Pond attributes influence competitive interactions between tadpoles and mosquito larvae
Abstract
Abstract Tadpoles and mosquito larvae often coexist in natural freshwater bodies. We studied competitive interactions between: (i) tadpoles of the striped marsh frog (Limnodynastes peronii) and larvae of the mosquito Culex quinquefasciatus; and (ii) tadpoles of the common eastern froglet (Crinia signifera) and larvae of the mosquito Aedes australis. These two sets of taxa occur in natural water bodies in the Sydney region. Laboratory trials revealed competition between mosquito larvae and tadpoles in both systems. For example, mosquitoes displayed reduced rates of survival, growth and development, and smaller size at metamorphosis, when they were raised with tadpoles. The intensity of competitive suppression was influenced by attributes such as pond size (and hence, larval density), the location of food (on the water surface vs the substrate), and the extent of opportunities for direct physical interactions between the two competing organisms. These effects differed between the two study systems, suggesting that the mechanisms of suppression also differed. Limnodynastes peronii tadpoles suppressed C. quinquefasciatus even when the two types of organisms were separated by a physical partition, suggesting that chemical or microbiological cues may be responsible. Pond attributes also affected the impact of C. signifera tadpoles on Aedes larvae, but (unlike the Limnodynastes–Culex system) these effects disappeared when densities were lowered or when the tadpoles and mosquito larvae were physically separated. Thus, direct physical interactions may suppress mosquitoes in the Crinia–Aedes system. Our results suggest that tadpoles suppress the viability of larval mosquitoes by multiple pathways.
Introduction
Ephemeral ponds are highly variable systems, changing constantly in volume, nutrient levels and in the number of species they support (Bronmark 1998). When ephemeral ponds fill, nutrient levels rise as the water dissolves decomposing plant and animal material left over from the last time the pool was filled (Wilbur 1997). Soon after the pond fills, organisms such as anurans and insect larvae arrive to take advantage of the opportunity to complete the free-living aquatic stage of their life cycles (Blaustein & Margalit 1995). At this stage, competition among the species present in the pond is likely to be low. However, as the pond ages, nutrients become locked up in the living biomass and the amount of water in the pond decreases (Alford 1999). Such changes in the attributes of the pool over time may increase the intensity of competitive interactions among species within the pond (Inger et al. 1986). Tadpoles and mosquito larvae are two examples of aquatic organisms that are influenced by such changes in ephemeral ponds over time (Day & Van Handle 1986; Cortwright & Nelson 1990; Pehek 1995).
Larval amphibians have been used in several studies to examine the role of pond attributes in modifying intraspecific and interphyletic interactions among organisms. For example, the outcome of competition between species can be modified by the position of food in the water column, because this differentially alters the ability of organisms to feed (Dickman 1968; Bronmark et al. 1991; Baker & Beebee 1997). Similarly, the size of the pond can influence interspecific interactions, as the rate of chance encounters and mechanical interactions increases in more confined spaces (Wilbur 1982; Morin 1983; Gascon & Travis 1992; Pearman 1993, 1995). The presence of refugia can influence competitive processes for the same reason (Morse 1980; Baker & Beebee 1997, 2000). However, the effects of pond attributes on competitive interactions will depend upon the mechanisms involved. For example, the presence of refuges will not alter competitive effects that result from chemical interference. Tadpoles separated from each other by physical partitions may thus still suppress each other's growth (Wong & Beebee 1994; Griffiths 1995) by producing algal (Petranka 1989) or yeast species (Steinwascher 1979) in their faeces. These substances can divert the other taxon from more nutritious food resources, effectively starving the competitors (Baker & Beebee 1997).
Although chemical interference between tadpoles has been extensively studied, the same process has not been examined in terms of interphyletic interactions. Although strong competitive interactions have been documented between tadpoles and mosquito larvae (Blaustein & Margalit 1994, 1995, 1996; Mokany 2001), most of these studies were conducted in similar-sized, replicated enclosures. Therefore, the effect of changes in the attributes of ephemeral ponds on the interaction between these taxa has not been determined. Here, we manipulate attributes of the water body (pond size, location of food, presence of refugia) and examine their influence on the extent of competitive interactions between tadpoles and mosquito larvae.
Methods
Study species
We studied two tadpole–mosquito systems from south-eastern Australia. These were the tadpole of the Striped Marsh Frog, Limnodynastes peronii, with the mosquito larvae Culex quinquefasciatus and the tadpole of the Common Eastern Froglet, Crinia signifera, with the mosquito larvae Aedes australis. We chose these two systems as the tadpoles are relatively common in the Sydney region and frequently co-occur with their respective mosquito larvae. These two systems allow for both comparison and contrast of general tadpole and mosquito interactions.
The Striped Marsh Frog, L. peronii (Myobatrachidae), is a medium-sized (65 mm) light brown frog that mainly breeds from August to March, laying 700–1000 eggs in a foam egg mass entangled in vegetation at the edge of freshwater creeks and backyard ponds (Barker et al. 1995; Robinson 1996; Cogger 2000). The duration of the tadpole stage varies from 3 months to 1 year, with tadpoles growing up to 65 mm in length (Barker et al. 1995).
The L. peronii tadpoles used in the present study were collected from a single egg mass in November 2000 from a backyard pond in the Sydney suburb of Ryde. We hatched this egg mass in the laboratory (in a 10-L plastic container) and transferred the tadpoles to experimental treatments at Gosner stage 25 (Gosner 1960).
The mosquito C. quinquefasciatus (Culicidae) has a wide global distribution, and inhabits all states of mainland Australia (Beehler et al. 1994). The adults of this species are generally active from November to March and although they mainly attack avian hosts, they can be a major pest to humans in some areas (Beehler et al. 1994). Culex quinquefasciatus is a known vector of Ross River Fever, Murray Valley Encephalitis and dog heartworm (Russell 1985). Blood from their hosts is used in the production of mosquito egg rafts, a process that takes approximately 7 days (Mpho et al. 2000). Culex quinquefasciatus generally take 14–21 days to develop from an egg to an adult mosquito (Reisen et al. 1991).
The C. quinquefasciatus mosquito larvae used in our study were collected as egg rafts from the rear of the Macleay Building at the University of Sydney, in November 2000. We hatched these egg rafts in the laboratory (in 1-L plastic containers) and transferred the mosquitoes to experimental treatments at the first instar stage.
Limnodynastes peronii and C. quinquefasciatus commonly co-occur at high densities in backyard ponds, creeks and streams of the Sydney region. Densities at such sites ranged from 3 to 21 tadpoles per litre for L. peronii and 4 to 54 per litre for C. quinquefasciatus mosquito larvae (Mokany 2001). Thus, the densities used in our laboratory studies (three tadpoles and 20 mosquito larvae per litre) were within the range seen in natural water bodies.
The Common Eastern Froglet, C. signifera (Myobatrachidae), is a small (35 mm) terrestrial frog, which breeds all year round with a peak in breeding activity during winter. Crinia signifera breeds in both permanent and temporary water bodies, where females lay clutches of 70–270 eggs in batches of 1–30, usually attached to emergent vegetation (Lemckert & Shine 1993). The tadpoles of this species are small (≤20 mm in length), and develop for 1 to 3 months prior to metamorphosis (Williamson & Bull 1996).
The C. signifera tadpoles used in our study were collected in August 2000, as tadpoles at Gosner stage 25 (Gosner 1960) from rock pools at the Sydney suburb of Cronulla. We held the tadpoles (in 10-L containers) in the laboratory for 2 days to acclimate them prior to placement into experiments.
Aedes australis (Culicidae) is a common mosquito that rarely bites humans (except in Tasmania) and is capable of producing eggs without blood feeding (autogeny; Brust 1997). This species breeds year-round, depositing its eggs above the high tide mark on coastal rock platforms. The larvae of A. australis commonly feed at the base of the water column, and are able to remain there for extended periods of time as they possess both gills and an air breathing tube (Merritt et al. 1992). The larval cycle of A. australis extends from 16 to 30 days (Russell 1993).
We collected the A. australis larvae used in our study in August 2000, from rock pools at Cronulla, at the first instar stage. The larvae were transported to the laboratory where they were housed (in 1-L containers) for 1 day to acclimate prior to use in the experimental treatments.
Crinia signifera and A. australis commonly co-occur at high densities in shallow brackish pools on sandstone rock platforms in the Sydney region. Densities ranged from 2 to 59 tadpoles per litre for C. signifera and 2 to 206 per litre for A. australis mosquito larvae (Mokany 2001). The densities used in our experiments (three tadpoles and 20 mosquito larvae per litre) were thus within the range recorded in natural water bodies where these taxa occur in sympatry.
Four experiments were conducted indoors in the laboratory (22°C, 8L:16D cycle) from August 2000 to March 2001, using polyethylene 1.5-L (10 cm × 10 cm × 15 cm) containers as artificial ponds. These containers were within the size range of ponds that naturally contain both tadpoles and mosquitoes in the Sydney region (Mokany 2001). To each tub we added 1 L aged tap water (Aqua-pet® water ager), 100 g of aquaria gravel and floating fish pellets. We assigned three treatments to these ponds, in a Latin square formation. These treatments were:
- 1
Three early stage tadpoles (Gosner stage 25: Gosner 1960).
- 2
Twenty first-instar mosquito larvae within 1 day of hatching from the egg.
- 3
Three early stage tadpoles (Gosner stage 25) plus 20 first-instar mosquito larvae (within 1 day of hatching).
There were three replicates of each treatment and each treatment was fed three floating fish pellets daily.
We weighed and measured the tadpoles prior to experimentation, by transferring tadpoles via a sieve into a Petri dish full of water, onto the pan of a digital balance. We measured the length (snout to tail-tip) and maximum width of each tadpole with digital calipers (± 0.01 mm). We checked the containers daily for mosquito pupae, which were then transferred to a separate container to metamorphose. The removal of pupae from our experimental containers should not influence the interaction between tadpoles and mosquito larvae as the pupae do not feed or excrete wastes. We froze the metamorphosed adult mosquitoes to enable later measurement of wing sizes. We terminated each experiment after 25 days, by which time most mosquito larvae had died or pupated. We then re-weighed the tadpoles and recorded their final length and width. These measurements were used to calculate the mean percentage changes in tadpole mass, length and width since the beginning of the experiment.
At the end of experimentation, we calculated the percentage of mosquitoes that had pupated, the duration of their larval periods (day to pupation from the beginning of experimentation), their percentage survival and their wing sizes (separately for male and female mosquitoes). We measured wing size from the alular notch to the tip of the wing margin excluding wing scales. The sex of the mosquitoes was determined by examining the mouthparts and antennae.
We then performed an additional three experiments, using the same basic protocol as above (henceforth, ‘basic competition’ experiment):
- 1
The ‘sinking food’ experiment used food that sank to the bottom of the container instead of food that floated. We made the sinking food by soaking the floating fish pellets in water for 24 h, so they sank directly to the bottom of the container. This was designed to simulate circumstances in a natural pond when food was available on the substrate rather than the water surface.
- 2
The ‘container size’ experiment was identical to the ‘basic competition’ experiment except that each treatment was conducted in a 10-L container instead of a 1.5-L container. This was designed to simulate circumstances in a large natural pond with lower densities of competing larvae.
- 3
In the ‘partition’ experiment, each 1.5-L container was divided vertically down the middle by a partition of nylon mesh. There were three treatments for the partition experiment: (i) three tadpoles on one side of the partition and only water on the other; (ii) 20 mosquito larvae on one side of the partition and only water on the other; and (iii) three tadpoles on one side of the partition and 20 mosquito larvae on the other. This was designed to simulate circumstances in a natural pond with abundant refugia, where tadpoles and mosquito larvae rarely or never come into physical contact.
Statistical analysis
All percentage data were transformed by arcsine. We analysed the combined data from the four experiments using the program Superanova 1.1 (Abacus Concepts 1989). To reduce the number of separate tests involved (and thus minimize the problem of artifactually ‘significant’ results; Cabin & Mitchell 2000), we used multivariate anova on combinations of functionally linked traits. Thus, the data on tadpoles included percentage changes in mass, length and width as three indices of overall growth, so our statistical interpretations are based on manova that include all three of these variables in a single analysis. For the mosquito data, we conducted two manova analyses for each species: one on duration to metamorphosis plus male and female wing sizes (because these traits are plausibly linked) and one on proportional survival plus proportional pupation rates.
We also examined results separately within each experiment, because in most cases the manova identified significant interaction terms and hence, we could not interpret main effects without further subdivision of the data. Thus, we used one-factor anova to analyse the data on tadpoles (change in size over the course of the experiment) and mosquitoes (percentage pupation and percentage survival). We used two-factor nested anova to analyse our data for day to pupation and wing size data for the mosquito larvae. These analyses tested the effects of experimental treatments against the error term for the nested factor (replicate containers within treatments) rather than against the residual error term (Snedecor & Cochran 1980).
We used Bonferroni corrections cautiously in these latter (univariate) analyses, applying correction factors only when two or more tests addressed a common hypothesis (Snedecor & Cochran 1980). For example, we evaluated the effect of mosquito larvae on tadpoles by recording the mass, width and length of the tadpoles. As we used three measurements to test the same hypothesis, we applied a conservative alpha of 0.017 as the critical level (a Bonferroni adjustment of 0.05/3) for each test. Some of the results for C. quinquefasciatus in the ‘food position’ experiment (percentage pupation, day to pupation and wing size) and ‘container size’ experiment (percentage survival and percentage pupation) violated the assumptions of homogeneity of variance and normal distribution (Cochran & Cox 1957). No transformations that we attempted were able to resolve the problem, so alpha was reduced to 0.01 for the C. quinquefasciatus‘food position’ and ‘container size’ data to minimize Type I error (Underwood 1997).
Results
Limnodynastes peronii and Culex quinquefasciatus
Effects on tadpoles
The growth rates of tadpoles raised with mosquito larvae were lower than were those of tadpoles raised without mosquito larvae (Fig. 1; Wilk's Lambda from manova, F3,62 = 2.88, P = 0.043). Pond attributes also affected growth rates of tadpoles (F9,151 = 13.82, P < 0.0001; Tukey–Kramer posthoc tests show that growth rates were higher in the basic experimental set-up than in larger containers, or in the partition experiment). The interaction between pond attributes and the presence of mosquito larvae did not significantly affect the growth rates of tadpoles (F9,151 = 1.16, P = 0.33). Univariate analyses did not detect any significant difference between treatments with and without mosquito larvae for mean change in body sizes (for basic competition experiment: mass F1,4 = 1.145, P = 0.34, width F1,4 = 0.211, P = 0.67, length F1,4 = 0.29, P = 0.62; for sinking food experiment: mass F1,4 = 3.35, P = 0.14, width F1,4 = 21.59, P < 0.01, length F1,4 = 0.80, P = 0.42; for container size experiment: mass F1,4 = 3.67, P = 0.13, width F1,4 = 0.79, P = 0.79, length F1,4 = 3.89, P = 0.12; for partition experiment: mass F1,4 = 1.52, P = 0.28, width F1,4 = 0.57, P = 0.49, length F1,4 = 0.07, P = 0.81; see Fig. 1).
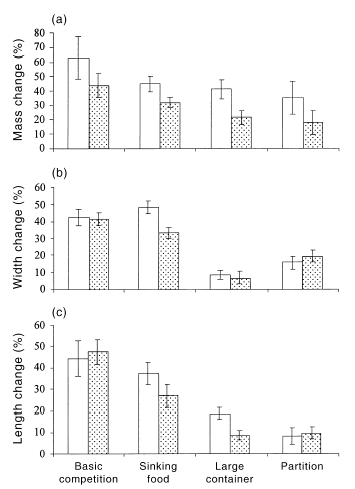
Effects of the presence of mosquito larvae on growth rates of tadpoles. The graphs show mean values (± SE) for percentage growth measured for (□) Limnodynastes peronii tadpoles raised without mosquito larvae and () L. peronii tadpoles raised with Culex quinquefasciatus mosquito larvae. The histograms represent: (a) percentage mass change; (b) percentage width change; and (c) percentage length change. The x-axis represents the various interaction experiments.
Effects on mosquitoes
All attributes of C. quinquefasciatus larvae that we measured were significantly affected by the presence of tadpoles, by attributes of the pond, and by the interaction between these two factors (for manova on rates of survival and pupation, interaction F6,30 = 11.50, P < 0.0001; for manova on date to pupation and wing sizes, interaction F6,114 = 6.93, P < 0.0001). In univariate analyses on the basic competition experiment, L. peronii tadpoles did not affect the mosquitos' developmental rates (day to pupation F1,5 = 0.49, P = 0.51), but the larvae raised with tadpoles exhibited significant reductions in percentage pupation (F1,5 = 34.97, P < 0.01), female wing size (F1,5 = 12.48, P = 0.02), male wing size (F1,5 = 9.8, P = 0.02) and percentage survival (F1,5 = 55.12, P < 0.01; Fig. 2). In the sinking food experiment, C. quinquefasciatus larvae raised with L. peronii tadpoles had a significant decrease in percentage survival (F1,4 = 67.06, P < 0.01) and failed to pupate in the tadpole treatments (percentage pupation F1,4 = 34.19, P < 0.01). In the larger containers, C. quinquefasciatus larvae demonstrated no significant difference between the treatments for percentage survival (F1,4 = 4.00, P = 0.12), but larvae raised with tadpoles took longer to pupate (F1,4 = 14.33, P = 0.02) and had a lower percentage pupation (F1,4 = 451.61, P < 0.01), female wing size (F1,4 = 68.08, P < 0.01) and male wing size (F1,4 = 297.15, P < 0.01; Fig. 2). In the partition experiment, the presence of tadpoles did not affect C. quinquefasciatus day to pupation (F1,4 = 0.27, P = 0.63), male wing size (F1,4 = 2.16, P = 0.22) or percentage pupation (F1,4 = 1.55, P = 0.28). However, larvae raised with tadpoles displayed a significant decrease in female wing size (F1,4 = 8.74, P = 0.04) and percentage survival (F1,4 = 192.32, P < 0.01; Fig. 2).
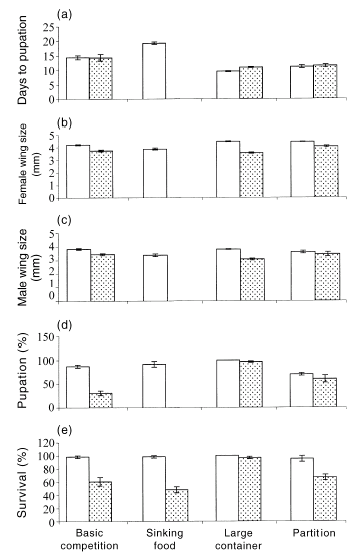
Effects of the presence of tadpoles on the traits of mosquitoes. The graphs display mean values (± SE) for variables measured for (□) Culex quinquefasciatus mosquito larvae raised without tadpoles and () C. quinquefasciatus mosquito larvae raised with Limnodynastes peronii tadpoles. The histograms represent: (a) day to pupation; (b) female wing size; (c) male wing size; (d) percentage pupation; and (e) percentage survival of the mosquitoes at the end of experimentation. The x-axis represents the various interaction experiments. The zero values shown for the food position experiment are due to the mosquitoes failing to pupate. Where error bars are not shown zero variance occurred between the replicates.
Crinia signifera and Aedes australis
Effects on tadpoles
Overall, the mean growth rates of tadpoles raised with mosquito larvae were similar to those of tadpoles raised without mosquito larvae (manovaF3,62 = 0.94, P = 0.43), but there was a significant interaction between pond attributes and the presence of mosquito larvae (F9,151 = 1.96, P < 0.05). Inspection of these data show that the presence of mosquito larvae did not suppress tadpole growth if the two organisms did not come into physical contact (partition experiment) or did so only rarely (in the larger ponds: Fig. 3). Univariate analyses on the basic competition experiment did not detect any effects of the presence of mosquito larvae in terms of growth rates, for mass (F1,4 = 2.8, P = 0.17), width (F1,4 = 1.55, P = 0.28) or length (F1,4 = 0.16, P = 0.71). The same was true for the sinking food experiment (mass F1,4 = 1.92, P = 0.24, width F1,4 = 2.39, P = 0.19, length F1,4 = 0.04, P = 0.85), and also in the larger pond (mass F1,4 = 1.34, P = 0.31, width F1,4 = 3.46, P = 0.14, length F1,4 = 0.13, P = 0.73), and in the partition experiment (mass F1,4 = 1.56, P = 0.28, width F1,4 = 1.82, P = 0.25, length F1,4 = 0.04, P = 0.84; Fig. 3).
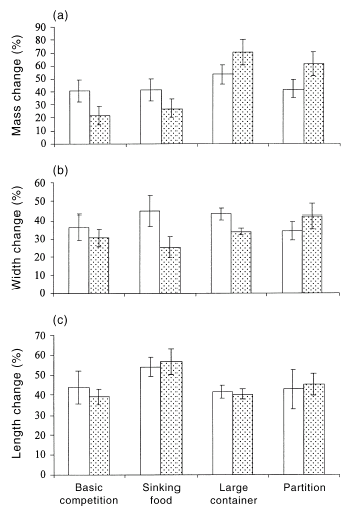
Effects of the presence of mosquito larvae on growth rates of tadpoles. The graphs show mean values (± SE) for percentage growth measured for (□) Crinia signifera tadpoles and () C. signifera tadpoles raised with Aedes australis mosquito larvae. The histograms represent: (a) percentage mass change; (b) percentage width change; and (c) percentage length change. The x-axis represents the various interaction experiments. There was no significant difference between any treatments.
Effects on mosquitoes
When raised with tadpoles, Aedes australis larvae demonstrated a marginally significant decrease in rates of survival and pupation (manovaF2,15 = 3.68, P = 0.05) with no significant effects of pond attributes (F6,30 = 1.17, P = 0.35) or any interaction between these factors (F6,30 = 0.81, P = 0.57). Effects were stronger for development rates and wing sizes, with a highly significant interaction between pond attributes and the presence of tadpoles in this respect (manovaF9,68 = 2.88, P < 0.007; Fig. 4). Univariate analyses on the basic competition experiment failed to detect significant effects of tadpoles on the mosquitoes' day to pupation (F1,4 = 2.33, P = 0.20), survival (F1,4 = 1.61, P = 0.23) or female wing size (F1,4 = 4.19, P = 0.11), but fewer mosquitoes pupated when they were raised with tadpoles (F1,4 = 12.10, P = 0.03) and the male mosquitoes that did pupate, did so with a reduced wing size (F1,4 = 7.56, P = 0.05). In the sinking food experiment, A. australis larvae demonstrated no significant difference among treatments for day to pupation (F1,4 = 7.24, P = 0.06), percentage survival (F1,4 = 0.27, P = 0.88) or percentage pupation (F1,4 = 0.39, P = 0.57). However, female wing size (F1,4 = 9.31, P = 0.04) and male wing size (F1,4 = 28.13, P < 0.01) were smaller in larvae raised with tadpoles compared with larvae raised with conspecifics only (Fig. 4). In a larger container, the phenotypic traits of mosquito larvae raised with tadpoles were similar to those of the mosquito larvae raised without tadpoles, for day to pupation (F1,4 = 0.01, P = 0.92), percentage pupation (F1,4 = 0.45, P = 0.54), percentage survival (F1,4 = 1.96, P = 0.23), female wing size (F1,1 = 4.32, P = 0.29) or male wing size (F1,3 = 3.83, P = 0.15). In the partition experiment, mosquito larvae raised with tadpoles were similar to larvae raised without tadpoles: for day to pupation (F1,4 = 0.03, P = 0.87), percentage pupation (F1,4 = 0.05, P = 0.84), percentage survival (F1,4 = 0.68, P = 0.46), female wing size (F1,4 = 0.73, P = 0.44), and male wing size (F1,4 = 0.60, P = 0.48; Fig. 4).
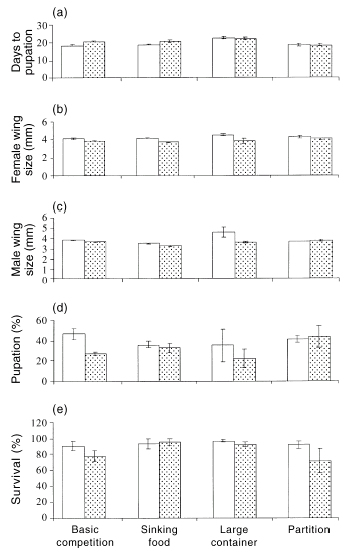
Effects of the presence of tadpoles on traits of mosquitoes. The graphs show the mean values (± SE) for variables measured for (□) Aedes australis mosquito larvae and () A. australis mosquito larvae raised with Crinia signifera tadpoles. The histograms represent: (a) day to pupation; (b) female wing size; (c) male wing size; (d) percentage pupation; and (e) percentage survival of the mosquitoes at the end of experimentation. The x-axis represents the various interaction experiments.
Discussion
In our experiments, growth rates of both tadpole species were reduced in the presence of mosquito larvae. However, this effect was independent of pond attributes in the case of one species (L. peronii); even separating the mosquitoes and tadpoles with a physical partition did not remove the suppression. In contrast, the extent to which mosquito larvae suppressed the growth of the other anuran (C. signifera) depended upon attributes of the pond. Suppression was minimal in larger ponds (where densities of organisms were lower) or when the competitors were separated by a partition (Fig. 3). The two mosquito species also differed in their vulnerability to competition with tadpoles. Neither pond attributes nor the presence of tadpoles affected survival or pupation success of A. australis, although both affected developmental rates and wing sizes at emergence. The other mosquito species, C. quinquefasciatus, proved to be extremely sensitive to all of the factors that we manipulated, including tadpole presence as well as container size, food location and the availability of refuges.
The effect of mosquito larvae on tadpoles in these experiments mirrors results from previous studies (Blaustein & Margalit 1994, 1996), but the magnitude of the effects was relatively weak and thus was not detected by most of our univariate analyses. These relatively weak effects may be due to the fact that the densities used in our study (three tadpoles and 20 mosquitoes per litre) were less than those used in the earlier work (five tadpoles and 50 mosquito larvae per litre; Blaustein & Margalit 1994, 1996). Our densities were chosen to mimic those that we measured in natural water bodies.
In contrast, mosquito larvae of both species were strongly affected by their interactions with tadpoles. The C. quinquefasciatus mosquitoes were most severely affected by tadpoles in the experiment where food was provided on the substrate rather than the water surface, as reflected by the reduction in percentage pupation and percentage survival. This effect may be due to the normally surface-dwelling mosquito larvae having to migrate to the base of the container to feed (Bern & Dahl 2000). This migration is energetically expensive and increases the potential rate of mechanical interactions between the mosquito larvae and the bottom-dwelling tadpoles. This finding is consistent with the idea that tadpoles may negatively affect other organisms by modifying the position of food within the water column (Bronmark et al. 1991).
The C. quinquefasciatus mosquito larvae were also affected by interactions with the tadpoles in our other experiments, as demonstrated by the reduction in wing size and in percentage pupation of the mosquitoes raised with tadpoles. The magnitude of the effect on the mosquitoes was similar in the experiments conducted in large (10-L) and small (1-L) containers, suggesting that C. quinquefasciatus is affected by the presence of L. peronii regardless of the size of the experimental container. Our results support previous conclusions that tadpoles can influence day to pupation and mosquito size at pupation (Blaustein & Margalit 1994), and that pond size affects tadpole growth (Pearman 1993, 1995).
Culex quinquefasciatus mosquito larvae were affected by the presence of tadpoles even when they were separated mechanically by a partition and fed abundant food. This result indicates that mosquito survival and female wing size are affected by chemical or micro–biological interference interactions with L. peronii tadpoles. A similar phenomenon has been reported from experiments investigating interactions between different tadpole species. When competing tadpole species were separated by a partition, they exhibited significant chemical interference induced by the presence of an algae, Prototheca richardsii (Biesterfeld et al. 1993; Baker & Beebee 1997, 2000) and a yeast, Candida humicola (Steinwascher 1979).
Like C. quinquefasciatus, the larvae of A. australis were affected by the presence of tadpoles (in their case, C. signifera). However, our experimental treatments affected the two mosquito species in different ways. Most importantly, tadpoles had no significant effect on A. australis in larger containers, or when the ‘competitors’ were separated by a partition (Fig. 4). This result suggests that when the mechanical interactions between A. australis and C. signifera are reduced (by increasing the container size, or by mechanically separating the species with a partition) the tadpoles no longer exert a negative impact on the mosquitoes. Thus, the interference interaction between A. australis and C. signifera is likely to be mechanical rather than chemical or microbiological. In turn, this may be due to the small size of the C. signifera tadpoles; such small animals may not be able to produce enough growth inhibitors to interfere with the mosquito larvae. The large size of this mosquito species and their bottom dwelling nature also may increase the potential for mechanical interactions between these species. Mechanical interactions between tadpoles and mosquito larvae have been reported by Blaustein and Margalit (1994, 1996).
The notion of significant mechanical interactions between these two species is further supported by the effect of the position of food. The adult wing size of Aedes australis was significantly reduced by interactions with C. signifera tadpoles when food was placed on the bottom of the container, compared with when floating food was used. This effect may reflect an increased rate of mechanical interactions between these species as a result of them both being present at the base of the container. This finding is consistent with the idea that tadpoles change the position of the food within the water column, making it unavailable to other aquatic species (Bronmark et al. 1991).
The trait most strongly affected by the interaction between mosquito larvae and tadpoles was the wing size of adult females. This is an important result, as the wing size of an adult female mosquito affects her longevity and ability to reproduce (Lyimo & Takken 1993). This in turn influences the size of the subsequent generation, and the ability to pass on mosquito-borne diseases (Lorenz et al. 1990). Thus, our results suggest that competition between tadpoles and mosquito larvae may have important consequences for the control of mosquitoes and mosquito-borne diseases. Given the magnitude of the effects, the results of our experiments indicate that it would be worth conducting additional studies to investigate further the interaction between tadpoles and mosquito larvae. In particular, possible mechanisms (such as chemical or mechanical interference) warrant attention; our work strongly suggests that multiple pathways may be involved in these competitive interactions.
Acknowledgements
We thank the Australian Research Council for funding this study.




