Trophic trickles rather than cascades: Conditional top-down and bottom-up dynamics in an Australian chenopod shrubland
Abstract
Abstract Despite continued interest in the relative roles of top-down (predation) and bottom-up (productivity) factors in structuring ecological communities, there have been few studies of diverse terrestrial arthropod systems. Top-down theory predicts that decreased predator populations will result in increased herbivore populations and reduced plant populations. Bottom-up theory predicts that a positive producer level response to nutrients will support greater herbivore and predator populations. Few studies simultaneously examine both theories. In the present study, the roles of predators and nutrients in structuring an Australian chenopod shrubland community were investigated, by using a complete 2 × 2 factorial design with three replicated plots with and without nutrients (fertilizer) and predators (two wolf spiders and two ants). Producer and major arthropod taxa responses, predator densities and soil nutrient levels were monitored from April 1996 to February 1998. In agreement with bottom-up theory, the abundances of some plants and herbivores increased with the addition of nutrients, but most effects varied temporally and did not increase the abundances of any predator taxa. The effects of predator removal propagated down the food web to influence some herbivore and producer taxa but, in contrast to top-down theory, effects were temporally dependent and varied in the direction of response within each trophic level. Predators and nutrients only interacted significantly to determine the abundance of one taxon. The findings suggest that true trophic cascades are not a primary determinant of chenopod community structure, with ‘conditional trophic trickles’ a better descriptor for the effects of predators and nutrients on this community. It is suggested that the expression of top-down and bottom-up effects is conditional on appropriate timing and extent of rainfall. The results provide further support for the theory that food webs are more complex in terrestrial than in aquatic systems, with abiotic and biotic heterogeneity, intraguild interactions and non-consumptive interactions between adjacent trophic levels all confounding the classic consumptive interactions that are required for the expression of top-down and bottom-up forces.
Introduction
Understanding the distribution and abundance of organisms is of paramount importance to implementing effective management practices in ecosystems. This was the central message of Andrewartha and Birch (1954), and nearly 50 years later it still remains one of the fundamental goals of ecology (e.g. Horppila et al. 1998). This is evidenced by vigorous debate on the relative roles of top-down (e.g. predation) and bottom-up (resource availability, e.g. nutrients) forces in controlling population levels and community structure (e.g. Polis & Strong 1996).
In their ‘green world hypothesis’Hairston et al. (1960) argued that in systems comprising three trophic levels (predators, herbivores, producers) the ‘world is green’ because strong predation directly reduces herbivore populations, allowing producers to increase in abundance (termed a ‘top-down cascade’). Extension of this theory leads to the prediction that in systems with four trophic levels (top predators, intermediate predators, herbivores, producers), similar processes will result in a ‘barren world’ with producers being consumer- rather than resource-limited, as effects on successive lower trophic levels are reversed (e.g. Fretwell 1977). Proponents of the ‘bottom-up’ view argue that resource availability and competition limit populations at all trophic levels (Sinclair 1975; White 1978). In this scenario, nutrient addition has a positive effect on the producer level, supporting larger herbivore and, therefore, larger predator populations (termed a ‘bottom-up’ cascade, e.g. Hunter & Price 1992).
Experimental manipulations of predators and nutrients in aquatic food webs with varying numbers of trophic levels have directly quantified the effects predicted by trophic cascade theory. They have also established that top-down and bottom-up forces can jointly, but to varying degrees, regulate community structure (e.g. Menge 1992; Rosemond et al. 1993). In contrast, there is no emerging consensus on the role of top-down and bottom-up forces in structuring terrestrial systems, in part because of procedural problems and arguments of greater food web complexity (e.g. Polis & Strong 1996). Greater biotic heterogeneity, frequent omnivory and subsequent high species interconnectedness can blur the alternating limiting processes in adjacent trophic levels, making unified consumption across a trophic level difficult. This may change the predicted cascading effects for each trophic level, or diffuse and dampen any intense consumptive processes across the trophic spectrum (Spiller & Schoener 1994; Mikola & Setala 1998). Bottom-up forces may take precedence in such cases because a system lacking primary producers lacks resources for consumers to constitute a trophic structure (Hunter & Price 1992).
Despite this, terrestrial experiments have demonstrated top-down cascading effects for lizards (e.g. Pacala & Roughgarden 1984), birds (Marquis & Whelan 1994), spiders (Riechert & Bishop 1990), mantids (Moran & Hurd 1998), parasitoids (Roininen et al. 1996), ants (Way & Khoo 1992) and predatory beetles (Clark et al. 1994), with resultant quantitative (abundance and/or biomass) and/or qualitative (community composition) changes at the herbivore and, in some cases, producer level. However, in contradiction of top-down theory, predators have been shown to have positive or no effects on herbivorous taxa. Some predators have significant impacts on other predatory taxa within the community. The outcome of these intraguild interactions often determines how, and to what extent, herbivorous taxa will be influenced by predators (Polis & McCormick 1986; Letourneau & Dyer 1998b).
Bottom-up control of the plant component of terrestrial communities has been identified in a variety of systems, including deserts (Gutierrez & Whitford 1987), grasslands (Rubio et al. 1995), forests (Wang et al. 1996), coastal dunes (Houle 1997), salt marshes (Kiehl et al. 1997) and heathlands (Alonso & Hartley 1998). The effects of nutrient addition include increased plant biomass, abundance and diversity, and changes in community composition. Studies are limited, but results so far indicate that nutrient-induced bottom-up effects may flow through to increase the abundances of predatory arthropods (Rypstra 1983). However, nutrients can also have negative effects on the abundances of different arthropod taxa, often within the same system (e.g. Larsen et al. 1996).
Most terrestrial-based cascade studies examine only one or two predator, herbivore and/or plant species. Most have also been conducted in agroecosystems, which have simpler food web structures as biota re-establish annually after harvesting. Such experimental approaches are attractive because their low complexity helps keep track of and interpret the results of experimental manipulations (Hurd & Eisenburg 1984; Carter & Rypstra 1995). However, such studies reveal little about the relative roles of top-down and bottom-up forces in the dynamics and structuring of more complex, species-rich systems (e.g. Roininen et al. 1996).
Specifically, there is a need for more manipulative experiments that have four components:
- 1
Investigation of the relative importance of generalist predators and resources in the structuring of more natural terrestrial communities.
- 2
Recognition and quantitative assessment of biotic heterogeneity within the food web.
- 3
Incorporation of the effects of temporal variation in biotic interactions.
- 4
Acknowledgement of any direct or indirect effects of abiotic heterogeneity that may influence the relative strength of top-down and bottom-up forces.
The present study was designed to address these four issues, by investigating the effects of nutrient addition and removal of generalist predators on the invertebrate and producer trophic levels of a semi-arid chenopod shrubland community. The specific aims were to investigate the separate and interactive effects of nutrient addition and predator removal on: (i) density, cover, biomass and diversity of producers; and (ii) abundance of different arthropod taxa.
I report here on the significance of the experimental effectiveness of the manipulations, the responses of manipulated predators to bottom-up manipulations, arthropod community responses at the ordinal and, for ants, generic level and producer species level responses. A preliminary analysis for the first year of this experiment only, examining trends in abundance for three arthropod taxa is in Dawes-Gromadzki (1999).
Methods
Study site and manipulated species
Experimental plots were established in relatively homogenous open chenopod shrubland 25 km north-west of Mount Mary (139°26′E, 34°06′S), South Australia (Fig. 1). The region receives a mean annual rainfall of 241 mm over a mean of 46 rain days (r.d.; for the period 1925–1998), and has warm summers (means: maximum 28°C, minimum 13.3°C) and cool winters (means: maximum 13.6°C, minimum 5.3°C; for the period 1880–1998; Adelaide Bureau of Meteorology). Preliminary soil analyses showed the soil to be a nutrient-poor (3 mg kg-1 nitrogen (N), 12 mg kg-1 phosphorus (P)) clay loam. The vegetation is dominated by blue bush (Marieana sedifolia) at a height of approximately 1 m, with an understorey of native perennial grasses (Danthonia caespitosa and Stipa nitida) and scattered forbs. The area has a history of low-intensity grazing by sheep.
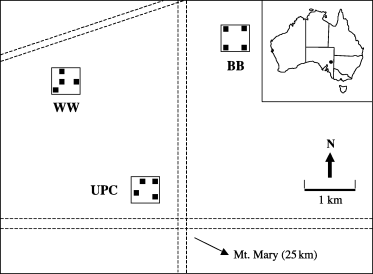
Location of the study site and relative locations of the Winters Dam (WD), UP Corner (UPC) and Bundey Bore (BB) replicate blocks in the study area. (▪), Experimental plots; (- - -), dirt roads.
At Eudunda, 36 km west-south-west of the study area, the average monthly maximum and minimum temperatures recorded over the study reflected the longer-term averages for the region. Annual rainfall and number of r.d. recorded at Bundey Bore Station, within the study area, were 284.2 mm over 80 r.d. in 1996 and 386.2 mm over 56 r.d. in 1997. The rainfall pattern varied considerably between years. In the January–June period, 128.4 mm fell over 27 r.d. in 1996, whereas 123 mm fell over 16 r.d. in 1997. In the July–December period, 155.8 mm fell over 53 r.d. in 1996, whereas 263.6 mm fell over 40 r.d. in 1997.
Generalist predatory ants (Hymenoptera: Formicidae) and spiders (Araneae) are among the most ubiquitous arthropod predators in terrestrial ecosystems, and often have many loose links to species in lower trophic levels (Wise 1993). Short-term studies have identified these generalists as ecologically important predators in agroecosystems, which exert strong top-down control of pest populations (Way & Khoo 1992; Clark et al. 1994). However, experimental evidence of their role in more natural ecosystems is lacking (Wise 1993). In the present study, I manipulated the common generalist ant species Iridomyrmex lividus (member of the purpureus group; workers to 10 mm length) and Rhytidoponera sp. (mayri group; workers to 15 mm length) and the only two wolf spider species present, Lycosa stirlingae (mean body length 32 mm) and Pardosa sp. (mean body length 13 mm).
Both L. stirlingae and Pardosa sp. inhabit burrows, have annual life cycles and are nocturnal. After breeding in autumn, females carry egg sacs attached to their spinnerets. The young hatch in spring and are initially transported about on the back of the female (T. Z. Dawes-Gromadzki, pers. obs.). Iridomyrmex lividus and Rhytidoponera sp. were selected for manipulation based on previous observations of their predatory nature, large colony sizes and prominence at the study site (Dawes-Gromadzki & Bull 1997a,b). Iridomyrmex lividus is the dominant ant species present, is diurnal and forages on the ground and vegetation. It displays the typical characteristics of Iridomyrmex, with high activity levels, aggressive behaviour and resource monopolization (Andersen & Patel 1994). Rhytidoponera sp. is nocturnal in summer and diurnal in cooler months, and is a solitary, opportunist forager for prey and carrion on the soil surface (Andersen 1991). Reference specimens are held by the author.
Experimental design and manipulations
The experiment was conducted from April 1996 to February 1998. Three replicate 25-ha areas (blocks) were established: Winters Dam (WD), UP corner (UPC) and Bundey Bore (BB). Four 45-m × 45-m plots separated by at least 200 m were randomly set up in each block (Fig. 1). The experimental design was a 2 × 2 factorial with the four plots per block randomly assigned one of the following four treatments: (i) control (nutrients and predators unaltered; –F +P); (ii) nutrients added, predators unaltered; +F +P; (iii) nutrients unaltered, predators removed; –F –P; (iv) nutrients added and predators removed; +F –P. Sampling was restricted to the central 25 m × 25 m of each plot to reduce any edge effects.
Target predators
In late April to early May 1996, all wolf spiders and nests of I. lividus and Rhytidoponera sp. were counted and removed from the two predator removal plots per block. Wolf spider searches were performed during the day when spiders were inactive, by walking transects 2 m apart across the 25-m × 25-m sampling area. Water was squirted down burrows, and emerging spiders were collected with forceps and permanently removed from the area. Larger L. stirlingae were ‘fished’ out by using a small length of fishing line and hook baited with a mealworm. Nests of I. lividus and Rhytidoponera sp. were excavated (to a depth of 0.3–0.5 and 0.5–0.8 m for I. lividus and Rhytidoponera sp., respectively) and the resultant hole was loosely refilled; ants appeared unable to reconstruct these nests. For both ant species, one nest represents one colony; therefore nest removal results in the removal of the entire colony. Following the last nest excavation, all nests excavated within a plot were revisited to collect any returning workers. Additional removals were conducted within the buffer zone by walking twice around at random distances from the edges of the 25-m × 25-m sampling area. Less than 4% of the total plot area was disturbed by nest excavation, so a sham treatment was not performed in other plots. Surveys of wolf spider abundance in plots with predators (discussed subsequently) were considered equivalent to a sham treatment for spider removal. Predators were removed eight times: in autumn, winter and spring 1996, summer 1996–1997, autumn, winter and spring 1997 and summer 1997–1998.
Nutrients
The low fertility of most Australian soils is attributed to high temperatures, low rainfall and low soil nutrient status (Spain & Hutson 1983). Generally, P and N are the most limiting nutrients and, therefore, these are the minerals most likely to regulate plant growth and soil fertility (Davidson & Morton 1981). An 8:4:8 N : P : K Complete D slow-release pebble fertilizer (Pivot Limited, Melbourne, Vic., Australia) was applied to the two nutrient-addition plots per block with a Scotts EasyGreen Rotary Spreader (model EG-3, Scotts, Marysville, OH, USA) at a rate of 580 kg ha-1. This was the maximum spreading rate recommended by the manufacturer, above which burning of the vegetation would result. Nutrient additions were conducted during winter; fertilizer breakdown was reliant on rainfall. Three applications were performed at 6-week intervals in 1996 (1 June, 12 July and 23 August), with two doses applied in the relatively dry 1997 winter (1 June, 12 July) because of slow degradation of the first application. This resulted in an annual addition of 14.4 g m-2 N, 7.2 g m-2 P and 14.4 g m-2 K in 1996 and 9.6 g m-2 N, 4.8 g m-2 P and 9.6 g m-2 K in 1997. The fertilizer spreader (containing no fertilizer) was run over the ground surface in unfertilized plots.
Sampling methods
Effectiveness of treatments
At each removal event, wolf spiders and ant nest numbers were counted in predator-present plots, using the same methodology as for predator-removal plots (except that predators were not removed). From March 1997, spider and ant nest numbers were also censused monthly between removal events in all plots, to monitor recolonization rates. Pitfall traps, used to sample ground-dwelling arthropods (see subsequent description), also collected predatory ants that were foraging, allowing comparison of removal and predator-present plots.
The effectiveness of the fertilizer treatment was measured indirectly through soil nutrient surveys, conducted in September 1996 and 1997. Four random soil cores of 10 cm diameter and 10 cm deep were collected per plot and pooled. For each block, samples from the two fertilized and two unfertilized plots were, respectively, pooled, mixed and single representative samples assayed. The resultant six samples were analysed for available N, P and K at the South Australian Soil and Plant Analysis Service (SASPAS) of Primary Industries South Australia.
If fertilization results in increased plant production, one possible outcome is a relative increase in vertebrate grazers in fertilized plots. The fertilizer effect on producers could then be confounded by associated physical disturbance (e.g. trampling), grazing and/or faecal deposition, which can lead to changes in abundance (Kelt & Valone 1995), cover (Bock et al. 1984), community composition (McInnes et al. 1992) and diversity (Waser & Price 1981) of plants, and soil nutrient enrichment (Landsberg et al. 1990). Vertebrate grazing can also affect herbivorous insect populations (e.g. Jepson-Innes & Bock 1989). To test for differences in grazer activity, vertebrate faecal pellets were surveyed six times, in August and December 1996, and March, June, September and December 1997. Three 5-m × 5-m quadrats were randomly selected in each plot, and faecal pellets were removed and sorted into three groups: sheep, kangaroo and other (rabbit, emu, wombat). Samples were oven-dried for 48 h at 60°C and weighed to the nearest milligram.
Plant responses
The vegetation surveys, conducted at the same times as the faecal pellet surveys, focused on quantifying the effects of experimental treatments on seasonal changes in grass-layer plants. There was little variation between plots (mean = 74; SD = ±15) in the density of M. sedifolia at the start of the experiment, and no recruitment during the study. In contrast, grasses and forbs had strong seasonal fluctuations, which has the potential to influence observed seasonal changes in the abundance of most arthropods. Plant species were classified into one of seven groups (Table 1); individual species were identified by the South Australian Herbarium. In each plot, four replicate 1-m × 1-m grids, each subdivided into 16 0.25-m × 0.25-m cells, were used to quantify plant density, percentage cover, biomass and species ‘group’ diversity (number of plant groups 1–7 present) at each vegetation census. Different grid positions, randomly located between blue bushes, were used for each survey.
| Group no. | Taxa | Life form |
|---|---|---|
| 1 | Danthonia caespitosa (Poaceae) | Grass |
| Stipa nitida (Poaceae) | Grass | |
| 2 | Eriochiton scherolaenoides (Chenopodiaceae) | Forb |
| 3 | Sida sp. (Malvaceae) | Forb |
| 4 | Convolvulus remotus (Convolvulaceae) | Forb |
| Convolvulus sp. (Convolvulaceae) | Forb | |
| 5 | Vittadinia gracilis (Asteraceae) | Forb |
| 6 | Brachycome ciliaris var. ciliaris (Asteraceae) | Forb |
| 7 | Echium plantagineum (Boraginaceae) | Forb |
- Family names and life form from Black (1986).
Plant density was measured as (i) total plant density (total number of plants in each grid) and (ii) absolute density of each species group. The total densities of grasses versus forbs were also determined. Above-ground plant biomass was harvested from a randomly selected 0.5-m × 0.25-m cell in each grid. Samples were oven-dried for 48 h at 60°C and weighed to the nearest mg for an estimation of dry above-ground biomass (subsequently referred to as biomass). No attempt was made to divide plant biomass into the different groups. Although destructive, by the end of the experiment less than 1% of the 25-m × 25-m plot area was removed. Plant cover was estimated using the 25 intersection points of the 1-m × 1-m grids. No foliar nutrient analyses were attempted.
In April 1996, numbers of target predator species, soil nutrient levels, arthropod abundances, vertebrate grazer activity and vegetation in all plots were censused to determine initial premanipulation conditions, followed by no sampling for four weeks to allow time for any disturbance to the community to subside and for any effects of the manipulations to begin.
Arthropod responses
Those orders for which a general feeding strategy can be assigned were classified into one of three trophic guilds: herbivores (Homoptera, Hemiptera, Lepidoptera, Orthoptera, Thysanoptera), predators (Araneae, Hymenoptera excluding Formicidae) or detritivores (Collembola). For the purposes of this study, parasitic Hymenoptera were classified as predators. Other taxa sampled were not categorized into a specific trophic guild, because they contained both herbivorous and predatory species (Coleoptera, Diptera, Acarina).
One premanipulation and 21 postmanipulation arthropod censuses were conducted. In each plot, ground-dwelling arthropods were sampled using nine pitfall traps laid out centrally in a 3 × 3 grid with 6.25-m intervals between traps. Traps were plastic vials (diameter 6 cm, depth 7.5 cm) sunk into sleeves of polyvinyl chloride (PVC) pipe, set flush with the soil surface. Pipes remained in place throughout the experiment, overcoming the problem of repeated digging-in effects (Greenslade 1973). Empty vials (with lids) were inserted into pipes at the start of the experiment and between trapping periods. Traps remained closed for 4 weeks prior to the first census to reduce initial digging-in effects. Traps were operated with open vials containing 50% ethylene glycol (3 cm depth) and drops of detergent to reduce surface tension, for 48 h monthly from April 1996 (premanipulation census) to February 1998. For each month in each plot, arthropods from five traps were pooled, sorted and counted to ordinal level. To overcome the confounding problem of ‘edge effects’ inherent in traps established in a grid pattern (Link & Barker 1994), the central trap was always sorted, with the other four traps randomly selected. Ants were further sorted and counted to the generic level, and also classified into functional groups following Andersen (1995; Table 2).
| Group | Common taxa | Relevant features |
|---|---|---|
| 1. Dominant Dolichoderinae | Iridomyrmex | Highly abundant, active, aggressive, monopolize resources |
| 2. Subordinate Camponotinae | Camponotus, Polyrhachis | Competitively subordinate to Iridomyrmex spp., relative abundance generally low |
| 3a. Hot climate specialists | Melophorus, Meranoplus, Monomorium rothsteini group | Physiological, morphological and behavioural specializations that enable coexistence with Iridomyrmex |
| 3b. Cold climate specialists | Notoncus, Stigmacros, Bothriomyrmex | Cooler regions where effects of Iridomyrmex are reduced |
| 4. Cryptic species | Small species from Myrmicinae and Ponerinae | Forage and nest mostly within soil and litter |
| 5. Opportunists | Rhytidoponera, Tapinoma, Tetramorium, Odontomachus | Unspecialized, sensitive to competition from other ants |
| 6. Generalized myrmicines | Pheidole, Crematogaster, Monomorium (excluding M. rothsteini group) | Unspecialized behaviour but highly abundant, show rapid recruitment and effective defences, successful competitors |
| 7. Specialist predators | Cerapachys, Anochetus, Bothroponera, Myrmecia | Larger-sized species, low foraging densities and/or specialist diets; reduced interactions with other ants |
- Modified from Andersen (1995).
Grass-layer arthropods were also sampled monthly using sweep nets (49 cm diameter). A sample in each 25-m × 25-m area consisted of 120 continuous sweeps, 60 of which were conducted along five 25-m parallel transects (12 sweeps per transect) in a back-and-forth fashion up and down the site, and the other 60 similarly conducted along five 25-m parallel transects, perpendicular to and overlaying the previous five transects. Sweep netting was conducted between 09.00 and 12.00 hours. Samples were sorted and counted to ordinal level. For both pitfall and sweep samples, the hymenopterans were further sorted into Formicidae and Other Hymenoptera (wasps, sawflies, bees).
The abundances of larger predators, primarily lizards and centipedes, were also monitored. Four 10-L buckets were dug flush with the soil surface in a central 2 × 2 grid, and operated monthly (without drift fences) for 48 h. Specimens were collected, identified and counted in the field prior to release.
Data analysis
Effectiveness of treatments
Three-way repeated-measures anova (ranova) was used to test for overall fertilizer and predator treatment effects on wolf spider numbers and the interactions between treatment effects and time. The repeated measures were the numbers per plot recorded on each postmanipulation census. The independent variables were block (WD, UPC and BB), predator treatment (predators present, predators removed), and fertilizer treatment (unfertilized, fertilized). Sample time (‘date’) was the within-subjects factor. A repeated-measures analysis of covariance (rancova) was initially performed with the premanipulation (April 1996) number in each plot used as the covariate. A P-value for the covariate of <0.05 indicates a significant effect of initial (pretreatment) differences in spider numbers among plots, in which case it was left in the analysis where it provided adjustment of the dependent variable scores, and output from the rancova was used (Tabachnick & Fidell 1996). The covariate was dropped if the P-value was non-significant and the unadjusted output from the ranova was used. Unless indicated, initial tests on premanipulation data were non-significant. For significant results, Huynh–Feldt adjustments were made in order to overcome the problem of circularity inherent in ranova (Von Ende 1993; Norusis & SPSS Inc. 1994).
A doubly multivariate repeated-measures design was used to test for treatment effects and time on nest numbers. This provides a joint analysis of treatment effects on nests of both ant species, and allows for any correlation of the responses of the two species (Von Ende 1993). Independent variables and the interaction factor were the same as for the ranova. To determine the dependent variables contributing to significant effects, tests of treatment effects were conducted separately for I. lividus and Rhytidoponera sp. nest abundances. For interaction effects, separate univariate tests are provided for I. lividus and Rhytidoponera sp., with Huynh–Feldt adjustment applied when necessary. For significant treatment effects a three-way manova was conducted on premanipulation values. For all multivariate analyses, the Wilks' lambda multivariate test statistic was used to test the significance of main effects (Tabachnick & Fidell 1996).
The same doubly multivariate repeated-measures design was used for ant abundance analyses. For all arthropod taxa, average abundances from the 3 months constituting each season were calculated, giving seven postmanipulation censuses (winter and spring 1996, summer 1996–1997, autumn, winter and spring 1997 and summer 1997–1998). The repeated measures were the seven mean values of the 3 months per plot for each season during postmanipulation censuses.
To test the effect of fertilizer treatment on N, P and K levels, the 1996 and 1997 data for each nutrient were analysed separately using two-way anova. The main effects were block and fertilizer treatment. Initially, premanipulation levels were included as a covariate. The effect of fertilizer treatment on vertebrate grazer activity was tested by using a doubly multivariate repeated-measures model with repeated measures on the average faecal biomass (of the three quadrats per plot) for kangaroos and sheep. Other faecal pellet types constituted less than 6% of total faecal biomass.
Plant responses
A doubly multivariate repeated-measures design was used, with repeated measures on the average density of grasses and forbs in each postmanipulation census. For all plant variables, values used were averages of the four grids per plot. Separate repeated-measures manova were conducted on the densities of each forb group. Initially a multivariate repeated-measures analysis of covariance (rmancova) was performed. Species groups 6 and 7 were not analysed separately as they were only recorded in one census and constituted less than 3% of the data. Plant biomass and species ‘group’ diversity data were analysed using multivariate repeated-measures three-way anova.
Arthropod responses
Pitfall and sweep data were considered separately, as they sampled different components of the arthropod fauna. For each, a univariate repeated-measures three-way anova was used to analyse treatment effects on total arthropod abundance, and the abundance of each order. Over the entire study, pitfall traps caught fewer than 20 individuals for each of Isoptera, Chilopoda, Psocoptera, Chelonethida, Mantodea, Thysanura and Scorpionida. For sweep samples the Formicidae, Acarina, Lepidoptera, Psocoptera and Odonata combined constituted less than 3.5% of the total number of arthropods caught. These taxa were not analysed separately.
Ant fauna analyses
The same univariate three-way repeated-measures anova model was used to analyse treatment effects on the abundance of each ant genus that contributed >40 individuals. The same doubly multivariate repeated-measures procedure used for target ant species abundances was applied to ant functional group data (incorporating all genera). All seven functional groups were represented but only six were entered into the analysis (only one individual of cryptic species (CrS) was recorded). For significant between- or within-subject effects for climate specialists (CS), separate repeated-measures anova were conducted on the abundance of hot (HCS) and cold climate specialists (CCS). For each plot, numbers of centipedes and lizards in the four buckets were pooled, and then pooled into seasons as for pitfall trap data. Data were used for descriptive purposes, with numbers insufficient for statistical analyses.
The general linear models (GLM) procedure in SPSS (version 8.0; Norusis & SPSS Inc. 1994) for Windows (1997) was used for all analyses and a critical alpha of 0.05 was used to reject null hypotheses. Type III sums of squares were used in all statistical inferences. For all variables, two-tailed tests were used. Variables with distributions not meeting the expectations of normality were appropriately transformed. Untransformed means and standard errors are reported in the results section.
Results
Effectiveness of treatments
Predator removals
Overall mean numbers of wolf spiders in each plot during postmanipulation censuses were twice as high in treatments with predators (40.95 ± 2.97, mean ± SE) as in treatments with predator-removal (20.95 ± 2.37; F1,6 = 11.264, P = 0.015). There was also a significant date × predator effect (F13,78 = 12.946, P < 0.001), with the effectiveness of removals increasing as the experiment progressed (Fig. 2a). Overall mean nest number of the two ant species was an order of magnitude higher in predator (10.61 ± 0.28) compared with predator-removal (1.61 ± 0.17) treatments (F2,5 = 59.055, P < 0.001) and this difference increased significantly over time (F26,154 = 3.878, P < 0.001), particularly early in the experiment (Figs 2b,c). Univariate tests for Rhytidoponera sp. (predator F1,6 = 76.183, P < 0.001; date × predator F13,78 = 4.025, P < 0.001) and I. lividus (predator F1,6 = 30.442, P = 0.001; date × predator F13,78 = 2.940, P = 0.002) nests showed that both species contributed significantly to these effects. The overall mean number of foraging individuals of the two ant species was higher in predator (13.2 ± 1.59) than predator-removal (8.4 ± 1.31) treatments (F2,5 = 8.312, P = 0.026), and this difference varied significantly over time (F12,70 = 3.362, P = 0.001). In summer (1996–1997 and 1997–1998) and autumn (1996 and 1997), and spring 1997, abundance was higher in treatments with predators. Rhytidoponera sp. contributed significantly to these effects (predator F1,6 = 17.575, P = 0.006; season × predator F6,36 = 7.001, P < 0.001) but I. lividus did not (predator F1,6 = 0.093, P = 0.771; season × predator F6,36 = 0.883, P = 0.517).
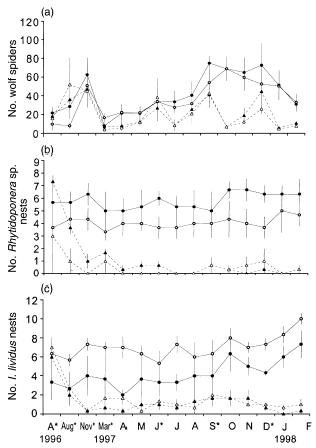
Mean numbers (± SE) of (a) wolf spiders, (b) Rhytidoponera sp. nests, and (c) Iridomyrmex lividus nests in each treatment. Dates indicate months starting with the April (A*) premanipulation census. *, Predator removal event; (●), fertilizer added; (▴), fertilizer added, predators removed; (), control; (▵), predators removed.
Nutrient addition
In September 1996 available N levels were higher in fertilized (5.33 ± 0.33 mg kg-1) than unfertilized (3.67 ± 0.33 mg kg-1) treatments (F1,2 = 21.816, P = 0.043), as were soil phosphorous levels (15.67 ± 1.20 mg kg-1 and 12.00 ± 0.58 mg kg-1; F1,2 = 65.463, P = 0.015). In September 1997 the overall fertilizer effect was not significant for N (P = 0.627) or P (P = 0.600). For both years K levels did not differ between fertilizer treatments (1996: F1,2 = 0.086, P = 0.797; 1997: F1,2 = 0.702, P = 0.490). There was no difference either overall or through time in mean faecal biomass between fertilized (4.54 ± 0.63 g m-2) and unfertilized (4.53 ± 0.40 g m-2) treatments (fertilized F2,7 = 0.017, P = 0.983; date × fertilized F10,78 = 0.667, P = 0.751).
Effects of nutrients on manipulated predators
Nutrient addition had no detectable effect on the manipulated predators. The overall mean number of wolf spiders was similar across fertilized (31.79 ± 2.85) and unfertilized (28.16 ± 2.64) treatments (F1,6 = 0.138, P = 0.723) with no difference between fertilizer treatments over time (F13,78 = 0.623, P = 0.828; Fig. 2a). There were no significant differences in overall numbers of Rhytidoponera sp. nests across fertilized (3.25 ± 0.32) and unfertilized (6.36 ± 0.56) treatments (F1,6 = 2.367, P = 0.175) or in numbers of I. lividus nests (F1,6 = 2.933, P = 0.138) across fertilized (2.61 ± 0.29) and unfertilized (4.14 ± 0.36) treatments (Figs 2a,c). There were also no significant differences in overall mean number of Rhytidoponera sp. in pitfall traps (F1,6 = 2.619, P = 0.157) across fertilized (4.94 ± 0.61) and unfertilized (3.97 ± 0.67) treatments, or number of I. lividus (F1,6 = 0.165, P = 0.699) across fertilized (5.85 ± 1.06) and unfertilized (6.84 ± 1.34) treatments. Neither number of nests (F26,154 = 1.164, P = 0.28) or trapped individuals (Rhytidoponera sp. F6,36 = 1.039, P = 0.416; I. lividus: F6,36 = 0.470, P = 0.826) varied significantly across fertilizer treatments over the experiment.
Producer responses
Mean plant density (F10,58 = 17.905, P < 0.001; Fig. 3a) and cover (F10,58 = 17.645, P < 0.001; Fig. 3b) both varied significantly over time, with density and cover higher in August 1996 and December 1997. Both grass density (P < 0.001) and cover (P < 0.001), and forb density (P < 0.001) and cover (P < 0.001) contributed significantly to these effects. Among the forbs, E. scherolaenoides density (P = 0.004) and cover (P = 0.036), and cover of Sida sp. (P = 0.005), were higher in December 1997, with Convolvulus spp. density (P = 0.003) and cover (P = 0.009) higher in August 1996.
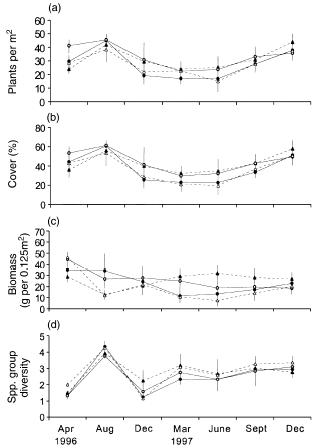
Mean (± SE) (a) density of plants, (b) plant cover, (c) plant biomass and (d) number of species groups in each treatment during the experiment. (●), Fertilizer added; (▴), fertilizer added, predators removed; (), control; (▵), predators removed.
Overall mean plant density was similar across fertilized (29.43 ± 1.75 individuals per m2) and unfertilized (30.26 ± 2.08 individuals per m2) treatments (F2,5 = 1.279, P = 0.356) and across predator (30.37 ± 2.04 individuals per m2) and predator-removal (29.33 ± 1.8 individuals per m2) treatments (F2,5 = 0.364, P = 0.712; Fig. 3a). Similarly, overall mean plant cover did not differ across fertilized (40 ± 2.4%) and unfertilized (40 ± 2.9%) treatments (F2,5 = 0.863, P = 0.466) and in predator (41 ± 2.8%) compared with predator-removal (39 ± 2.6%) treatments (F2,5 = 0.154, P = 0.861; Fig. 3b). Mean E. scherolaenoides density increased in fertilized compared with unfertilized treatments over the last three censuses (F5,2 = 64263.2, P = 0.003). There was also a date × fertilizer effect (F5,2 = 103.895, P = 0.010) and date × predator effect (F5,2 = 50.286, P = 0.020) for cover of Sida sp. From September to December 1997 Sida sp. cover was higher in fertilized compared with unfertilized treatments. In August 1996 and December 1997, Sida sp. cover was lower in predator-removal compared with predator-present treatments.
Mean plant biomass did not change significantly over the course of the experiment, and overall biomass did not vary significantly across fertilized (24.12 ± 2.18 g per 0.125 m2) and unfertilized (22.46 ± 2.45 g per 0.125 m2) treatments (F1,6 = 1.081, P = 0.339) or across predator (24.31 ± 2.49 g per 0.125 m2) and predator-removal (22.26 ± 2.11 g per 0.125 m2) treatments (F1,6 = 0.255, P = 0.631; Fig. 3c).
Variation in species group diversity over time was significant, increasing in August 1996 (F5,2 = 41.809, P = 0.024; Fig. 3d). Overall mean group diversity did not differ across fertilized (2.62 ± 0.16 species groups per m2) and unfertilized (2.67 ± 0.17 species groups per m2) treatments (F1,6 = 0.058, P = 0.817) or across predator (2.51 ± 0.17 species groups per m2) and predator-removal (2.78 ± 0.16 species groups per m2) treatments (F1,6 = 1.288, P = 0.300). No other treatment or interaction effects were significant.
Responses of ground-dwelling arthropods
A total of 492 436 arthropods from 17 orders was sorted from pitfall traps. Collembola was numerically dominant, constituting 83% of all arthropods collected (Table 3). Mean abundance of all ground-dwelling arthropods did not vary significantly over the experiment; however, with Collembola removed, mean abundance was greatest in spring and summer. There were no significant differences in mean number of all ground-dwelling arthropods across fertilized (2411.48 ± 598.9) and unfertilized (1468.81 ± 639.14) treatments (F1,5 = 1.200, P = 0.323), or across predator (2449.72 ± 708.01) and predator-removal (1430.57 ± 515.07) treatments (F1,5 = 1.378, P = 0.293). Neither the fertilizer × predator or season × treatment interactions was significant.
| Pitfall traps | % | Sweeps | % |
|---|---|---|---|
| Collembola | 82.7 | Thysanoptera | 42.8 |
| Hymenoptera | 15.2 | Orthoptera | 11.9 |
| Formicidae | 14.9 | Collembola | 9.3 |
| Other Hymenoptera | 0.3 | Hymenoptera | 7.8 |
| Acarina | 0.5 | Other Hymenoptera | 6.9 |
| Diptera | 0.3 | Coleoptera | 6.8 |
| Coleoptera | 0.3 | Hemiptera | 6.7 |
| Homoptera | 0.2 | Diptera | 6.1 |
| Lepidoptera | 0.2 | Araneae | 4.0 |
| Araneae | 0.2 | Homoptera | 2.0 |
| Thysanoptera | 0.1 | ||
| Orthoptera | 0.1 | ||
| Hemiptera | 0.1 |
- Listed taxa constitute 99.9% and 97.5%, respectively, of total arthropod catch.
For every taxon, mean number of individuals varied with season (P < 0.01), with peak abundance in spring and/or summer for the majority of taxa identified. For each taxon there were no overall treatment effects on mean abundance (Table 4). Mean thysanopteran abundance increased significantly in fertilized compared with unfertilized treatments in spring 1996 (season × fertilizer F6,36 = 3.557, P = 0.007; Fig. 4a). There was also a strong trend for mean orthopteran abundance to be lower in predator-removals in spring 1996 and summer 1996–1997 but higher in this treatment compared with the predator-present treatment in spring 1997 (season × predator F6,36 = 2.357, P = 0.051; Fig. 4b).
| Taxon | +F +P | +F –P | –F +P | –F –P |
|---|---|---|---|---|
| Total | 2281.08 ± 654.27 | 2541.89 ± 1008.81 | 2618.37 ± 1261.87 | 319.25 ± 110.58 |
| Collembola | 1338.59 ± 622.47 | 2388.70 ± 1011.65 | 2519.71 ± 1261.96 | 197.44 ± 108.46 |
| Hymenoptera | 905.40 ± 280.16 | 112.79 ± 22.74 | 57.41 ± 5.86 | 84.56 ± 19.38 |
| Formicidae | 900.83 ± 280.10 | 105.86 ± 22.51 | 52.06 ± 5.74 | 78.40 ± 19.36 |
| Other | 4.57 ± 0.57 | 6.94 ± 0.96 | 5.35 ± 0.73 | 6.16 ± 0.94 |
| Acarina | 7.92 ± 0.95 | 8.75 ± 1.20 | 11.14 ± 1.50 | 10.29 ± 1.69 |
| Diptera | 4.84 ± 0.68 | 6.32 ± 1.04 | 6.46 ± 1.46 | 5.22 ± 0.76 |
| Coleoptera | 3.22 ± 0.53 | 4.83 ± 0.88 | 6.38 ± 3.55 | 4.44 ± 0.75 |
| Homoptera | 4.79 ± 0.58 | 4.54 ± 0.49 | 4.83 ± 0.55 | 4.22 ± 0.47 |
| Lepidoptera | 4.22 ± 0.76 | 4.49 ± 0.71 | 3.41 ± 0.49 | 3.75 ± 0.64 |
| Araneae | 3.87 ± 0.29 | 3.49 ± 0.39 | 3.43 ± 0.35 | 3.84 ± 0.42 |
| Thysanoptera | 3.32 ± 1.06 | 3.03 ± 0.78 | 1.94 ± 0.48 | 1.71 ± 0.44 |
| Orthoptera | 2.97 ± 0.73 | 2.70 ± 0.51 | 2.00 ± 0.41 | 1.95 ± 0.40 |
| Hemiptera | 1.63 ± 0.34 | 1.98 ± 0.74 | 1.37 ± 0.28 | 1.63 ± 0.32 |
- Taxa not analysed (due to low numbers caught) were Isoptera, Chilopoda, Psocoptera, Chelonethida, Mantodea, Thysanura, Scorpionida. +F +P, fertilized, predator-present treatment; +F –P, fertilized, predator-removal treatment; –F +P, unfertilized, predator-present treatment; –F –P, unfertilized, predator-removal treatment. Excludes data for the target species manipulated. Values are means (±SE) of the three replicates for each treatment. Total includes all orders sampled throughout the experiment.
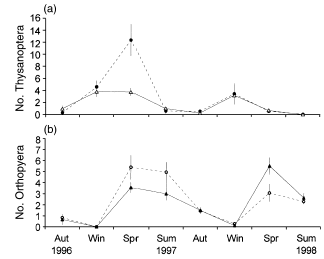
Mean numbers (± SE) of (a) Thysanoptera in (▵) unfertilized and (●) fertilized treatments and (b) Orthoptera in () predator and (▴) predator-removal treatments, collected in pitfall traps. Aut, autumn; Win, winter; Spr, spring; Sum, summer.
Responses within the ant fauna
A total of 73 258 ants, representing 20 genera, were sorted and identified. Iridomyrmex was numerically dominant, accounting for 78% of all ants sampled (Table 5). Temporal variation in mean number of individuals among postmanipulation censuses was significant for almost all genera (P < 0.001), with mean abundance highest during spring and summer. Mean number of Monomorium was higher in fertilized (37.19 ± 7.40) compared with unfertilized (14.46 ± 5.00) treatments (fertilizer F1,6 = 17.916, P = 0.005), and mean Tetramorium abundance was higher in predator-removal (1.34 ± 0.35) compared with predator-present (0.71 ± 0.13) treatments (F1,6 = 12.451, P = 0.012). The predator effect on Tetramorium also varied with season (F6,36 = 2.596, P = 0.034), increasing in spring and summer. There was also a temporally dependent fertilizer effect (F6,36 = 3.650, P = 0.006), with Tetramorium abundance lower in fertilized treatments in spring 1996 but higher in fertilized treatments in summer 1997–1998. For Melophorus there was a significant fertilizer × predator interaction with overall mean abundance lowest in the predator-present and fertilized treatment (F1,6 = 24.403, P = 0.003).
| Genera | Functional groups | ||
|---|---|---|---|
| Iridomyrmex | 78.4 | Dominant Dolichoderinae | 78.4 |
| Monomorium | 9.1 | Climate Specialists | 12.6 |
| Pheidole | 3.6 | Hot | 12.5 |
| Melophorus | 3.3 | Cold | 0.1 |
| Rhytidoponera | 1.8 | Generalized myrmicines | 5.4 |
| Crematogaster | 1.8 | Opportunists | 3.3 |
| Tapinoma | 1.1 | Subordinate Camponotini | 0.2 |
| Tetramorium | 0.3 | Specialist predators | 0.1 |
| Meranoplus | 0.2 | ||
| Camponotus | 0.1 | ||
| Cerapachys | 0.1 | ||
| Notoncus | 0.1 |
- Listed genera constitute 99.9% of total ants caught.
The 20 genera identified represented all seven functional groups, with 78% of ants caught representative of dominant Dolichoderinae (DD; Table 5). Overall mean numbers of CS were higher in fertilized (47.65 ± 8.55) compared with unfertilized (24.21 ± 5.65) treatments (P = 0.012), with HCS responsible for this effect (P = 0.012). There was a trend for a higher mean number of SP in treatments without predators (0.50 ± 0.12) compared with treatments with predators (0.24 ± 0.07; P = 0.049). For all other genera and functional groups, mean numbers of individuals did not differ significantly among treatments.
Responses of grass-layer arthropods
Sweep samples yielded 29 325 arthropods from 13 orders, dominated by Thysanoptera (43% of the sample; Table 3). Mean abundance of all grass-layer arthropods varied significantly over the seasons, with abundance high in summer censuses (F6,30 = 3.925, P = 0.005). Overall mean abundance did not differ significantly between fertilized (92.86 ± 16.74) and unfertilized (105.10 ± 23.84) treatments (F1,5 = 0.004, P = 0.950) or between predator (100.27 ± 21.56) and predator-removal (97.69 ± 19.63) treatments (F1,5 = 1.486, P = 0277; Fig. 5). The fertilizer × predator and season × treatment interactions were not significant. For all orders, variation in mean number of individuals with season was significant (P < 0.001), with peak abundance for most taxa occurring in spring and/or summer.
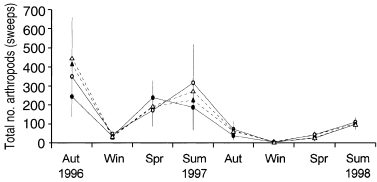
Mean numbers (± SE) of arthropods in each treatment during the experiment, collected from sweep samples. (●), Fertilizer added; (▴), fertilizer added, predators removed; (), control; (▵), predators removed. Aut, autumn; Win, winter; Spr, spring; Sum, summer.
Overall mean numbers of Homoptera were greater in fertilized (2.61 ± 0.52) rather than unfertilized (1.74 ± 0.34) treatments (F1,5 = 13.874, P = 0.010). For all other taxa, overall mean abundance did not differ significantly among treatments (Table 6). However, there was a fertilizer effect on mean thysanopteran abundance that varied with season (F6,30 = 2.670, P = 0.034), with abundance being lower in fertilized compared with unfertilized treatments during summer censuses. The mean abundance of Hemiptera was lower in predator-removal compared with predator-present treatments during summer 1997–1998 (F6,36 = 3.651, P = 0.006).
| Taxon | +F +P | +F –P | –F +P | –F –P |
|---|---|---|---|---|
| Total | 90.54 ± 26.29 | 95.17 ± 21.37 | 110.00 ± 34.71 | 100.21 ± 33.52 |
| Thysanoptera | 32.60 ± 14.29 | 34.05 ± 13.19 | 49.87 ± 25.58 | 47.13 ± 25.17 |
| Orthoptera | 13.59 ± 3.88 | 14.87 ± 2.85 | 13.32 ± 2.83 | 12.49 ± 2.97 |
| Collembola | 6.57 ± 1.88 | 10.78 ± 2.93 | 7.90 ± 2.36 | 5.43 ± 1.95 |
| Hymenoptera | 7.67 ± 1.67 | 8.37 ± 1.70 | 8.70 ± 1.76 | 8.21 ± 1.84 |
| Other | 6.54 ± 1.45 | 7.35 ± 1.45 | 7.84 ± 1.65 | 7.44 ± 1.58 |
| Coleoptera | 6.86 ± 3.19 | 6.79 ± 2.38 | 6.86 ± 2.44 | 10.06 ± 4.53 |
| Hemiptera | 8.86 ± 3.02 | 7.51 ± 2.36 | 8.38 ± 2.88 | 6.14 ± 1.95 |
| Diptera | 4.86 ± 1.20 | 5.21 ± 0.93 | 5.73 ± 1.29 | 4.59 ± 1.10 |
| Araneae | 3.92 ± 0.70 | 3.32 ± 0.73 | 4.54 ± 1.11 | 3.73 ± 1.24 |
| Homoptera | 2.68 ± 0.89 | 2.54 ± 0.57 | 2.02 ± 0.49 | 1.46 ± 0.46 |
- Taxa not analysed (due to low numbers caught) were Formicidae, Acarina, Lepidoptera, Psocoptera and Odonata. Key for column headings as in Table 4. Values are means (±SE) of the three replicates for each treatment.
Responses of centipedes and lizards
A total of 198 centipedes were collected, with no difference in overall mean number observed between fertilized (0.87 ± 1.05) and unfertilized (0.95 ± 1.31) treatments or between predator (0.86 ± 1.12) and predator-removal treatments (0.96 ± 1.25; Fig. 6a). A total of 65 lizards (and one blind snake) were collected, representing five species from three families (Table 7). Morethia adelaidensis and Menetia greyii constituted 41 and 43% of the reptiles caught, respectively. Reptiles were most common in spring and summer and were not captured during winter. No difference in mean number of reptiles was observed between fertilized (0.24 ± 0.24) and unfertilized (0.21 ± 0.21) treatments or between predator (0.25 ± 0.25) and predator-removal treatments (0.2 ± 0.2; Fig. 6b). No differences in mean numbers of centipedes or lizards were observed between the four treatments.
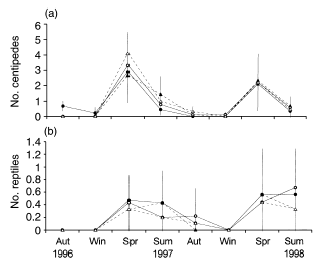
Mean numbers (± SE) of (a) centipedes and (b) reptiles in each treatment during the experiment. (●), Fertilizer added; (▴), fertilizer added, predators removed; (), control; (▵), predators removed. Aut, autumn; Win, winter; Spr, spring; Sum, summer.
| Species | Common name | Family | Head–body length (cm) | No. caught |
|---|---|---|---|---|
| Morethia adelaidensis | Saltbush Morethia Skink | Scincidae | 6.0 | 27 |
| Menetia greyii | Common Dwarf Skink | Scincidae | 3.8 | 22 |
| Tympanocryptis lineata | Lined Earless Dragon | Agamidae | 6.8 | 11 |
| Ctenotus leonhardii | Leonhardi's Ctenotus | Scincidae | 7.8 | 5 |
| Ramphotyphlops australis | Southern Blind Snake | Typhlopidae | 46 | 1 |
- Nomenclature follows Ehmann (1992).
Discussion
The overall objective of this study was to illuminate the broader types of trophic interactions that may be observed in a more natural terrestrial arthropod community and the effects of biotic and abiotic heterogeneity on the relative strength of top-down and bottom-up forces. The lack of specific information on biology precluded a comprehensive analysis at species level. However, I wanted to sample as inclusive an assemblage as was feasible rather than limit the investigation to a subset of species. I therefore used a taxon-based approach by classifying all species to ordinal level, and focused my attention on those ‘orders’ with clear trophic positions. I recognized the possibility that not all species within an order belong to their designated trophic guild, but such ordinal level analyses should nevertheless provide a good indicator of community structure.
Effectiveness of treatments
Predator removals
Spider removals were most effective in the month following a removal event and least effective in the month immediately prior to each removal event. Because spider numbers were reduced after each removal, this pattern resulted from the cumulative immigration of spiders since last removal. This trend suggests that numbers may have also been lower in removal treatments for at least some months between the removal events of 1996. Juvenile spiders constituted the majority of spiders in the November 1996 removal, which resulted from ineffective removal of female spiders with egg sacs in August 1996. Within spider populations, hatching of spiderlings can result in high cannibalism and intraspecific competition, leading to self-regulation and subsequent stability of the population size (Riechert & Lockley 1984). In removal plots towards November 1996, increased spiderling survival due to previous reductions in adult spider numbers may have led to population self-regulation, at which time numbers in removals mirrored that of the unaltered predator treatment. Therefore top-down spider manipulation may have remained more effective at this time than indicated by total spider abundance, as spiderlings were likely to exert a weak top-down effect, acting more as prey to other arthropods in the system, rather than predators on them.
Ant nest removals successfully reduced numbers of I. lividus and Rhytidoponera sp. nests but had no influence on numbers of I. lividus foragers. Despite meat ants having fast nest turnover rates, often less than 3 months (Lobry de Bruyn & Conacher 1994), minimal nest rebuilding on the refilled loose soil was observed. The majority of I. lividus individuals in removal plots were more likely to be a result of immigration of foraging individuals from outside colonies, with meat ants known to expand into vacant territories when neighbouring colonies are lost (Greenslade 1975). The lack of nest reconstruction by Rhytidoponera sp. probably resulted from the considerable investment in nest construction, and resultant slow nest turnover rate, as noted for other larger Rhytidoponera spp. (e.g. Lobry de Bruyn & Conacher 1994). In contrast to I. lividus (and other meat ant species), Rhytidoponera sp. forages singly and over relatively short distances. Thus their minimal immigration from outside suggests, unlike I. lividus, that the majority of the home range of Rhytidoponera sp. may have been encompassed within the experimental plots.
Nutrient addition
The above-average winter rainfall of 1996 facilitated the breakdown of each fertilizer dose prior to the next application. The sporadic, below-average winter rainfall probably caused the lack of an effective increase in soil N and P under fertilization in 1997. Heavier rainfall events followed the soil nutrient census of September 1997, so a positive fertilizer effect may have occurred later that year. Others have documented similar results, with below-average rainfall insufficient to move added fertilizers down into the soil to make them available to plant roots, with resultant lack of plant growth (e.g. Gutierrez 1992).
Bottom-up effects
Producers
Many studies have quantified the overall positive effects of nutrients on the producer level as a whole (e.g. Siemann 1998). However, in the current experiment, nutrient addition in the first year increased soil nutrients, but not overall production. In contrast to bottom-up theory, nutrient addition did not result in any overall change in producer density, cover, biomass or diversity. However, there were temporally dependent positive effects of nutrients within components of the plant community.
Density of E. scherolaenoides and cover of Sida sp. increased in fertilized plots in the later half of 1997, when nutrient manipulations appeared ineffective. This may have resulted from the utilization of nutrients stored during 1996. In other studies of N and P additions over a single growing season, increased plant quality in the absence of plant growth has been found (e.g. Rice et al. 1994). Such nutrient accumulation has been documented in low nutrient status systems and is thought to be an essential buffer from variable nutrient supply. Any effects of nutrients in terms of growth are then only seen in the following growing season(s), provided conditions are suitable (Chapin et al. 1990; Lipson et al. 1996). Sufficient winter rainfall in 1996 may have mobilized the added nutrients and led to increased nutrient accumulation, and thus plant quality. Eriochiton scherolaenoides and Sida sp. may have then tolerated the drier conditions in 1997 and utilized previously acquired nutrients and the late rainfall to show a progressive increase in density/cover in fertilized plots from June to December 1997.
Arthropods
Past studies have predicted strong indirect positive bottom-up effects of fertilization on total arthropod abundance (Siemann 1998). In the present study, nutrient addition did not lead to any overall change in abundance of ground-dwelling or grass-layer arthropods, although within these groups some positive changes in abundance were detected. However, the observed changes at the producer level did not adequately explain these results.
Within ants, fertilizer addition led to an overall increased abundance of Monomorium, with the majority identified as belonging to the rothsteini group. Given that these are seed harvesters, I suggest that this may be a result of increased seed production under fertilization. Species of Monomorium are particularly efficient in exploiting and defending temporary abundances of resources resulting from vegetation growth after good rains, with these ants most likely to show increased abundances when local food resources increase (Greenslade & Greenslade 1984). This was reflected in their increased abundance in spring 1996 (following above-average winter rains) and summer 1997–1998 (following the late rains of 1997). Despite the ineffective fertilizer manipulation in 1997, seed production may have remained higher under fertilization if plants utilized their stored nutrients. Seed production probably occurred in both years as the plants display a ‘semiperennial’ life history, completing their life-cycle within a single season, as documented for other plants in semi-arid systems (Davidson & Morton 1981). The overall higher abundance of HCS ants under fertilization was a consequence of the increased abundance of the Monomorium rothsteini group, which constituted the majority of HCS. The other major HCS genera, Meranoplus and Melophorus, also showed a trend of increased abundance under fertilization, with both also including seed-harvesting species.
As Homoptera are plant-sucking insects, I suggest that the overall increased grass-layer homopteran abundances under fertilization are a result of increased host plant nutritional quality, if plants in this system stored excess nutrients. Such an effect has been well documented in other studies (e.g. Honek 1994). Similarly, herbivorous Thysanoptera collected in pitfalls showed increased abundance under fertilization in spring 1996 when Thysanoptera abundance peaked. Given that many are leaf- and pollen-feeders (Mound & Heming 1991), this may also reflect increased plant resource quality in 1996. It is possible that the effect of increased plant quality continued through 1997 but, given the drought, that Thysanoptera abundances remained too low for a response to increased plant quality to be detected (see Andrewartha & Birch 1954; Cepeda-Pizarro et al. 1996). Other studies have demonstrated some increased abundances of phloem- and seed-feeding insects in response to increased nitrogen content of plant tissues and seed production under fertilization, rather than in response to associated increases in plant growth (e.g. Strauss 1987).
In contrast to cascade theory, there were no positive effects of nutrient addition detected among the predator taxa. With respect to producer level changes, the positive responses of E. scherolaenoides and Sida sp. do not appear to have propagated up the food web to increase the abundance of any herbivorous or predator taxa significantly.
Top-down effects
Predator manipulations did not result in an overall change in abundance of ground-dwelling or grass-layer arthropods, or producers. Only one plant taxon showed the predicted top-down effects. Sida sp. cover was reduced in predator removal plots in August 1996 and December 1997. Other studies have documented similar variation in the strength of top-down forces on producers, suggesting that there may be a herbivore density threshold below which predators may be unable to exert strong top-down control on herbivore populations, and therefore have no impact on producers (Spiller & Schoener 1994; Carter & Rypstra 1995). Alternatively, temporal changes in the relative strength of top-down forces on producers may relate to changes in the abundance or activity of the predators themselves, with predators only exerting an impact on producers at times of high abundance and/or activity (Power 1992). However, there were no associated increases in abundance of any herbivorous taxa in August 1996 or December 1997, and the abundances of all manipulated species were also higher at other times when no top-down effect on Sida sp. was detected.
Interaction effects
Other studies have reported the importance of the interaction of top-down and bottom-up effects in determining community structure. Although most of these studies involve vertebrate herbivores (e.g. John & Turkington 1995), only under nutrient addition has lycosid removal lead to increased grass biomass through herbivore biomass reduction (Schmitz 1994). Similarly, Letourneau and Dyer (1998a) suggested that the negative effect of predatory beetles on producers was mitigated in sites with high soil fertility. In the present study, predators and nutrients interacted to determine the abundance of only one taxon. Melophorus abundance was lowest under fertilization with predators present, with this effect strongest in summer 1997, when Melophorus abundance was high. Melophorus includes species that are predators and scavengers as well as seed harvesters, so their reduced abundance may have resulted from interference and/or exploitation competition with Monomorium, whose abundance increased under fertilization. The higher abundances of Monomorium compared with Melophorus throughout the study are likely to have facilitated such an effect. The effect of increased abundance in removals suggests reduced predation or competition from the manipulated predators.
Intraguild and non-consumptive interactions
As predicted by early cascade theory, investigations of trophic cascades in simpler aquatic systems have demonstrated continual control by predators and/or nutrients over time, and showed that predator and nutrient effects are driven by direct consumptive interactions between adjacent trophic levels (e.g. Rosemond et al. 1993; Kuitek et al. 1998). Although the responses of taxa discussed so far at least partially conform to the former and show support for the latter, other results were suggestive of alternative, non-conforming interactions, which may have acted to dampen or complicate predicted top-down and bottom-up effects in this system.
The results of the present study suggest that effects are also propagated sideways, within the predator guild. Generalist predators can be involved in intraguild interactions such as predation, exploitation and interference competition, and predator-avoidance behaviour (e.g. Human & Gordon 1996; Moran et al. 1996). In the current system the main generalists include other Hymenoptera, Araneae and some Formicidae taxa, with intraguild interactions among these and the manipulated predators likely. Some of the results support this proposition. For example, if predatory and scavenger species are represented within Melophorus, there was the observed decrease in Melophorus abundance under predator removal (in fertilized treatments), discussed previously herein. The majority of Tetramorium were predatory and there was an overall increase in Tetramorium abundance under predator removal. There was also an overall increased abundance of SP ants under predator removal. For both Tetramorium and SP the predator effect was strongest at the time when abundances of these two taxa peaked. These results suggest the existence of intraguild interactions between these taxa and the manipulated predators. However, often more than one type of intraguild interaction will operate (e.g. Polis et al. 1998), and it can be difficult (and rare) to distinguish the mechanism(s) responsible for negative intraguild effects, requiring different manipulative experiments from the one performed here (Wise 1993).
However, such intraguild interactions can then have important consequences for the overall expression of top-down forces. In some cases, intraguild interactions can dampen cascading behaviour when removal of one predator group leads to an equivalent increase in another, so that prey arthropod taxa and/or producer populations remain unchanged (e.g. Holway 1998). This may explain the lack of responses of some herbivorous taxa to predator removal. In other cases, the effects of manipulated predators may cascade down to have indirect positive effects on herbivores through their negative effects (either direct or indirect) on other predators (Rosenheim et al. 1993; Letourneau & Dyer 1998a). For example, Hurd and Eisenburg (1984) demonstrated the positive indirect effect of predatory mantids on crickets through their direct negative effect on the spiders that consumed the crickets. Such effects represent non-consumptive interactions between adjacent trophic levels.
In the present study there were temporally dependent negative effects of predator removal on some herbivorous taxa. The abundance of grass-layer Hemiptera was lower in removal plots in summer 1997–1998 when hemipteran abundance peaked. This may have resulted from associated increases in the abundance of predatory taxa. For example, the overall increase in Tetramorium in removals was strongest in summer 1997–1998, with an eightfold increase in Tetramorium abundance in predator-removal compared with predator-present treatments. Smaller, non-significant increases in more than one predatory taxon in response to removals could also have occurred, which collectively acted on hemipteran abundance.
Orthopteran abundance in pitfalls was lower in removal plots in spring 1996 and summer 1996–1997 but greater in removal plots in spring 1997. These opposing predator effects may relate to differences in the strengths of intraguild interactions each year. In removal plots, abundances of other Hymenoptera and Araneae in pitfalls increased in spring 1996, which may have had a direct negative effect on orthopteran abundance. In contrast, under the drier spring of 1997, other Hymenoptera and Araneae abundances remained low. For Orthoptera the manipulated predators may then have had a direct negative effect on abundance. Although the changes in abundance of other Hymenoptera and Araneae in removal plots were not significant, their joint effects may still have been sufficiently strong to affect significantly orthopteran abundance.
In contrast to bottom-up theory, the results also suggest non-consumptive interactions between adjacent trophic levels in response to fertilizer addition. Nutrients had a negative effect on the abundance of grass-layer Thysanoptera during summer, particularly in 1996–1997 when thysanopteran abundance peaked. There were no recorded increases in any predatory taxon under fertilization that could then have reduced thysanopteran abundance. It seems more likely that fertilizer addition could have led to increased levels of plant secondary defence compounds in both years, as has been noted in other studies (Quinn & Walgenbach 1990; Roininen et al. 1996).
The bottom-up induced changes observed for some herbivorous taxa also did not influence the abundances of the target predators. For lycosids this contrasts with the results of previous studies, which have demonstrated negative (Kajak 1981) as well as the predicted positive (Dobel 1987 as described by Wise 1993) changes in abundance in response to nutrients. In the current system, abiotic factors and intraguild interactions may be more important regulators of the abundance of these predators than bottom-up factors. Intraguild interactions have played important roles in determining the abundance of other top predators (Polis & McCormick 1986; Floyd 1996), including lycosids (Hurd & Eisenburg 1990) and ants (Risch & Carroll 1982). For ants and lycosids, intra- and interspecific competition for food, nesting sites and territories, as well as cannibalism for lycosids, can have a significant impact on the abundance of different species (e.g. Andersen & Patel 1994), with wandering spiders and ants also known to prey on each other (Halaj et al. 1997).
Lack of producer responses
Larger quantities of limiting nutrients than I used may have been required to elicit detectable responses from plants in the present system. However, the quantity of fertilizer used was similar, if not greater, than that used in other fertilizer addition experiments conducted in low nutrient status systems in which responses were documented (e.g. Siemann 1998). In the absence of explanatory changes at the producer level, I propose that much of the bottom-up nutrient effects on herbivorous taxa resulted from increased plant quality under fertilization, although this was not assessed. It is also possible that fertilization had stronger effects on the fresh plant biomass than dry matter production (Kiehl et al. 1997). Top-down effects may have also indirectly cascaded down to producers in the form of changes in plant damage (Atlegrim 1989; Letourneau & Dyer 1998a), but such an effect would have gone undetected. Buffering of potential cascading effects of predators on producers through predator compensation may have also occurred. Alternatively, herbivorous arthropods that foraged on producers in this system may not constitute a large proportion of the diet of the manipulated predators, as has been noted elsewhere (Pacala & Roughgarden 1984).
Conditional cascades
Top-down and bottom-up effects were inconsistent for many of the taxa that showed significant effects, and I suggest that this periodicity is linked to variation in weather, specifically the timing and extent of rainfall. The high variability of annual rainfall and unpredictability of drought is of particular significance in Australian systems (Drake 1994), with rainfall a major climatic factor that often influences the size, behaviour, activity and continuity of terrestrial arthropod populations (Tigar & Osborne 1997).
In the present experiment, although annual rainfall in both years was above average, the abundances of many taxa peaked in the first spring/summer following above-average winter rainfall, but remained depressed in the second spring/summer after below-average winter rainfall. In turn, predator effects were strongest in the first year, with minimal to no predator effect in the second. Below a certain threshold prey density, predators may be unable to influence prey populations significantly. In some cases this threshold density may be influenced by rainfall (e.g. Spiller & Schoener 1995).
Other studies have documented no changes in overall plant parameters under nutrient addition conditions (e.g. Wang et al. 1996). In the current experiment the lack of fertilizer effects in 1997 may be attributable to low winter rainfall, as previously documented across a wide range of systems (Gutierrez 1992; Houle 1997; Kiehl et al. 1997). Alternatively, as for other more arid systems, rainfall (as opposed to nutrients) may be the major limiting factor for the current plant community, producing year-to-year variation in the strength of bottom-up forces through variable effects on plant productivity (e.g. Gutierrez & Vasquez 1996). In contrast to the first year, the below-average winter rainfall and subsequent late rains in 1997 appear to have led to a delayed growth response. Density and cover reached similar levels to 1996, but peaked in summer as opposed to winter/spring. In turn, there was a delayed increase in the abundances of some arthropod taxa from spring 1996 to summer 1997. For other taxa, abundances remained depressed for the rest of that year.
Overall, in this chenopod shrubland system, temporal variation in the abundances of the manipulated predators, other arthropod taxa and producers, and the effectiveness of added nutrients, all of which may be linked to inter- and intra-annual variation in weather conditions, appear to have had important consequences for the timing and strength of top-down and bottom-up control. Such biotic and abiotic heterogeneity makes continual control by predators and nutrients unlikely. For some taxa, variation in abiotic factors may be the major determinant of abundance, upon which top-down and/or bottom-up forces then act. For others, variability in abiotic factors alone, or in conjunction with other factors apart from the manipulated predators and nutrients, may determine abundance.
Conclusions
The current work provides some insight into the effects of predators and nutrients on different taxa within a diverse arthropod community, and the effects of biotic and abiotic heterogeneity on the strength of these interactions. Such a synthesis in a complex terrestrial system is rare. However, despite the associated complexities, such investigations are needed in order to provide a basis of comparison with investigations from simpler terrestrial and aquatic systems. Such comparisons then contribute toward the development of a general conceptual framework that aims to predict which kinds of organisms will play key roles in different types of ecosystems (Brown & Heske 1990).
Although top-down and bottom-up effects were detected, the results suggest that ‘trophic trickles’ (Strong 1992) as opposed to trophic cascades may be a more applicable metaphor to describe the impact of nutrients and predators in ecosystem structuring. True trophic cascades do not appear to be the primary determinant of community structure. Temporal variation is not a characteristic of true trophic cascades, and although many effects were detected, these represented relatively diffuse and often isolated interactions throughout the food web. The effect of nutrient addition did propagate up to increase abundances of some herbivorous taxa, but further positive effects at the predator level were not detected. There were negative predator effects on the abundances of some herbivorous taxa but no producer-level effects were detected.
However, the variety and nature of the effects of the nutrient and predator perturbations illustrated the complexity of the interactions within the arthropod food web. In agreement with Strong (1992), the higher diversity of this assemblage may have contributed to providing buffers against any strong, continual, cascading behaviour. Timing and extent of rainfall appeared to be an important determinant of the extent to which different components of the community were then affected by top-down and bottom-up factors. This created ‘conditional trophic trickles’ in this system as opposed to ‘trophic cascades’. These results support the view of Hunter and Price (1992) that incorporation of biotic and abiotic heterogeneity is essential in order to understand more comprehensively the role of top-down and bottom-up forces in community structuring. The nature of some of the results from the present study also contradict the predictions of cascade theory, and support the view that other interactions as well as the classic consumptive interactions should be considered when investigating the source and outcome of indirect trophic-level effects (Schmitz et al. 1997).
Acknowledgements
A. S. Gromadzki assisted in the field. C and R. Jaensch and the late D. Eberhardt and family provided access to their properties. C. M. Bull and D. A. Mackay provided guidance and advice throughout the study. A. N. Andersen and C. M. Bull provided constructive comments on the manuscript. I thank U. G and G. F. Dawes and A. S. Gromadzki for their contribution in many ways during this study. This research was supported by an Australian Postgraduate Award scholarship, The Flinders University of South Australia research budget, two Doreen McCarthy Bursary Awards to T. Dawes-Gromadzki from The Australian Federation of University Women (SA Inc.), an Ecological Society of Australia Travel Award and the Tropical Savannas CRC.




