Spatial ecology of arboreal snakes (Hoplocephalus stephensii, Elapidae) in an eastern Australian forest
Abstract
Abstract Stephens' Banded Snakes (Hoplocephalus stephensii Krefft 1869) are large (to 1 m), highly arboreal elapid snakes, restricted to mesic forested areas along the eastern coast of Australia. Radiotelemetric monitoring of 16 individuals at Whian Whian State Forest in north-eastern New South Wales over 25 months provided the first data on spatial ecology of this threatened taxon. Two major influences on movements by Stephens' Banded Snakes were identified: the distribution of large hollow-bearing trees, and the avoidance of conspecifics. Radiotracked snakes were sedentary inside tree hollows for extended periods (mean = 8 days) during their active season, interrupted by occasional long (mean = 124 m) nocturnal movements to another shelter tree. Snakes travelled on the ground rather than within the canopy, and thus were potentially exposed to terrestrial predators. Although the home ranges of the radiotracked snakes overlapped substantially (mean = 27%), simultaneous occupancy of ‘shared’ shelter trees was less common than expected by chance. Hence, we conclude that adult Stephens' Banded Snakes generally avoid the presence of conspecifics. Snakes used from five to 30 shelter trees and home ranges of male snakes were larger than those of females (mean = 20.2 vs 5.4 ha). The large spatial scale of these movements, and limited overlap among individuals, means that a viable population of this taxon requires a large area of contiguous forest. This requirement may explain why the species has not persisted in small forest fragments.
Introduction
Most of the Australian continent is characterized by nutrient-poor soils and low, unpredictable rainfall, with the result that forests occupy approximately 5% of the land mass (Lunney 1991). However, there is great spatial heterogeneity in these characteristics, and a wide latitudinal band of higher, more equable precipitation and (in patches) relatively rich soils extends along the eastern coastline (Flannery 1994). Parts of this region support a rich and diverse forest fauna and flora (CSIRO 1996). Although the forests were widely distributed in coastal areas of the continent when Europeans invaded Australia 200 years ago, these areas were soon subjected to intense harvesting (for timber) and clearing (for agriculture). The inevitable result has been a massive decline in the area of contiguous moist forest, and throughout much of the coastal range the only surviving forest is highly fragmented (Lunney 1991). The fauna and flora that were specialized to live in forests have also declined (Recher & Lim 1990). We urgently need to understand more about the biology of such taxa to clarify why some species have persisted whereas others have disappeared even from relatively large forest fragments.
Although the mammals (Lindenmayer et al. 1990; Ward 1990; Bennett et al. 1991) and birds (Kavanagh et al. 1985; Milledge et al. 1991) of Australian forests have attracted increasing scientific attention, the reptiles remain poorly known. Logistical obstacles, such as dense vegetation and cryptic behaviour, preclude many kinds of study methods and impede most kinds of visual census techniques. Radiotelemetry offers a partial solution to such problems, and in recent years has provided new insights into the ecology of forest lizards (Rummery et al. 1995; Klingenböck et al. 2000). We have applied this technique to a snake species that is largely restricted to moist forest areas, and hence has become of increasing conservation concern as these areas have been degraded by anthropogenic effects (Neave & Norton 1991). The present paper focuses on patterns of movement and spatial ecology, because these attributes can influence the ability of a species to persist in fragmented landscapes.
Methods
Study species
Stephens' Banded Snakes (Hoplocephalus stephensii) are relatively slender-bodied proteroglyphs (elapids), growing to approximately 1.0 m total length and weighing up to 250 g (Wilson & Knowles 1988; Fitzgerald 2002). The species is distributed patchily along the coast and hinterland of eastern Australia, from the central coast of New South Wales to south-eastern Queensland (Longmore 1986). Stephens' Banded Snakes feed on a diverse array of small vertebrates, especially lizards and small mammals (Shine 1983; Fitzgerald 2002). Females bear two to nine live young (Wells et al. 1988; Fitzgerald 2002), but reproduce on a less-than-annual schedule (Shine 1983).
Study area
Whian Whian State Forest (WWSF) in north-eastern New South Wales comprises the eastern part of an 11 000 ha forested plateau, at elevations of 200–600 m a.s.l. Vegetation is predominantly wet sclerophyll forest and rainforest with small areas of drier open forest and heath. The study area has a varied history of logging and is described in detail elsewhere (CSIRO 1996; Fitzgerald et al. 2002). The climate is mild with mean daily maximum temperatures of 17.7°C in June and 26.5°C in December. Mean annual rainfall at Rummery Park in WWSF is 236.8 cm. Total rainfall in WWSF for the study period (April 1997 to May 1999) was 448.7 cm.
Monitoring regime
Snakes were mainly captured as they crossed roads at night. Sixteen snakes (six males; 10 females) were surgically implanted with miniature temperature-sensitive radiotransmitters (Holohil, Canada; BD2-GT1, PD-2T and SB-2T models) and released at the point of capture, usually within 48 h of implantation. Transmitter preparation and implantation techniques are described elsewhere (Fitzgerald et al. 2002). Work was focused at three main locations, each approximately 1 km apart. Radiotracking was conducted from April 1997 to April 1999, with each individual tracked for 41–635 days (mean = 287; SE = 43 days). Each animal was located two to three times per week during the active season (September to May) and less frequently during winter. The total number of observations per snake varied from 9 to 143 (mean = 76, SE = 11). Radiotracked snakes were located using a Regal 2000 receiver and three-stage Yagi antenna (Titley Electronics, Ballina).
Mapping locations and distances moved
Radiotracked snakes were visible only occasionally, regardless of whether they were in trees or in terrestrial locations. Thus, we used triangulation to identify the location of origin of the transmitter signal. When snakes were in trees, their height above ground was estimated using an optical height meter (Sunto, Finland). Snake locations were identified with labelled flagging tape and distances between successive locations were measured either by pacing or by plotting locations against known reference points. All recorded distances and bearings were checked for accuracy against maps of home ranges. A network of logging tracks, streams and escarpment features allowed snake locations to be reliably determined to ± 10 m. Movements of less than 10 m are not included in the current analysis. We used straight line distances between successive locations, because we had no information on actual paths followed by the snakes. Limited nocturnal tracking indicated that overnight moves between trees sometimes involved near-direct linear movements, as described for an arboreal lizard (Thompson et al. 1999), as well as meandering foraging behaviour. However, sample sizes are too small for analysis.
Home-range analysis
Analysis of home-range areas of radiotracked snakes is complicated by variation in the number of observations and the implicit assumption of independence of successive observations. Four snakes were tracked for two full active seasons, seven snakes for more than one full active season and five snakes for less than one complete active season. Most observations were made in the afternoon, when diurnal activity by snakes was most likely. The mean interval between observations satisfies the usual ‘rule of thumb’ criteria for statistical independence (i.e. sufficient time elapsed between observations for the snake to traverse its home-range diameter; White & Garrott 1990). The Ranges IVm software was used to analyse home-range characteristics (Kenward 1990).
Results
Use of shelter sites
All radiotracked snakes overwintered in large trees from June to August or later. There were occasional minor shifts in vertical position (including infrequent basking) but the only snake to leave its tree during winter was a male that briefly (in early winter) visited a tree later found occupied by another male and female snake in spring. Although the mean duration of this overwinter inactivity period was longer for females (mean = 132 days, SE = 12.4) than for males (mean = 105 days, SE = 8.6) this difference did not attain statistical significance (single-factor anova with sex as the factor, F1,8 = 3.23, P = 0.11). Transmitters expired while three snakes were in overwinter trees, so we have ingress dates only for these individuals. Snakes entered overwinter trees from 27 April to 10 June. Males entered overwinter trees slightly later than did females on average (mean male ingress date = 24 May, female = 19 May; one-way anova with sex as the factor, F1,11 = 0.51, P = 0.49). Males left overwinter trees significantly earlier on average than did females (mean dates of egress = 6 September for males and 3 October for females; anova, F1,8 = 6.71, P = 0.03) The sexes selected shelter sites at similar heights above ground (anova, F1,11 = 0.51, P = 0.49).
The activity patterns of radiotracked snakes were similar in spring, summer and autumn, so we collectively term this period the ‘active season’ (i.e. September to May). During the active season, snakes were most frequently (65% of locations) found sheltering in hollows within trees (n = 328) or in dense vines in trees (n = 109). When in terrestrial situations, snakes in rainforest habitat were located in litter (n = 15) or vines (n = 6), whereas snakes in wet sclerophyll forest were most often located in tall sedges (n = 74), especially Lepidosperma clipeicola K.L. Wilson. Details of shelter site usage have been provided elsewhere (Fitzgerald et al. 2002).
Residence times at shelter sites were calculated as the midpoint between maximum possible residence and maximum observed residence time. Because most observations were made diurnally, residence times represent the number of nights spent at a location. Overall, during the active season, radiotracked snakes spent more time sequestered in trees than in terrestrial situations (Table 1; single-factor anova, both sexes F1,24 = 27.12, P < 0.001). Mean and maximum active season residence times did not vary significantly between sexes according to season (mean tree residence times, F1,10 = 0.83, P = 0.38; mean terrestrial residence times, F1,10 = 0.73, P = 0.41; maximum tree residence times, F1,8 = 1.14, P = 0.32; maximum terrestrial residence times, F1,8 = 0.12, P = 0.74).
| Snake ID no. | Sex | Arboreal sites | Terrestrial sites | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | n | Mean | SE | n | ||
| 271 | M | 9.61 | 3.74 | 9 | 3.79 | 0.96 | 7 |
| 228 | M | 7.36 | 1.59 | 18 | 2.57 | 0.48 | 7 |
| 057 | M | 15.3 | 3.29 | 15 | 2.17 | 0.20 | 44 |
| 938 | M | 6.5 | 1.06 | 35 | 4.29 | 2.12 | 7 |
| 919 | M | 5.18 | 0.95 | 33 | 3.21 | 0.85 | 19 |
| Mean male | 8.79 | 1.78 | 130 | 3.21 | 0.39 | 84 | |
| 141 | F | 7.00 | 2.99 | 10 | 2.72 | 1.36 | 9 |
| 978 | F | 6.17 | 1.33 | 29 | 3.08 | 0.74 | 36 |
| 019 | F | 5.17 | 1.79 | 15 | 2.36 | 0.45 | 7 |
| 299 | F | 7.06 | 2.25 | 26 | 2.86 | 0.59 | 7 |
| 200 | F | 7.64 | 1.93 | 25 | 2.82 | 0.44 | 20 |
| Mean female | 6.61 | 0.43 | 105 | 2.77 | 0.18 | 79 | |
- Table shows mean values and associated standard errors and sample sizes for residence times in arboreal and terrestrial sites.
The radiotracked snakes were highly arboreal. Even when located in terrestrial sites, they were generally on slightly raised perches rather than on the ground surface. Table 2 summarizes data on the use of terrestrial and arboreal microhabitats during the active season, based on 1099 locations from 13 radiotracked snakes. No significant sex difference was apparent in the distribution of mean height above ground across these six microhabitat categories (F1,12 = 0.003, P = 0.99). Females averaged higher than males when the snakes were found in shrubs or dead trees (stags), but lower when the snakes were in live trees or vines (Table 3).
| Snake ID no. | Sex | Ground | Sedge | Log | Shrub | Stag | Live tree | Vines | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | n | ||
| 271 | M | 0.24 | 0.08 | 9 | 0.00 | 0.00 | 2 | 4.00 | 1.15 | 3 | 19.31 | 0.90 | 13 | 10.00 | 10.00 | 10 | ||||||
| 228 | M | 0.00 | 0.00 | 10 | 7.29 | 0.38 | 17 | 20.00 | 1.08 | 18 | 7.87 | 0.38 | 16 | |||||||||
| 057 | M | 0.22 | 0.06 | 19 | 0.29 | 0.07 | 22 | 1.09 | 0.36 | 7 | 15.80 | 0.70 | 64 | 33.42 | 0.86 | 19 | 0.60 | 0.20 | 2 | |||
| 938 | M | 0.00 | 0.00 | 7 | 2.58 | 0.24 | 6 | 10.00 | n/a | 1 | 21.11 | 0.89 | 93 | 1.65 | 1.35 | 2 | ||||||
| 919 | M | 0.20 | 0.20 | 5 | 0.71 | 0.14 | 18 | 0.80 | 0.20 | 2 | 3.15 | n/a | 1 | 15.97 | 1.04 | 39 | 21.35 | 1.12 | 34 | |||
| 141 | F | 0.00 | 0.08 | 4 | 0.73 | 0.29 | 7 | 14.74 | 3.61 | 5 | 23.79 | 0.73 | 33 | 1.00 | n/a | 1 | ||||||
| 978 | F | 0.04 | 0.08 | 4 | 0.18 | 0.04 | 25 | 1.05 | 0.29 | 11 | 7.00 | 0.00 | 5 | 12.85 | 1.59 | 13 | 10.42 | 0.69 | 32 | 6.36 | 0.99 | 19 |
| 019 | F | 0.37 | 0.07 | 3 | 0.35 | 0.35 | 2 | 4.67 | 0.97 | 6 | 27.30 | 1.48 | 27 | 18.50 | 2.36 | 4 | 2.00 | 1.00 | 2 | |||
| 299 | F | 0.13 | 0.08 | 4 | 0.30 | 0.00 | 2 | 1.00 | n/a | 1 | 3.00 | 1.00 | 2 | 22.49 | 0.71 | 76 | 18.94 | 3.61 | 9 | 1.6 | n/a | 1 |
| 200 | F | 0.03 | 0.03 | 9 | 0.38 | 0.15 | 10 | 1.89 | 0.90 | 4 | 3.93 | 0.37 | 12 | 21.16 | 1.09 | 77 | 15.00 | 5.00 | 2 | 3.72 | 0.30 | 9 |
| 121 | F | 0.00 | n/a | 1 | 1.00 | 1.00 | 2 | 2.00 | 1.00 | 2 | 2.00 | 0.00 | 2 | 16.40 | 4.00 | 5 | 12.41 | 1.00 | 22 | |||
| 338 | F | 0.00 | 0.00 | 2 | 0.48 | 0.16 | 6 | 31.13 | 0.69 | 61 | 30.71 | 1.73 | 14 | |||||||||
| 190 | F | 0.03 | 0.09 | 7 | 0.00 | n/a | 1 | 1.00 | n/a | 1 | 0.65 | 0.15 | 2 | 5.50 | 2.06 | 4 | 22.81 | 1.96 | 29 | 1.65 | 0.15 | 2 |
- The table shows mean heights (in metres) above ground and associated standard errors and sample sizes. Some snakes were not observed in particular microhabitats.
| Sex | n | Duration of tracking (days) | No. observations | No. moves | Mean distance moved (m) | Total distance moved (m) | Maximum overnight move (m) | No. moves per observation | No. moves per day | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Male | 5 | 395.20 | 80.31 | 105.40 | 19.38 | 40.80 | 7.59 | 152.53 | 21.14 | 5982 | 1517.99 | 244 | 41.55 | 0.38 | 0.03 | 0.11 | 0.01 |
| Female | 8 | 290.38 | 51.19 | 77.88 | 13.83 | 32 | 6.6 | 116.23 | 8.95 | 3392 | 574.90 | 160.60 | 17.10 | 0.38 | 0.06 | 0.13 | 0.02 |
| All | 13 | 330.69 | 44.55 | 88.46 | 11.48 | 35.38 | 4.96 | 130.19 | 10.58 | 4388.15 | 739.17 | 192.70 | 21.50 | 0.28 | 0.04 | 0.12 | 0.01 |
Movements during the active season
Radiotracked snakes in arboreal shelter sites were relatively sedentary. Using the average of individual values for 13 snakes, 47% of locations (SE = 4%) were in the same tree as the previous observation (for males mean = 44%, SE = 6%, for females mean = 49%, SE = 5%). Less often, they had moved from one tree to another (overall mean = 30%, SE = 4%; for males mean = 33%, SE = 9%, for females mean = 28%, SE = 3%). However, snakes moved around more often when they were on the ground. A total of only 5% of records, SE = 2% (for males mean = 4%, SE = 1%, for females mean = 5%, SE = 2%) were of snakes found in the same terrestrial sites on successive locations, whereas in a further 18% (SE = 3%; for males mean = 19%, SE = 4%; for females mean = 18%, SE = 4%) snakes had moved from one terrestrial site to another.
Although most movements occurred between trees, the snakes used terrestrial rather than arboreal pathways for this purpose. Even when contiguous crown vegetation was available, radiotracked snakes instead descended to the ground when moving to a new location. The distances moved between successive locations were similar regardless of whether movements were from one tree to another (mean = 124 m, SE = 7.9, n = 163), from a tree to the ground (mean = 143 m, SE = 11.4, n = 94), from the ground to a tree (mean = 144 m, SE = 11.5, n = 87) or from one terrestrial perch to another (mean = 102 m, SE = 8.9, n = 92; anova, F3,20 = 0.51, P = 0.68). The mean values for all these categories were greater for males than for females, but the difference did not attain statistical significance in terms of the mean distance moved (anova, F1,6 = 0.91, P = 0.38) or the distribution of movements across the previously mentioned four categories (F1,6 = 0.29, P = 0.61).
On average, radiotracked snakes moved between arboreal shelter sites approximately once every 8 days (Table 1) and thus moved approximately 20 or 30 times during a 9-month active season (Fig. 1). Seasonal patterns of movement frequency differed slightly between the 2 years of our study (Fig. 1), suggesting that proximate stimuli (such as rainfall, temperature or prey abundance) might influence activity levels. Overall, the mean number of active season moves was greatest in summer 1998–1999 and lowest in spring 1997 (Fig. 1).
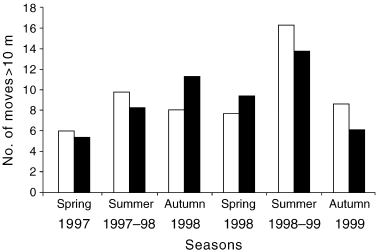
Mean number of moves by sex, (□) female and (▪) male, per season by radiotracked Stephens' Banded Snakes at Whian Whian State Forest.
On average, moves by males were longer than those by females; this difference was statistically significant in the first but not the second active season (single-factor anova with sex as the factor; 1997–1998 active season, F1,4 = 9.38, P = 0.03; 1998–1999 active season, F1,4 = 1.27, P = 0.32). Total distances moved were also greater for males than for females, but the difference between sexes was not statistically significant in either active season (1997–1998 active season, anova, F1,5 = 0.59, P = 0.48; 1998–1999 active season, F1,7 = 1.27, P = 0.30). The greatest distances moved between successive locations were 900 m for a male and 380 m for a female, and the maximum distances moved within a single night were 400 m for a male and 200 m for a female.
Home ranges
We defined a snake's home range as the area that it occupied during radiotracking, regardless of sample size variation (14–63 fixes; locations that were occupied more than once constituted only a single fix in estimating home-range size). Given the potential ecological importance of marginal locations to snakes, simple minimum convex polygon (MCP) estimates may best represent the areas needed for conservation planning and management purposes. We also calculated several other home-range descriptors (Table 4): (i) the 95% MCP estimate to facilitate comparisons with previous studies (White & Garrott 1990); (ii) the harmonic mean estimate to provide an index of central tendency; (iii) kernel analysis to clarify the possible role of multiple centres of activity; and (iv) range-width estimates as a further index of space use (Harris et al. 1990).
| Sex | 100% MCP (ha) | 95% MCP (ha) | Harmonic mean (ha) | Kernel (ha) | No. active seasons | No. fixes | Range width | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Male | 20.19 | 5.52 | 18.46 | 5.35 | 1.94 | 0.77 | 25.67 | 7.51 | 1.40 | 0.27 | 40.40 | 7.11 | 715.06 | 90.21 |
| Female | 5.44 | 0.85 | 4.63 | 0.79 | 1.26 | 0.52 | 5.26 | 0.90 | 1.25 | 0.19 | 30 | 5.96 | 375.38 | 36.61 |
| All | 11.11 | 2.91 | 9.95 | 2.77 | 1.52 | 0.43 | 13.11 | 3.97 | 1.30 | 0.15 | 34 | 4.62 | 506.23 | 61.71 |
- MCP, minimum convex polygon.
Home ranges as calculated by the MCP method were large (mean = 11 ha), with no significant overall correlation between home-range size and the number of fixes for either sex (males, r = 0.24; females, r = 0.47, both have P > 0.05). This result suggests that our sample sizes were sufficient to quantify overall space use by each snake although, undoubtedly, longer monitoring would eventually detect more extensive movements. On average, the home ranges of male snakes were almost four times larger than those of females (Table 4; anova, F1,11 = 7.55, P = 0.007). Home-range sizes were not significantly correlated with the body size (mass) of snakes within either sex (males, r = −0.36; females, r = −0.22, overall r = 0.08; all P > 0.05).
Home range overlaps among individuals
Spatial overlaps in home-range areas were observed for 15 of the 16 snakes monitored and for all 13 used in the spatial analysis. Because additional uncaught snakes were occasionally observed within the home ranges of radiotracked snakes, the real extent of spatial overlap is unknown. Of the radiotracked animals, home ranges of males overlapped those of other males by an average of 38% (n = 2 pairs) and those of females by an average of 11% (n = 8 pairs). The radiotracked females overlapped other females by 39% (n = 6 pairs), and males by 30% (n = 7 pairs). anova shows that differences between the extent of home-range overlaps (same sex, different sex) were not significant compared across all categories (n = 20 overlaps; F3,16 = 1.79, P = 0.19). Figure 2 depicts the home-range area overlaps of six radiotracked snakes at one of the study sites.

Home range overlaps for six radiotracked Stephens' Banded Snakes at the Rummery Road study site in Whian Whian State Forest.
Radiotracked snakes tended to remain well separated from each other, even when their home ranges overlapped substantially. Of the 162 trees occupied by snakes during the course of the study, only 10 were used by more than one snake. Only once did we record two snakes less than 60 m apart on the same or consecutive radiotracking days. For four pairs of snakes monitored synchronously, the mean distances maintained between snakes were 328, 264, 291 and 209 m. Nearest same-day distances between these snakes were 96, 61, 70 and 24 m. We examined this apparent avoidance behaviour by estimating the probability of shared occupation of each ‘use’ tree (i.e. used at least once by a snake) within a home-range overlap zone under the null hypothesis of no avoidance. To perform this test, we calculated the probability that a given snake would be in any given one of the overlap trees at any given time (= 1/total number of trees used by that snake overall), and then did the same for the snake with which its home range overlapped. The resulting probabilities of co-occurrence were multiplied by the number of times we simultaneously located both snakes, to give an expected number of observed co-occurrences within the same tree. For 13 pairs of snakes, the probabilities ranged from 0.0 (where one snake had no use tree in the overlap zone) to 0.77. Where both snakes had at least one tree in the overlap zone, probabilities of co-occurrences ranged from 0.03 to 0.77. In practice, no instances of co-occurrence were found. This result thus enables us to reject the null hypothesis of no avoidance (sign test, n = 0 of 13 cases where expected value exceeds observed value, P < 0.01; Siegel 1956). Some terrestrial areas (mainly sedge stands) were also utilized by snakes with overlapping home ranges but (as was the case with trees), radiotracked snakes were never located in these areas simultaneously.
Discussion
Our radiotracked snakes displayed very consistent patterns in terms of habitat selection and movement patterns. They were highly arboreal for most of the year, spending the winter inactive within tree hollows. During the active season they generally remained within a specific shelter site (most often a tree hollow) for more than a week before descending to the ground and travelling more than 120 m to a new shelter tree. The frequency and duration of such movements were broadly similar in spring, summer and autumn, and in males and females. However, males had much larger home ranges than females overall (means of 20 vs 5 ha). Although home ranges often overlapped by approximately one-third, snakes rarely occupied that overlap area at the same time. We first discuss factors that may have generated these patterns. We then consider the consequences of these results for the conservation of Stephens' Banded Snakes.
The radiotracked snakes exhibited a high degree of arboreality in their selection of shelter sites. Prey were probably secured in these retreat sites; several of the small mammal species consumed by H. stephensii are known to nest in tree hollows (Ward 1990; Dickman 1991; Woodside 1995; Gibbons et al. 2002). Telemetered snakes inside tree hollows were sometimes coiled within mammal nests, presumably awaiting the occupants' return (Fitzgerald et al. 2002). Thus, the presence of prey species may influence tree selection by snakes. Snakes in wet sclerophyll forest were also observed foraging for 1 to 33 nights in dense tall sedges. Two snakes captured prey (weaner Bush Rats, Rattus fuscipes) in these locations.
Two aspects of these movement patterns are intriguing: (i) why did the snakes remain sequestered within sites for long periods; and (ii) why did they move so far between successive sites? The answer to the first question almost certainly involves foraging mode. Detailed studies on a congeneric species, Hoplocephalus bungaroides, have revealed similarly long residency times punctuated by long-distance movements, and have attributed this pattern to the snakes' reliance on ambush predation (Webb & Shine 1998).
Turning to the second aspect, why do the snakes move so far (on average, more than 120 m) between successive shelter sites? The answer presumably involves the spatial distribution of suitable ambush-sites and associated prey animals. Radiotracked snakes disproportionately used very large, old, hollow-bearing trees for this purpose (Fitzgerald et al. 2002), and such trees are typically rare within forests such as WWSF, which have been managed for timber production over long periods (Gibbons & Lindenmayer 1997). This scarcity inevitably increases the distances that snakes must move to find a suitable tree. However, our radiotracked snakes frequently moved past many other large hollow-bearing trees, and we do not know why these were not used (perhaps because of the presence of predators, or the absence of mammalian prey). The other factor reducing the availability of suitable trees may be that waiting in ambush at a specific site is unlikely to be productive if the site's owner has recently been either consumed or frightened away (‘resource depression’: Charnov et al. 1976; Gregory et al. 1987). Thus, snakes may benefit from visiting a given site only infrequently.
The same phenomenon (a preference for ambush sites not recently used) may lead snakes to avoid sites that other snakes have recently occupied. Avoidance of conspecifics has also been reported in the congeneric H. bungaroides (Webb & Shine 1997), as well as in another Australian elapid species, Pseudonaja textilis (Whitaker 2001). Although the conventional wisdom in this field has been that social interactions have only a trivial effect on spatial patterns of snakes (Gregory et al. 1987; but see Carpenter 1984), these studies suggest that the location of conspecifics may exert a significant effect on movements and habitat selection.
Another potential determinant of movement patterns (and especially distances and directions moved) might involve the snakes' preference (or reluctance) to utilize specific types of habitats within the mosaic available at WWSF. For example, although radiotracked snakes frequently crossed unsealed roads, which ran through the study area, they avoided cleared areas such as log dumps, and generally remained within dense vegetation. The snakes' readiness to travel long distances on the ground (although almost always at night) also meant that the three-dimensional canopy structure (and especially, physical connectivity between the branches of adjacent trees) was not an important constraint on movement patterns.
Last, what are the consequences of these aspects of spatial ecology for the conservation of Stephens' Banded Snakes? Terrestrial movement may expose individuals to the risks of predation (Shine & Fitzgerald 1996; Bonnet et al. 1999; Reinert & Rupert 1999), of accidental killing by vehicles on roads, and of deliberate killing by humans (Whitaker & Shine 2000). Thus, management should address the abundance of feral predators such as cats and dogs. However, habitat quality may be the most important consideration. Apart from the obvious issue of reliance upon a scarce resource (large, old, hollow-bearing trees: Fitzgerald 2002), the most significant issue concerns the absolute amount of suitable habitat required per individual in this highly vagile species. Home ranges for the snakes in our study (mean of 11.1 ha) were larger than for the congeneric H. bungaroides (3.3 ha; Webb & Shine 1997) or for much larger elapid species such as the Eastern Brown Snake, Pseudonaja textilis (5.8 ha; Whitaker & Shine 2001) and Common Black Snake, Pseudechis porphyriacus (males 9.6 ha, females 2.4 ha; Shine 1987). Presumably, the large home range size for Stephens' Banded Snakes reflects both the scarcity (and thus wide dispersion) of suitable trees, and the snakes' preference for trees not recently occupied by conspecifics.
One consequence of this large home range size is that a substantial area of contiguous forest may be required to sustain a viable population of H. stephensii. All records acquired during the present study of the present-day distribution of Stephens' Banded Snakes (n = 44 records since 1997) derive from large forested areas. The smallest state forest from which this species has recently been recorded was 5.5 km on its longest axis, and the mean longest axis of 13 state forests where populations were recorded was 20.4 km (range 5.5–35 km). The vast majority of existing forest remnants are much smaller than these values, suggesting that populations of H. stephensii are retained only in relatively large patches of remnant forest. For example, in a survey of remnant bushland in south-east Queensland, it was found that 52% of bushland areas surveyed were less than 50 ha in size (Catterall & Kingston 1993). Thus, future planning for the conservation of this species should aim not only for the retention of suitable habitat types, but also for the maintenance of large contiguous areas of such habitat.
Acknowledgements
State Forests of New South Wales and the Australian Research Council provided logistic and financial support. We thank the Fitzgerald family, M. Whicker, R. Kooyman, S. Phillips, P. Harlow and P. Whitaker for help and advice. Field work was carried out under NSW National Parks and Wildlife Scientific Licence No. B1712, State Forests of NSW Special Purposes Permit 97/1 and University of Sydney Animal Care and Ethics Committee Approval L04/5–97/3/2561. An earlier draft of this manuscript was much improved by the suggestions of two anonymous reviewers.




