Role of GAP-43 in mediating the responsiveness of cerebellar and precerebellar neurons to axotomy
Abstract
To determine whether the competence for axonal sprouting and/or regeneration in the cerebellar system correlates with GAP-43 expression, we have studied GAP-43 mRNA and protein expression in the postlesioned cerebellum and inferior olive. Purkinje cells transiently express GAP-43 during their developmental phase (from E15 to P5 in the rat) which consists of fast axonal growth and the formation of the corticonuclear projection. Adult Purkinje cells, which in control adult rats do not express GAP-43, are extremely resistant to the effects of axotomy but cannot regenerate axons. However, a late and protracted sprouting of axotomized Purkinje cells occurs spontaneously and correlates with a mild expression of GAP-43 mRNA. In contrast, inferior olivary neurons, despite their high constitutive expression of GAP-43, do not sprout but retract their axons and die after axotomy. Furthermore, mature Purkinje cells in cerebellar explants of transgenic mice that overexpress GAP-43 do not regenerate after axotomy, even in the presence of a permissive substrate (cerebellar embryonic tissue) and, contrary to the case in wild-type mice, they do not survive in the in vitro conditions and undergo massive cell death. These results show that the expression of GAP-43 is not only associated with axonal growth, but also with neuronal death.
Introduction
There is mounting evidence suggesting the importance of the cellular environment for axonal regeneration (Schwab & Bartholdi, 1996). It is, however, generally accepted that successful regeneration results from an interplay between intrinsic and environmental factors, and is associated with specific changes in gene expression of the injured neurons (Caroni, 1997). Present knowledge on the genetic cascade allowing adult injured neurons to regenerate their axons is still very limited. GAP-43 (growth associated protein of 43 kDa) is the protein that has been the most frequently associated with axonal growth during development and axonal regeneration in the adult. GAP-43 is expressed at high levels during development, and is down-regulated as the neurons mature (Skene, 1989), although its expression persists in a number of adult neurons (Kruger et al., 1993).
The cerebellar system is a good model system in which to gain insight into the role of GAP-43 during axonal lesion and regeneration. Studies on lesioned cerebellum have shown that mature Purkinje cells are remarkably resistant to axotomy, and that they do not regenerate even in the presence of permissive environments, such as transplanted sciatic nerve or embryonic neurons (David & Aguayo, 1981; Dusart & Sotelo, 1994; Rossi et al., 1995; Bravin et al., 1997). On the other hand, precerebellar inferior olivary neurons, that give rise to the climbing fibers, exhibit an impressive ability to regenerate when confronted with growth permissive environments (Armengol et al., 1989; Rossi et al., 1995; Bravin et al., 1997). Inferior olivary neurons, contrary to Purkinje cells, have a constitutive expression of GAP-43 (Kruger et al., 1993; Console-Bram et al., 1996). One could therefore hypothesize that GAP-43 expression is necessary for a successful regeneration in the cerebellar system.
With an in vitro approach using cerebellar explants, we have previously shown that Purkinje cell survival and axonal regeneration are age-dependent (Dusart et al., 1997). Furthermore, despite the lack of regeneration, lesioned Purkinje cells in adult cerebella are capable of a late and protracted process of terminal sprouting that begins only 3 months after axotomy, and becomes maximal around 12–18 months (Dusart et al., 1999). The aim of the present study was, therefore, to determine whether the neuronal competence for axonal sprouting and/or regeneration, in the cerebellar system, correlates with the expression of GAP-43.
First, we show that embryonic and perinatal Purkinje cells – during the formation of the corticonuclear projection – express GAP-43, an expression which completely disappears by P5 in rats and P7 in mice. Second, we show that the late and protracted sprouting of axotomized Purkinje cells in adult rats is temporally correlated with a mild expression of GAP-43. In contrast, inferior olivary neurons, despite their GAP-43 expression, do not sprout after the lesions but follow a progressive process of retrograde cell death. Finally, we have tested the effect of GAP-43 in mature Purkinje cells, using cerebellar explants taken from transgenic mice constitutively expressing this growth protein in neurons (Aigner et al., 1995). Our results show that, as it has been previously shown in vivo (Buffo et al., 1997), the expression of GAP-43 is not sufficient to promote regeneration in Purkinje cells and also, unexpectedly, that in the explants, the vulnerability of these Purkinje cells to cell death increases. Thus, GAP-43 is involved in the ‘essential choice’ of the signalling pathway that will provoke either axonal growth or cell death, two opposite cellular ‘decisions’ which are surprisingly linked (Herdegen et al., 1997).
Materials and methods
In vivo studies
Animals
All procedures were carried out in accordance to the guidelines approved by the French Ministry of Agriculture, following European Standards. Fifty-four female Wistar rats (weighing 200 g at the time of lesioning) were used in this study. The lesioning procedure has been described previously (Dusart & Sotelo, 1994). The rats were anaesthetized with chloral hydrate (400 mg/kg; i.p.), and after the lesion, the animals were returned to their cages and given free access to food and water. The lesioned rats were divided in two principal groups which were either prepared for immunohistochemistry or for in situ hybridization. At each time point, 8 and 15 days, 1, 2, 3, 6, 12 and 18 months after the operation, three animals were perfused transcardially for immunohistochemistry and three animals were decapitated for in situ hybridization. For controls, six non-operated animals were processed in the same way. For the study of GAP-43 expression during development, rat fetuses at E15, E18, E20 (E0 is the first day of gestation), and rat and mouse pups at P0, P3, P5, P7, P10 (P0 is the day of birth) were decapitated. At least, three animals were used for each age.
Immunohistochemistry of GAP-43
GAP-43 distribution was studied by using 9-1-E12 mouse monoclonal antibody against GAP-43 (kind gift of Dr P. Skene, Schreyer & Skene, 1991; also sold by Boehringer, Mannheim, Germany). Rats were anaesthetized and perfused through the aorta with 0.12 m phosphate buffered (pH 7.4) 4% paraformaldehyde. Brains were removed, postfixed for 4 h and cryoprotected in 30% sucrose for 2 days. The cerebella were cut in the sagittal plane and the inferior oliviary neurons in the coronal plane (24-µm-thick free-floating sections) on a freezing microtome.
The sections were incubated overnight in the first antibody against GAP-43 (1 : 20 000 in phosphate buffer, 0.12 m, pH 7.4) containing 0.9% NaCl with 0.25% triton, 0.2% gelatin and 0.1% sodium azide (PBSGTA). The sections were then incubated for 2 h in a biotinylated horse anti-mouse IgG antibody adsorbed with rat serum (Vector Laboratories, Burlingame, CA; dilution 1 : 100 in PBSGT). The anti-mouse IgG antibody used was adsorbed with rat serum due to the opening of the blood brain barrier. Then, streptavidin–biotin–horseradish peroxidase complex (Amersham, Arlington Heights, IL; dilution 1 : 400) was applied. Histochemical detection of the peroxidase activity was carried out in 0.1 m Tris buffer (pH = 7.4) containing 0.03% 3,3′diaminobenzidine tetrahydrochloride (Sigma, Saint Louis, MO) with 0.005% hydrogen peroxide. Sections were mounted on gelatin-coated slides, dehydrated, covered with Eukitt mounting medium and analysed using a Zeiss Axiophot microscope.
In situ hybridization
A GAP-43 mouse cDNA fragment of 740 bp subcloned into the pCDNA3 plasmid was linearized with Xba1 (Boehringer, Mannheim, Germany) for antisense RNA synthesis by Sp6 polymerase (Boehringer) and with EcoR1 (Boehringer) for sense RNA synthesis by T7 polymerase (Pharmacia Biotech, Orsay, France). The in vitro transcription was carried out by using the Riboprobe Gemini System II buffers (Promega, Madison, WI, USA) and probes were labelled with 35SdUTP (1000 Ci/mM; Amersham, Arlington Heights, IL, USA) as described in Fontaine & Changeux (1989). In situ hybridization for cRNA probes was performed on fresh frozen cerebellar and inferior olivary sections, taken from cerebella and brainstems rapidly removed after decapitation. The sections (15 µm) were cut serially at −18 °C, mounted on gelatinized coated glass slides and stored at −80 °C until the hybridization was complete. Tissue sections were postfixed for 15 min in 4% paraformaldehyde in PBS, washed in PBS, acetylated, washed in PBS, dehydrated and air dried. Sections were covered with hybridization buffer containing 0.1 × 106 c.p.m./µL of the GAP-43 probe (50 µL per section) and then incubated overnight in a humid chamber at 48 °C. Washes were then performed as described in Fontaine & Changeux (1989). From P0, the in situ hybridization of the cerebellum was combined with an immunostainin for anticalbindin (CaBP) antibody (gift of Dr E.M. Lawson; Spencer et al., 1976) to visualize the Purkinje cells. After several washes, the slides were rinsed in PBSGTA and incubated overnight at room temperature in the CaBP antibody (dilution 1 : 10 000). The sections were then incubated for 2 h in a biotinylated goat anti-rabbit IgG (Vector dilution 1/200 in PBSGT), and revealed with streptavidin–biotinylated–horseradish peroxidase complex (Amersham, dilution 1/400 in PBSGT). Histochemical detection of the peroxidase activity was carried out in the same way as for GAP-43 immunohistochemistry described above. The slides were then dehydrated and dried. For autoradiography the slides were dipped once in NTB2 emulsion (Kodak Integra Biosciences, Cergy le Haut, France) warmed at 40 °C, air dried overnight and conserved in a dry light-tight box with desiccant at 4 °C for 7 days. Then, slides were developed at 14 °C in D19 (Kodak, Rochester, NY, USA) for 4 min, rinsed in the water bath, fixed for 5 min in AL4 (Kodak) and subsequently rinsed, dehydrated and mounted with cytoseal 60 (Stephens Scientific, Riverdale, NJ, USA), and analysed in bright- and dark-fields using a Zeiss Axiophot microscope. Sections of the embryonic developing cerebellum and the adult inferior olive were counterstained with cresyl violet prior dehydration. With this double-labelling method the CaBP antigenicity was slightly modified, as CaBP-immunoreactivity was observed not only on Purkinje cells, but also slightly on interneurons of the molecular layer and also on deep nuclear neurons.
Quantitative analysis
Quantification of silver grains in Purkinje cells
To quantify temporal changes in the expression of GAP-43 in Purkinje cells after axotomy, silver grains were counted in 1/5 Purkinje cells on one cut lobule (lobule 5) and one intact lobule (lobule 2) on the same section for each animal (Fig. 3). In control animals, the same counting was performed in lobules 2 and 5. Quantitative analysis was performed using an image analysis system (Morphostar software, Imstar, Paris, France) under bright field illumination at high magnification (×40) on an Olympus microscope. The Purkinje cells were classified into three different classes: class 1, Purkinje cells, with < 36 silver grains, per 100 µm2 (< 4 times the background noise); class 2, between 36 and 70 grains and class 3, > 71 grains. Then, for each survival time the percentages of Purkinje cells in each class were calculated.
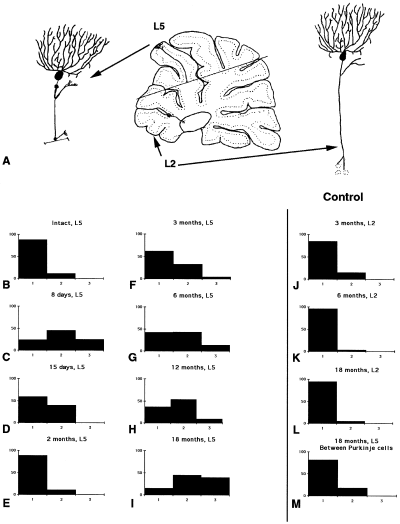
Quantification of silver grains in autoradiograms indicating GAP-43 expression in Purkinje cells after axotomy. (A) Diagrammatic representation of the lesion that divided the cerebellum into two parts containing either, the axotomized Purkinje cells (for example in lobule 5) or non axotomized ones (for example in lobule 2). Graphs show percentage of Purkinje cells in the three different classes (class 1 Purkinje cells with less than 36 silver grains per 100 µm 2, class 2 between 36 and 70, and class 3 more than 71). (B) intact, lobule 5; (C) 8 days after the lesion, lobule 5; (D) at 15 days, lobule 5; (E) 2 months, lobule 5; (F) 3 months, lobule 5; (G) 6 months, lobule 5; (H) 12 months, lobule 5; (I) 18 months, lobule 5. Note that more than 30% of Purkinje cells are in class 3. (J) Three months, lobule 2; (K) 6 months, lobule 2; (L) 18 months, lobule 2. Note that, at any time after the lesion, in the intact lobule there is the same proportion of Purkinje cell bodies that did not express GAP-43. (M) at 18 months, silver grains have been counted between Purkinje cells, the number of silver grains follow the same distribution as the one observed in intact lobule 5 and in lobules 2.
Count of inferior olivary neurons expressing GAP-43
In our lesion model, the vermian lobules IV, V and VI were systematically sectioned. Thus, only neurons in the medial accessory olive (MAO) were axotomized (Buisseret-Delmas & Angaut, 1993). To quantify changes in the number of olivary neurons, the total number of MAO neurons expressing GAP-43 mRNA was counted in lesioned rats and compared with the total number of MAO neurons in control rats.
In vitro studies
Organotypic cultures
Wild-type mice and a transgenic mouse line overexpressing GAP-43 were used. In the mouse line, chick-GAP-43 is expressed constitutively and selectively in neurons by the use of a mouse Thy1.2 expression cassette (Aigner et al., 1995). The transgenic mice were heterozygous, and were mated with wild-type mice. Thus, in the litters, half of the animals were transgenic and half of them wild-type. The genotypes were determined by PCR. After decapitation, P10 cerebella were used for the production of explants (Stoppini et al., 1991; Dusart et al., 1997). Cerebellar parasagittal slices (350 µm) were cut on a McIlwain tissue chopper. Some of the cultures were transected with a glass knife passing through lobules III and VIII, under a dissecting microscope. The two parts were gently separated to ensure a complete axotomy, then apposed and kept for one week in vitro. In other instances, the dorsal regions of the amputated explants were co-cultured, also for one week, with an apposed slice of fetal cerebellum (see Dusart et al., 1997).
Antibodies
Rabbit polyclonal antibodies against CaBP (diluted 1 : 5,000; Swant, Bellinzona, Switzerland) and mouse monoclonal antibody against CaBP (diluted 1 : 10,000; gift of Dr W. Hunziker, Pinol et al. 1990) were used to label Purkinje cells.
Rabbit polyclonal antibodies against chick-GAP-43 (1 : 500 dilution; Aigner & Caroni., 1993) were used to specifically label chick-GAP-43.
Immunostaining procedures
The cultures and co-cultures were fixed in 4% paraformaldehyde in phosphate buffer (0.12 m, pH 7.4) for 1 h at room temperature. Explants were incubated for 1 h in PBSGTA, with 0.1 m lysine, before immunostaining. The first antibodies were applied in PBSGTA overnight. For double-labelling the mouse monoclonal antibody was incubated together with the rabbit polyclonal antibodies. The first antibodies were revealed with the following secondary antibodies: goat anti-rabbit CY3 (1 : 200 dilution; Jackson ImmunoResearch Laboratories, Inc), sheep anti-mouse FITC (fluorescein isothiocyanate; 1 : 50 dilution, Amersham, Arlington Heights, IL, USA), and sheep anti-rabbit FITC (1 : 200 dilution; Silenus Laboratories, Hawthorne, Australia). After 2 h incubation in buffer containing the secondary antibodies, the slices were washed several times with PBS, mounted in mowiol (an anti-fade agent; Calbiochem, La Jolla, CA, USA) and analysed using a Leica DMR microscope and a Leica confocal microscope.
Crude evaluation of Purkinje cell survival
Using criteria previously defined (Dusart et al., 1997), we identified three groups of slices: group 1 with few and dispersed Purkinje cells, i.e. with no compact clusters of more than 20 Purkinje cells (with the exclusion of lobule X, generally well preserved and with more than 20 Purkinje cells). Group 3, slices containing an almost continuous compacted layer of Purkinje cells all over the cortex (with less than three discontinuities in the CaBP-immunostaining; regions devoid of Purkinje cells). Group 2 includes all the slices that do not satisfy the criteria of the other two groups. For each age, we calculated the percentage of slices in groups 1, 2 and 3.
Results
Expression of GAP-43 in the developing and adult rat cerebellum: transient expression in developing Purkinje cells
To determine whether GAP-43 is expressed during Purkinje cell development, we performed in situ hybridization analyses from E15–P10. At E15, GAP-43 mRNAs were detected in two well defined zones, ‘dz1’ and ‘dz3’ that contained, respectively, postmitotic Purkinje cells and neurons of the deep cerebellar nuclei (Fig. 1A and B; Altman & Bayer, 1997). After E19, strong GAP-43 expression was confined to the external granular layer (EGL) and deep nuclei (Fig. 1C). In addition, up to P3, a moderate expression was evident in the ‘Purkinje cell plate’ corroborated by colocalization with calbindin (CaBP) immunoreactivity (Fig. 1C and D). By P5, CaBP-positive neurons, already aligned into a monolayer, exhibited only a background autoradiographic noise, as it is the case at P10 (Fig. 1E). From P3 to P10, GAP-43 expression remained in the whole EGL, although it was much stronger in the deeper postmitotic than in the upper mitotic half (Fig. 1D and E). In the adult cerebellum, GAP-43 mRNA were abundant in molecular layer interneurons (basket and stellate) and in granule cells (Fig. 2A). Thus, developing rat Purkinje cells have a transient expression of GAP-43, starting at least by E15 and disappearing by P5. Similarly, numerous mouse Purkinje cells expressed GAP-43 during development, particularly by P3 (Fig. 1F). However, in the mouse this expression is delayed and Purkinje cells could still express GAP-43 at P5 (Fig. 1G), whereas, the expression completely disappeared by P7.
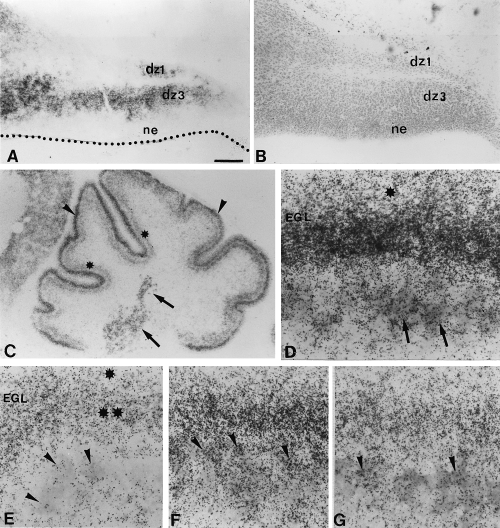
Developmental expression of GAP-43 in the rat (A, C, D and E) and mouse (F–G) cerebellum. Photomicrographs (A and B) are taken from adjacent sections. (A) E15 parasagittal section, the cerebellar primordium possesses two areas with cells strongly expressing GAP-43, these areas have been identified as dz1 and dz3 on an adjacent cresyl violet section (see B and Altman & Bayer, 1997). Note that the neuroepithelium (ne) is devoid of GAP-43. B is an adjacent section of A, differentiating zones dz1 and dz3, and neuroepithelium (ne) have been identified. (C) P3 parasagittal sections, GAP-43 expressing cells are observed in external granular layer (arrowheads), Purkinje cells layer (stars), and deep nuclear cells (arrows). (D) High magnification of C, in situ hybridization for GAP-43 is coupled with an immunostaining against CaBP to identify Purkinje cells (arrows). Note that the external granular layer (EGL) is divided into two parts in regard to the intensity of GAP-43 expression. The upper part (star) exhibits a lower GAP-43 expression. (E) P10 parasagittal section, note that no more labelling is present in Purkinje cells (arrowheads), and the two levels of GAP-43 expression in the EGL remains (upper part one star, bottom part double stars). (F) P3 mouse section, GAP-43 expressing cells are observed in the external granular layer (EGL) and in Purkinje cells (arrowheads). (G) P5 mouse section, GAP-43 expressing cells are observed in external granular layer (EGL) and in a few Purkinje cells (arrowheads). Bar, A 130 µm (A and B), 270 µm (C), 25 µm, (D and E) and 18 µm (F and G).
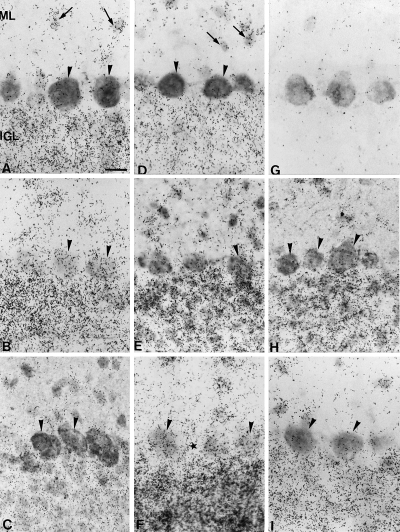
Expression of GAP-43 mRNA in lobule 5 (L5, axotomized) and lobule 2 (L2, non axotomized) of the anterior vermis of the rat cerebellum. The autoradiograms of GAP-43 mRNA are always doubled with CaBP immunohistochemistry. (A) Intact cerebellum (lobule 5), GAP-43 mRNA is present in interneurons (arrows) of molecular layer (ML) and internal granule cell layer (IGL) but not in Purkinje cells (arrowheads). (B) Eight days after lesion in lobule 5, some Purkinje cells (arrowheads) express GAP-43 mRNA. (C) One month after the lesion in lobule 5, GAP-43 mRNA is not expressed again in Purkinje cells (arrowheads). (D) Intact cerebellum (lobule 2, same section as in A), GAP-43 mRNA is present in interneurons (arrows) of molecular layer and internal granule cell layer but not in Purkinje cells (arrowheads). (E) Three months after the lesion in lobule 5, few Purkinje cells (arrowhead) reexpress GAP-43 mRNA. (F) Eighteen months after the lesion in lobule 5, many Purkinje cells expressed GAP-43 mRNA (arrowheads). Note that between 2 Purkinje cell bodies no labelling is visible (star). (G) Control autoradiogram of the GAP-43 mRNA in situ hybridization with a sense riboprobe. Note the absence of silver grains. (H) Three months after the lesion in lobule 2 (same section as in E), Purkinje cells (arrowheads) do not express GAP-43 mRNA. (I) Eighteen months after the lesion in lobule 2 (same section as in F), Purkinje cells (arrowheads) do not express GAP-43 mRNA. Bar in A, 25 µm (A–I).
Expression of GAP-43 in the adult rat cerebellum after axotomy: protracted upregulation in some Purkinje cells
In vivo studies have shown that axotomized Purkinje cells are capable of a late and protracted process of terminal sprouting (Dusart et al., 1999). We examined whether this process was correlated with an upregulation of GAP-43 expression. Because of the geometry of the cerebellum, each of our parasagittal vermal sections has axotomized (lobule 5) and intact lobules (lobule 2), providing an internal control for the quantitative analysis of the in situ hybridization results (Fig. 3A). In the intact adult cerebellum, lobule 5 exhibited high autoradiographic activity (indicative of GAP-43 expression) only in stellate, basket and granule cells (Fig. 2A). This activity disappeared when the sections were hybridized with the sense GAP-43 riboprobe (Fig. 2G). The number of silver grains, over CaBP-positive Purkinje cell bodies, was too low (less than 36 grains per 100 µm2 of perikaryal surface; < 4 times the background noise) to be significant. These Purkinje cells were considered as belonging to class 1, and devoid of GAP-43 expression, corroborating the lack of GAP-43 mRNA in mature Purkinje cells. In a small percentage of lobule 5 Purkinje cells (about 12%, see Fig. 3B), the number of silver grains fluctuated between 37 and 70 per 100 µm2. These cells were considered as class 2, and corresponded to a few Purkinje cells with a low constitutive level of GAP-43 expression. Finally, Purkinje cells with more than 71 silver grains per 100 µm2 of perikaryal surface, the only ones with moderate to high GAP-43 expression (class 3), were missing as expected (Fig. 3B).
Temporal changes in cerebellar GAP-43 expression were quantified at 8 and 15 days and 1, 2, 3, 6, 12 and 18 months after axotomy. In all dorsal lobules this expression increased progressively in molecular layer interneurons and granule cells (Fig. 2B, E and F). Whereas, in nonaxotomized lobules it remains unchanged (Fig. 2D, H and I). Concerning Purkinje cells, class 3 cells were encountered in lobule 5 by 8 days after the lesion (Fig. 2B and 3C). This fast increase was transient, and by 15 days, class 3 Purkinje cells had disappeared 2, 3. A second and protracted wave of GAP-43 expression started by 3 months after the lesion 2, 3, and progressed up till 18 months 2, 3. At all survival times analysed, silver grains in lobule 2 Purkinje cells did not change (Fig. 3J–L). Moreover, at 18 months after the lesion, silver grains in the spaces separating Purkinje cell bodies in lobule 5 remained constant (Fig. 3M), discarding the possibility that the observed increase of silver grains in Purkinje cell perikarya could be the consequence of the upregulation of GAP-43 expression in nearby granule cells.
These quantitative results reveal that the terminal axonal sprouting previously reported (Dusart et al., 1999) is temporally correlated with a second, protracted period of GAP-43 re-expression. However, the highest expression of GAP-43 concerns only a minority of Purkinje cells (25% for the first increase, Fig. 3C; 40% in the later increase Fig. 3I).
Inferior olivary neurons did not sprout after axotomy but died
Adult inferior olivary neurons are known to express GAP-43 (Kruger et al., 1993; Console-Bram et al., 1996; Fig. 5A–C), and die after axotomy (Barmack & Simpson, 1980; Ito et al., 1980; Buffo et al., 1998). This study mainly focused on the fate of the proximal stumps of inferior olivary amputated axons, to compare the behaviour of olivary axons with that of Purkinje cells after axotomy. The study was carried out with anti-GAP-43 antibodies, that in the white matter of cerebellum stain selectively the olivocerebellar fibers (the immunostaining disappeared after lesion of this system; Buffo et al., 1998). In control rats, fibers in the olivocerebellar pathway exhibit GAP-43 immunoreactivity (GAP-43-IR). These thin fibers are moderately GAP-43-immunoreactive at their entry point in the inferior cerebellar peduncle (Fig. 4D), and only weakly GAP-43-IR within the white matter axes (Fig. 4A).
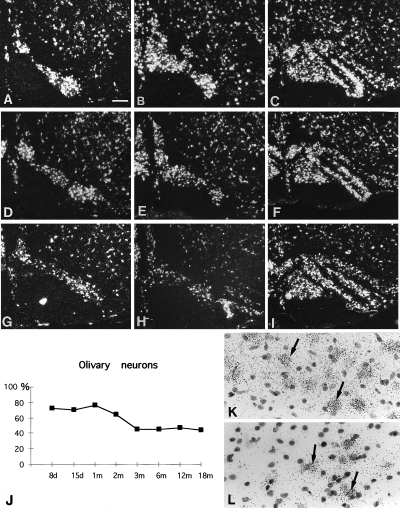
Expression of GAP-43 mRNA in olivary neurons. Darkfield photomicrographs. (A–C) Expression of GAP-43 mRNA in the intact animal, (D–F) 1 month after the lesion and (G–I) 18 months after the lesion. (A, D and G) The most caudal region of the Medial Accessory Olive (MAO); (B,E and H) MAO at the level of β nucleus; (C and F and I) the rostral half of the inferior olive. Note that 1 month and 18 months after the lesion there is a decrease in the number of olivary neurons in the caudal MAO (D–E and G–H), whereas the rostral part of the inferior olive does not seem to be affected (F–I). (J) The counting of olivary neurons expressing GAP-43 mRNA shows that there is at first a decrease in the number of cells at 8 days after the lesion and from 3 months to 18 months another decrease of 50% neurons is observed. (K) Brightfield photomicrograph of cresyl violet sections after in situ hybridization 8 days after the lesion in the caudal part of the MAO. (L) Brightfield cresyl violet sections after in situ hybridization 18 months after the lesion in the caudal part of the MAO. Note that after the lesion all the olivary neurons expressed GAP-43 mRNA (arrows). Bar in A is 200 µm (A–I), 20 µm (K and L).
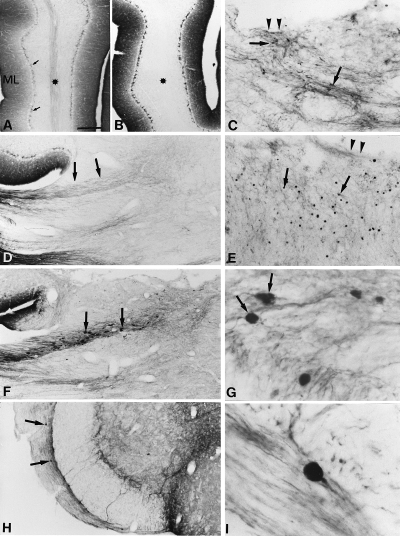
GAP-43 immunoreactivity (GAP-43-IR) in intact and lesioned cerebella. (A) Sagittal section of an intact cerebellum, GAP-43-IR is weak in the white matter (star), more intense in the molecular layer (ML) and in the axon terminals of the basket cells (arrows). (B) 18 months after the lesion, the lesioned lobule is devoid of labelling in the white matter (star). (C) Eight days after the lesion, in the white matter under the cut (arrowheads), some fibers showed intense GAP-43-IR (arrows). (D) Mild GAP-43-IR in olivocerebellar axons entering the intact cerebellum (arrows) through the inferior peduncle (E) 1 month after the lesion, numerous small varicosities (arrows) appear near the lesion site (arrowheads) corresponding to retraction bulbs of axotomized olivocerebellar axons. (F) Three months after the lesion, large round intensely GAP-43-IR varicosities (arrows) appeared in the distal part of olivocerebellar tract at its entry through the inferior peduncle. (G) Three months after the lesion, some large GAP-43-IR varicosities appeared to be terminal varicosities (arrows). (H) Six months after the lesion, the GAP-43-IR varicosities appears in the deeper portion in the white matter of the brain stem (arrows). (I) Twelve months after the lesion, a terminal varicosity GAP-43-IR in the deeper portion in the white matter of the brain stem. Bar in A is 400 µm (A, B and E), 175 µm (H), 160 µm (D and F), 80 µm (C), 20 µm (G) and 15 µm (I).
In the dorsal part of the lesioned lobules, where the distal amputated segments of the olivocerebellar fibers were located, GAP-43-IR disappeared from the white matter as early as one month after the lesion (Fig. 4B), and remained undetectable up until 18 months, our longest survival time. Interestingly, the fate of the proximal segments (located ventrally, under the cavity) evolved with time. Eight days after the lesion, these segments increased their GAP-43-IR, and became more disorganized (Fig. 4C). Only very few of them bore terminal varicosities (Fig. 4C). By 1 month on, olivary axons underwent a retraction process, because there was less GAP-43-IR near the cavity, except for small terminal varicosities (Fig. 4E). By 3 months, these terminal varicosities were in the inferior peduncle (Fig. 4F and G), and thereafter they disappeared from the cerebellum to be observed only in the olivocerebellar pathway close to the inferior olive (Fig. 4H and I).
Our cerebellar lesions only affected the vermis and, therefore, axotomized neurons were only located in the caudal part of the medial accessory olive (MAO; Buisseret-Delmas & Angaut, 1993). The fate of these neurons was determined by counting perikarya expressing GAP-43 mRNA in the MAO. By counterstaining the sections with cresyl violet after the in situ hybridization, we checked that at all the time points after the lesion, all the neurons expressed GAP-43 (Fig. 5K and L). Thus, the loss of GAP-43 expressing olivary neurons corresponded to the death of these neurons. As expected, cell loss was only found in the caudal MAO (compare Fig. 5D–E and G–H with Fig. 5A and B), whereas, the number of cells did not change in the rostral MAO (compare Fig. 5F and I with Fig. 5C). Already 8 days after the lesion, there was a 20% drop in caudal MAO neurons (Fig. 5J). This drop remained unaltered up until 2 months after the lesion, but thereafter, the drop progressively increased (Fig. 5J). Thus, by 18 months, 50% of caudal MAO neurons were missing. Even if the lesions did not affect the collaterals of the olivocerebellar axons projecting to the deep cerebellar nuclei, at least half of the olivary neurons projecting to the vermal cortex died by a process of retrograde degeneration, involving axon retraction.
GAP-43 expression by mature Purkinje cells does not promote axonal regeneration but neuronal death: In vitro study with a transgenic mouse line overexpressing GAP-43
To determine whether Purkinje cells expressing GAP-43 were capable of axonal growth and regeneration or would die as with the olivary neurons, we have performed a series of in vitro experiments with cerebellar explants taken from a transgenic mouse line that overexpresses GAP-43 in neurons (Aigner et al., 1995). We used the in vitro model previously documented for rat cerebellar explants, in which 10-day-old Purkinje cells had an excellent survival rate of up to one month (Dusart et al., 1997; Fig. 6D). Surprisingly, most CaBP-positive Purkinje cells in the P10 explants taken from the transgenic mice disappeared after 7 days in vitro (7 DIV, Fig. 6E). In these explants, chick-GAP-43 protein was present not only in Purkinje cell terminals (Fig. 6C), but also in their axons (Fig. 6B) and cell bodies (Fig. 6A). To determine whether the Purkinje cell death in the explants was the consequence of the GAP-43 overexpression, we compared Purkinje cell survival in explants taken from P10 wild-type or transgenic mice after 7 DIV. Because of the difficulties in performing accurate cell counts in the cultures, we used the semiquantitative approach previously validated for rat cerebella in cultures (Dusart et al., 1997). In wild-type mouse cerebella, Purkinje cells had an excellent survival rate. Indeed, CaBP-immunoreactive cells almost completely filled the Purkinje cell layer (group 3), the vast majority of the explants (more than 80%) belong to group 3 and none of the 114 explants belong to group 1 (with no clusters of more than 20 CaBP-positive cells; Fig. 6D and H). In explants taken from GAP-43 overexpressing mice, the survival of Purkinje cells was surprisingly poor, i.e. none of the explants belonged to group 3 (Fig. 6E and H). Thus, the expression of GAP-43 by almost mature Purkinje cells is associated with a high vulnerability of these neurons to the in vitro conditions.
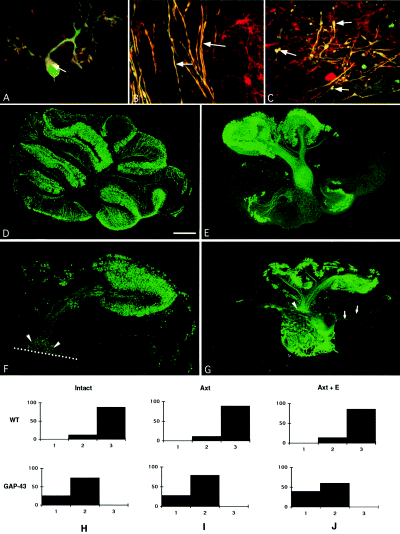
(A–C) Confocal microscopy images of CaBP (green) and GAP-43 (red) inmmunofluorescence of P10 cerebellar slices maintained 7 days in vitro. GAP-43 is present in Purkinje cell body (A, arrow), in some axons (B, arrows) and in terminals (C, arrow). Bar in C 30 µm in A, and 17 µm in B and C. (D–G) Microphotographs of postnatal day 10 (P10) cerebellar slices maintained 7 days in vitro and immunostained with anti-CaBP antibodies from (D) wild-type, and (E–G) GAP-43 overexpressing mice. (D) Purkinje cells are present all over the cortex, this slice was therefore included in group 3 (see below). (E) Purkinje cells are much less numerous and not present all over the cortex (group 2, see below). (F) Microphotograph of an explant which has been cut in two parts. Axons from Purkinje cells in the cerebellar lobules, dorsal to the lesion, are arrested at the lesion line (dotted line). The amputated axons terminate in large rounded varicosities (terminal bulbs, arrowheads). (G) Co-culture of the dorsal region of a split P10 GAP-43 overexpressing transgenic cerebellar slice and of an intact E17 fetal cerebellar slice. Embryonic axons (arrows) enter into the mature slices whereas mature axons do not regenerate into fetal slices, but terminate in large rounded varicosities (arrowhead). (H–J) Quantitative evaluation of Purkinje cells survival in the different types of cultured slices, (H) intact wild-type (WT) and GAP-43 overexpressing mice (GAP-43); (I) axotomized (Axt) wild-type and GAP-43 and (J) co-culture of a P10 wild-type axotomized slice and an embryonic slice (Axt + E) and co-culture of a P10 transgenic axotomized slice and an embryonic slice (Axt + E). The different groups of slices were defined according to their content in Purkinje cells: group 1, with no compact clusters of more than 20 Purkinje cells (with the exclusion of lobule X); group 3, slices containing an almost continuous layer of Purkinje cells all over the cortex (with less than three discontinuities in the CaBP-immunostaining, D) and Group 2 that includes all the slices that do not satisfy the criteria of the other two groups (E). Bar in D is 530 µm (D), 570 µm (E and G), 300 µm in (F).
In a series of explants, the cerebellar slices were transected and separated into a ventral and a dorsal part. After 7 more DIV, the distribution of CaBP-immunostained cells in the dorsal parts (the only ones containing axotomized cells) mimicked that obtained from intact transgenic explants (compare Fig. 6F with 6E), and the percentage of explants of groups 2 and 1 did not change with axotomy (Fig. 6I). Moreover, the axons of these Purkinje cells terminated in rounded varicosities without apparent sprouts (Fig. 6F). Finally, the regenerative capabilities of transgenic Purkinje cells were tested in co-culture experiments, where the transgenic P10 + 7 DIV transected cerebellar slices were apposed to fetal cerebellar explants (a permissive environment). Despite the overexpression of GAP-43 and the permissive environment, the axons of the surviving Purkinje cells did not regenerate (Fig. 6G). Furthermore, the Purkinje cells did not survive in these conditions as well (Fig. 6J).
Discussion
This study reveals the existence of a strong correlation between the expression of GAP-43 in neurons and their reactions to axotomy, which can lead to regeneration or sprouting in the presence of a favourable environment, and to cell death in its absence. First, we show that immature Purkinje cells express GAP-43 during their phase of fast axonal growth and the formation of the corticonuclear projection. Second, we show that there are two phases of upregulation of GAP-43 mRNA in adult Purkinje cells after axotomy. The second phase of expression is correlated with a protracted process of intense axonal sprouting. Third, we show that, contrary to the case where Purkinje cell axons do not retract after axotomy, the transected axons of the olivocerebellar pathway, which express high levels of GAP-43, do retract. This axonal retraction correlates with a loss of about half of the affected inferior olivary neurons. Finally, using cerebellar organotypic cultures, from 10 day-old transgenic mice with constitutive neuronal overexpression of GAP-43, we show that GAP-43 provokes a dramatic increase in neuronal vulnerability. Although wild-type mice Purkinje cells have an excellent survival rate, the transgenic mice Purkinje cells do not survive the culture conditions.
Purkinje cells transiently express GAP-43 during developmental axon elongation and synaptogenesis
Most developing neurons transiently express GAP-43 during the phase of axonal outgrowth (Skene, 1989). This transient expression has not yet been well established for developing Purkinje cells; only one study (Dani et al., 1991) reports low levels of GAP-43 immunostaining by E21 in the region containing the multilayered Purkinje cells bodies, whereas, in other published studies either embryonic cerebellum was not included and only negative results were reported for postnatal Purkinje cells (Oestreicher & Gispen, 1986; Rosenthal et al., 1987; McGuire et al., 1988; Meberg & Routtenberg, 1991; Console-Bram et al., 1996). Here, by correlating a distinct differentiating zone (‘dz3’, known to contain the migrating Purkinje cells, Altman & Bayer, 1997) with a cerebellar plate region containing numerous GAP-43 mRNA or, later on, by visualizing Purkinje cells by double labelling of the in situ hybridization autoradiograms with CaBP-immunostaining, we show that developing Purkinje cells do express transiently GAP-43 mRNA. This expression is high during the initiation of the axon outgrowth (E15), and remains high until the entry of the growing axons into their terminal domains (E18, Eisenman et al., 1991), the deep cerebellar nuclei. Thereafter, the expression rapidly decreases up until P3, when it is low and almost completely disappears by P5, when the synaptogenesis of Purkinje cells with deep nuclear neurons is close to its end (Gardette et al., 1985). The known temporal shift particular to mouse development also occurs for GAP-43 expression in Purkinje cells, with initiation of a down-regulation by P5 and loss of expression by P7. Thus, in mouse, the end of GAP-43 expression parallels the end of a period of extreme vulnerability of these neurons in organotypic culture (Ghoumari et al. 2000).
Axotomy of adult Purkinje cells upregulates GAP-43 mRNA expression: correlation with protracted axon sprouting
In situ hybridization with GAP-43 riboprobes in the lesioned cerebellum showed that Purkinje cells upregulate the synthetic machinery for this protein shortly after axotomy, and that the response only affects a subset of the injured neurons. In this respect, Purkinje cells respond to axotomy like some other central neurons that also upregulate GAP-43 expression even in the absence of axon regeneration (Doster et al., 1991; Tetzlaff et al., 1991; Vaudano et al., 1995). There are, however, important differences between the response of Purkinje cells and that of most of the central neurons studied so far.
Moderate and uneven GAP-43 expression
Contrary to what has been reported for retinal ganglion cells, rubrospinal and thalamic neurons (Doster et al., 1991; Tetzlaff et al., 1991; Vaudano et al., 1995), the upregulation of GAP-43 mRNA in injured Purkinje cells was mild, and did not increase the protein content to detectable immunohistochemical levels. This quantitative difference could be related to the fact that GAP-43 expression completely disappears in Purkinje cells older than P3 (see above), whereas the other central neurons that upregulate GAP-43 have a low constitutive level of expression in adulthood (Tetzlaff et al., 1991; Vaudano et al., 1995; Fournier et al., 1997). It is also noteworthy that only a subset of injured Purkinje cells re-expressed GAP-43 mRNA. This was not related to the distance between the lesion site and the cell body (data not shown). Moreover, this different response is not exclusive to Purkinje cells and has been reported in other injured central systems (Vaudano et al., 1995; Fournier et al., 1997). The reasons for this differential behaviour in the response of axotomized neurons within the same population remain unknown, but it could reveal those neurons with a larger potential for axonal growth.
Timing of GAP-43 expression
In most neuronal systems studied, the initiation of a transient GAP-43 upregulation occurs 6–8 days after the lesion (Doster et al., 1991; Tetzlaff et al., 1991; Vaudano et al., 1995), coincident with the first wave of expression that we observed in Purkinje cells. The second wave of expression, reported here for the first time, despite its protracted onset, is temporally correlated with the intense axonal sprouting of injured Purkinje cells, which starts by 3 months, becomes very dense 12 and 18 months later and leads to neosynaptogenesis (Dusart et al., 1999). In this instance, the regenerative attempts, represented by the late sprouting, parallel a mild to moderate expression of GAP-43 mRNA. We have previously correlated this late sprouting with changes in the permissivity of the glial scar (Dusart et al., 1999). The permissive environment of the injured axons may, thus, regulate the production of retrograde signals, which in turn govern the genetic program for axonal regeneration in axotomized neurons. Thus, our results, together with those obtained with transgenic mice overexpressing GAP-43 (Aigner et al., 1995; Holtmaat et al., 1995; Buffo et al., 1997), strongly indicate that GAP-43 expression is correlated with axonal sprouting and neosynaptogenesis (Caroni, 1997).
GAP-43 expression and cell death.
Purkinje cells with GAP-43 overexpression do not increase their ability to regenerate (present study, Buffo et al., 1997). Therefore, as proposed by Buffo et al. (1997), in Purkinje cells GAP-43 overexpression alone may not be sufficient to drive the expression of other growth-associated genes, and/or respond to growth-promoting environmental cues. Interestingly, however, Purkinje cells with GAP-43 overexpression in vivo (Buffo et al., 1997), and more particularly in vitro (present study) are extremely vulnerable to lesion. Furthermore, in the absence of a permissive environment, about half of the axotomized olivary neurons die after axotomy (Barmack & Simpson, 1980; Ito et al., 1980; Buffo et al., 1998; present study). Thus, GAP-43 is not only associated with axon growth, but also with pathways that trigger cell death. It has been known for years, that intense perikaryal reaction prepares injured neurons for regeneration although it often provokes a retrograde cell death (Cragg, 1970). More recent evidence suggests that the pathways leading to regeneration and cell death can be interrelated (see References in Herdegen et al., 1997). Thus, GAP-43 could also be involved in this genetic cascade. Indeed, in GAP-43 overexpressing mouse lines, an association between overexpression of GAP-43 and death of lesioned motoneurons has been reported recently (Harding et al., 1999); furthermore, absence of GAP-43 can protect neurons from death (Gagliardini et al. 2000). Moreover, neuronal systems with high GAP-43 expression and regenerative capacity after injury are also those that undergo a more severe neuronal degeneration (Doster et al., 1991; Tetzlaff et al., 1991; Herdegen et al., 1997). Last, around the period of synaptogenesis, Purkinje cells and other neurons have a high potential for regeneration, but are also very sensitive to axotomy-induced cell death (for review see Schwab & Bartholdi, 1996). The end of GAP-43 expression correlates with the end of a critical period of high dependency of neurons for contact with their targets.
Last, we propose that GAP-43 could be involved in the cascade of genes that inform neurons about the potentiality of their environment (Fig. 7). In this study, we show that inferior olivary neurons, which constitutively express GAP-43 (Kruger et al., 1993; Console-Bram et al., 1996) that retracted their axons after axotomy instead of sprouting, and eventually a large proportion of them died. In contrast, when the axotomized olivary neurons are confronted with a permissive environment, they exhibit robust regeneration and survive (Armengol et al., 1989; Rossi et al., 1995; Bravin et al., 1997). Inferior olivary neurons have the remarkable ability to produce long-terminal sprouts in the intact cerebellum when provided with grafted extra-Purkinje cells devoid of climbing fibers (Rossi et al., 1994). Therefore, the environment and the presence of targets seem responsible for the growing or retracting behaviour of the olivocerebellar axons. The present comparative study on the reactions of Purkinje cells and inferior olivary neurons to axotomy, together with previous results from the literature, suggest that neurons that do not express GAP-43 fail to mount a robust response to axotomy. As a consequence, they survive but do not regenerate, irrespective of the presence or absence of a favourable environment and/or target cells, as happens for Purkinje cells (David & Aguayo, 1981; Dusart & Sotelo, 1994; Rossi et al., 1995; Bravin et al., 1997; Dusart et al., 1997; present study, Fig. 7). In contrast, neurons that express GAP-43 respond to axotomy. The quality of this response depends on the local environment, and the presence or absence of targets. In the absence of targets, some die, as is the case for inferior olivary neurons (Barmack & Simpson, 1980; Ito et al., 1980; Buffo et al., 1998; present study), while in the presence of targets, some of them are able to regenerate and establish new synaptic contacts (neosynaptogenesis: Armengol et al., 1989; Rossi et al., 1995; Bravin et al., 1997; Fig. 7). Thus, in line with the role of GAP-43 in structural synaptic plasticity (Benowitz & Routtenberg, 1987, 1997; Strittmatter et al., 1992), we propose that GAP-43 is among the genes that operate as ‘intrinsic target sensors’ and convey messages to neurons to adapt their axonal arbors to an increase or a decrease in target size.
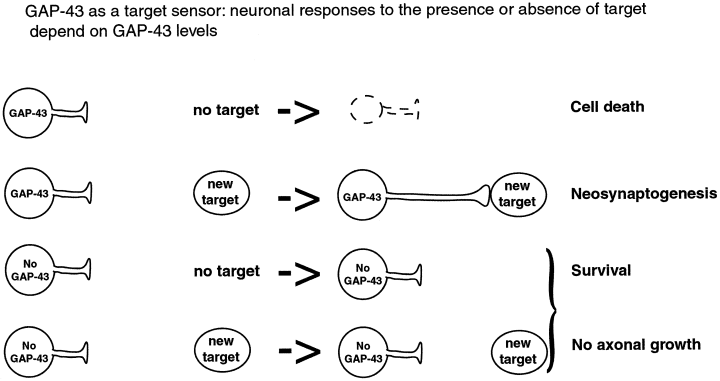
Schematic diagram showing that the neuron's responses to the presence or absence of targets is dependent of GAP-43 expression.
Acknowledgements
We thank Drs E. M. Lawson and P. J. H Skene for the gifts of the anti-CaBP and anti-GAP-43 respectively; Drs P. Gaspar, S. Marty and J. P. Rio for critical reading of the manuscript and Mr D. Le Cren for photographic assistance. Grant sponsors: Institut National de la Santé et de la Recherche Médicale (INSERM, grant U106); European Community (EC), Biotechnology Programme (ERBB104-CT96-0774 and CT98-0293). C. Sotelo and I. Dusart are Centre National de la Recherche Scientifique (CNRS) investigators.
Abbreviations
-
- CaBP
-
- calbindin
-
- DIV
-
- days in vitro
-
- EGL
-
- external granular layer
-
- FITC
-
- fluorescein isothiocyanate
-
- GAP-43
-
- growth associated protein (43 kDa)
-
- MAO
-
- medial accessory olive
-
- PBS
-
- phosphate-buffered saline.




