subunits, whereas a voltage-independent inhibition of ICa in sympathetic neurons involves G-protein α-subunits (Kammermeier et al. 2000). Thus, the inhibitory action of ADP on ICa can be assumed to be mediated by βγ subunits of inhibitory G-protein heterotrimers. Taken together, these results indicate that ADP acted via a G-protein-coupled receptor, but not via P2X receptors.
The group of G-protein coupled receptors for purines and pyrimidines consists of receptors for adenosine (P1 receptors) and of receptors for nucleotides (P2Y receptors; Fredholm et al., 1994; Ralevic & Burnstock, 1998). In cells that showed a pronounced inhibitory effect of ADP, agonists at adenosine receptors did not alter ICa. Furthermore, the inhibition of ICa by ADP was not altered by adenosine receptor antagonists, but was largely attenuated by the P2 receptor antagonist, reactive blue 2. Thus, ADP diminished ICa via a G-protein-coupled nucleotide receptor, i.e. via a P2Y receptor, and not via a P1 (adenosine) receptor.
ADP inhibits VACCs in PC12 cells via P2Y1-like receptors
Currently, four different subtypes of rat P2Y receptors have been characterized by molecular means: P2Y1, −2, −4 and −6 (Ralevic & Burnstock, 1998). Amongst these, the P2Y1 receptor is the only one that is not activated by uridine nucleotides, and uridine nucleotides did not affect ICa in PC12 cells. In contrast, ADP and ATP at micromolar concentrations equipotently inhibited ICa. Therefore, we tested additional agonists that are known to activate P2Y1 receptors, such as 2-methylthio-ADP and -ATP and ADPβS, for their capacity to inhibit ICa. ADPβS was about four-fold more potent than ADP in inhibiting ICa, and the 2-methylthio-derivatives were at least 300-fold more potent than ADP. These pharmacological characteristics resemble those of cloned P2Y1 receptors (Ralevic & Burnstock, 1998). In some, but not all, experimental systems, adenosine triphosphates were found to be antagonists at heterologously expressed P2Y1 receptors, and this fact may be occluded by diphosphate contaminations in commercially available ATP preparations (e.g. Hechler et al., 1998b; Palmer et al., 1998; Filippov et al. 2000). However, an ATP regenerating system, that is known to minimize ADP contaminations, reduced the potency, but did not prevent the agonistic acitivity, of ATP and 2-methylthio-ATP.
So far, the results are compatible with the ADP-induced inhibition of ICa in PC12 cells being mediated by P2Y1 receptors. However, the selective P2Y1 receptor antagonists A2P5P and A3P5P (Boyer et al., 1996; Filippov et al. 2000) failed to antagonize the effect of ADP. In contrast, ATPαS an antagonist at P2Y1-like receptors, which inhibit adenylyl cyclase activity in platelets (Hechler et al., 1998a), but not at P2Y1 receptors themselves (Hechler et al., 1998a; Vöhringer et al. 2000), attenuated the inhibitory action of ADP. Similarly, benzoyl-ATP, which blocks P2Y1 as well as cyclase-inhibiting P2Y1-like receptors (Vigne et al., 1999), also attenuated the inhibition of ICa by ADP. Additional data also argue against a role of P2Y1 receptors in the modulation of ICa by ADP: (i) Suramin and PPADS are antagonists at recombinant P2Y1 receptors (e.g. Leon et al., 1997; King et al., 1998), but did not alter the inhibition of ICa by ADP; (ii) In heterologous expression systems, P2Y1 receptors are activated by ADP in submicromolar, rather than micromolar (see above), concentrations, and 2-methylthio-ADP is about 10-fold, not 1000-fold (as above), more potent than ADP (Leon et al., 1997; Palmer et al., 1998; Filippov et al. 2000); (iii) PC12 cells have been reported not to express P2Y1 receptors (Arslan et al. 2000), and a RT-PCR analysis performed in the PC12 clone we used detected transcripts for P2Y2, −4, and −6, but none for P2Y1 (Moskvina and Boehm, unpublished observation). Thus, the receptor mediating the inhibition of ICa by ADP is a P2Y1-like receptor, but not P2Y1 itself.
Apart from P2Y1 receptors, the human P2Y11 receptor is activated by adenine, but not uridine, nucleotides (Communi et al., 1997). Hence, a homologous receptor in the rat might be involved in the effects described above. However, ADP and 2-methylthio-ADP are only weak agonists at P2Y11. Furthermore, this receptor stimulates the formation of cyclic AMP and is thus linked to stimulatory, rather than inhibitory, G-proteins (Communi et al., 1997).
Significance of the P2Y receptor-mediated inhibition of VACCs
Sympathetic neurons, chromaffin cells, and PC12 cells store and release not only catecholamines and peptides, but also ATP (von Kügelgen & Starke, 1991; Winkler, 1993). Hence, upon vesicle exocytosis, endogenous ATP may act on receptors located in the membrane of those cells which the nucleotide has been released from. In addition, nucleotidases rapidly hydrolyse released ATP (Todorov et al., 1997) and degradation products, such as ADP and adenosine, may also act on these receptors. Indeed, in chromaffin cells VACCs were found to be under control of an autocrine modulator that was suggested to be ATP (Currie & Fox, 1996), although the receptors involved remained unknown.
Transmitter release relies on transmembrane Ca2+ entry via VACCs, and the receptor-dependent inhibition of Ca2+ channels was suggested to be the only mechanism mediating the autoinhibitory modulation of sympathetic transmitter release via presynaptic α2-adrenoceptors (Boehm & Huck, 1996). Given that the receptors described above are also expressed in sympathetic neurons, these P2Y1-like receptors may underly the ATP-dependent presynaptic autoinhibition previously described for the sympathetic nervous system (Fuder & Muth, 1993; von Kügelgen et al., 1993; Boehm, 1999a).
Acknowledgements
The authors are indebted to M. Freissmuth for careful reading of the manuscript. This work was supported by grants from the Austrian Science Fund (FWF; P12997-MED and P13920-MED), from the Jubiläumsfonds der Österreichischen Nationalbank (8377) and from the Anton-Dreher-Gedächtnisschenkung. N.V. received a PhD fellowship from the University of Vienna.
Abbreviations
-
- A2P5P
-
- adenosine-2‘-phosphate-5’-phosphate
-
- A3P5P
-
- adenosine-3’-phosphate-5’-phosphate
-
- ATPαS
-
- Sp-isomer of adenosine-5’-O-(1-thiotriphosphate)
-
- CGS 21680
-
- 2-p-(2-carboxyethyl)phenethylamino-5’-N-ethylcarboxamidoadenosine
-
- CPA
-
- N6-cyclopentyladenosine
-
- DPCPX
-
- 8-cyclopentyl-1,3-dipropylxanthine
-
- EGTA
-
- ethylene glycol bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid
-
- HEPES
-
- 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
-
- Ica
-
- voltage-activated Ca2+ currents
-
- NGF
-
- nerve growth factor, PPADS, pyridoxal-phosphate-6-azophenyl-2’,4’-disulphonic acid tetrasodium
-
- VACCs
-
- voltage-activated Ca2+ channels
-
- XAC
-
- xanthine amine congener.




