The effects of willow and eucalypt leaves on feeding preference and growth of some Australian aquatic macroinvertebrates
Abstract
The effect of leaf species (willow, Salix fragilis L., and white gum, Eucalyptus viminalis Labill.) and leaf state (senescent or green) on the feeding selectivity and growth rates of three species of macroinvertebrate Notalina sp. Mosely (Trichoptera: Leptoceridae), Koorrnonga sp. Campbell and Suter (Ephemeroptera: Leptophlebiidae) and Physastra gibbosa (Gould) (Mollusca: Planorbidae) were tested in the laboratory. All three species of macroinvertebrate selected green willow most strongly over the other leaf types (senescent willow, green eucalypt and senescent eucalypt). Growth rates of P. gibbosa and Notalina sp. were significantly greater on green willow than on the other leaf types. We were unable to measure the growth of Koorrnonga sp. Invertebrates had access to softer internal tissues of leaf material during preference trials, therefore we do not think that leaf structure was the main influence on selection between these materials. Green willow material may have been a better food source because of the noticeably thicker biofilm that it supported, and this material may also retain higher levels of nutrients than abscissed leaves. We speculate that willow leaves may provide a preferred source of food but will be available for less time than native eucalypt detritus.
INTRODUCTION
Allochthonous leaf litter is an important source of organic matter for invertebrates living in streams, and several Northern Hemisphere studies have shown that many invertebrate species have life histories closely linked to autumn leaf fall ( Bärlocher & Kendrick 1974; Cummins 1974; Petersen & Cummins 1974; McArthur et al. 1988 ). Temperate Australian streams differ from those in deciduous biomes in the Northern Hemisphere in that they occur in evergreen, eucalypt-dominated woodlands and forests that produce well-defined summer inputs of litter ( Lake 1982; Bunn et al. 1986 ; Swain et al. 1993 ; Campbell & Fuchshuber 1994). Several authors have speculated that the widespread introduction of willows into Australian temperate riparian zones could change the trophic processes and feeding ecology of Australian streams by altering the timing, quantity and quality of allochthonous detritus ( Barmuta et al. 1992 ; Walker et al. 1992 ; Campbell 1993; Walker 1993) but empirical evidence is scant.
Recently, Schulze & Walker (1997) reported only minor differences in the composition of littoral benthos between sites lined with willow (Salix babylonica) and river redgum (Eucalyptus camaldulensis) in the River Murray, South Australia. In New Zealand, Lester et al. (1994a) found lower densities of benthic macroinvertebrates in reaches lined by Salix fragilis, and felt that willows may influence stream invertebrates through altering food supplies (through a decrease in sunlight and an autumnal input of leaves) or habitat (by a reduced substrate particle size and reduced flow). By contrast, Glova & Sagar (1994) found a richer fauna in reaches lined by willows than in reaches with bare banks. Further research into how aquatic fauna uses and responds to introduced willow leaf litter is clearly warranted, and we chose to focus on the feeding preferences and growth rates of three numerically dominant detritivores from southeastern Tasmania.
Feeding preferences were assessed using multiple-choice feeding experiments to determine the probability that a particular leaf type would be selected ( Johnson 1980). Because these experiments used approximately equal quantities of each food type, we follow Johnson’s (1980) nomenclature in calling these ‘preference experiments’. We also examined growth rates of each species reared on pure diets of each type of detritus to determine whether any of the displayed preferences led to increased growth. We included both green and abcissed, senescent leaves in our experiments because large inputs of green leaves often result from frequent but unpredictable wind events and because there is some speculation that green material not only contains more nutrients but also more potentially toxic secondary compounds (e.g. Rowell-Rahier 1984).
METHODS
Three invertebrate species were selected for growth and preference experiments: Notalina sp. Mosely (Trichoptera: Leptoceridae), which is a large-particle detritivore with shredding mouthparts, the mayfly, Koorrnonga sp. Campbell and Suter (Ephemeroptera: Leptophlebiidae), which is a browser and has sweeping/ scraping mouthparts and the snail, Physastra gibbosa (Gould) (Mollusca: Planorbidae), which has scraping mouthparts and rasps material from the surface of the leaves. These species were selected because they were numerous and all were observed to eat terrestrially derived detritus but each differed in the way that they consumed leaves.
Preference experiments
We used green and abscissed senescent leaves from introduced willows (S. fragilis L.) and native white gum or manna gum (Eucalyptus viminalis Labill.), which are the two prominent riparian tree species near Hobart, Tasmania. Freshly abscissed, senescent leaves of both species were collected from the ground and green material was collected from randomly selected trees in late summer/early autumn. Because of substantial variation in the amount of water-soluble materials remaining in the leaves, all leaves were leached in water for 10 days prior to use to ensure that this did not influence the results of the feeding experiments. Leaves were then cut into 13-mm-diameter disks, using a cork borer, so that the mid-rib of the leaf was included in the centre of each disk. Disks were dried at 40°C to constant weight and one disk of each leaf type (green willow, senescent willow, green eucalypt and senescent eucalypt) was placed in each replicate container. There were 15 replicates (each containing one animal) for each invertebrate species and 15 controls with no invertebrates (to determine loss from decomposition). Trials lasted for 14 days and were conducted in a constant temperature room (12 ± 1°C). None of the choices offered was completely consumed within the trial period.
The null hypothesis of no preference was tested using Manly’s (1993) modified version of Yao’s (1965) approximate degrees of freedom test. The modified version of the Chesson–Manly selection index was used ( Chesson 1983; Manly 1995) to determine which leaf type was preferred:
where βi is the preference for food-type i;αj is the proportion of food-type j consumed; and the denominator represents the sum of all proportions of the K food-types consumed.
Because the leaf material can undergo autogenic change, the average proportion of food-type i consumed, is estimated by:
where ci— and fi— are the means of the natural logs of the amounts of weight lost in control and feeding trials, respectively.
If the null hypothesis is true, then βi = 1/K indicates that all food types were consumed equally; βi≥ 1/K indicates selection for food type i; and βi≤ 1/K indicates avoidance of that food type. Confidence intervals of βi were computed using the corrected t distribution recommended by Manly (1995).
Growth experiments
Air-dried leaves of all four leaf types were leached for 10 days before use in all growth experiments. Ten replicate containers (750 mL for P. gibbosa, 2 L for the other species) were set up for each food type and each invertebrate species and held in a constant temperature room (12 ± 1°C). Each container was supplied with five large leaves of either green eucalypt, green willow, senescent eucalypt or senescent willow; this was an ample quantity for all animals because all containers still had leaf material at the end of the growth experiments. Because P. gibbosa was found to survive easily on biofilms alone, an additional treatment was included with 10 containers with no leaves but provided with a pebble with a rich biofilm formed after incubation for 20 days in water containing leachates from all four species. Animals were maintained for 60 days and were measured at the beginning and end of the experiment. The shell height of P. gibbosa was measured to the nearest 0.02 mm with Vernier callipers. The width of the anterior end of the case of Notalina was measured to the nearest 0.01 mm using a dissecting microscope. This was a more reliable measure than case length because the posterior end of cases broke readily (cf. Towns 1991). We were unable to get sufficient nymphs of Koorrnonga sp. to survive long enough to obtain any valid growth rates. We suspect that the handling required to measure the initial lengths of these fast-moving and delicate individuals was too stressful, often resulting in the loss of gills or caudal filaments.
Analysis of variance ( ANOVA) was used to determine whether there was a significant effect of food type on growth, and Tukey’s honestly significant difference test was applied to determine which food treatments differed significantly from one another. The death of some individuals resulted in slightly uneven sample sizes but inspection of residuals showed normality and homogeneous variances. All ANOVA were carried out using SYSTAT (SPSS, Chicago, IL, USA) ( SPSS 1996).
RESULTS
All species of invertebrate preferred green willow over all of the other leaf types ( Fig. 1). The preferences of all three invertebrate species were highly significant: P. gibbosa, F(3, 15.5) = 6.73, P = 0.004; Notalina sp., F(3, 18.7) = 8.5, P = 0.001; and Koorrnonga sp. F(3, 14.9) = 8.07, P = 0.002. All three species appeared to avoid eucalyptus leaves and only Notalina sp. did not, to avoid senescent willow ( Fig. 1). During the experiment, all invertebrates were well able to disperse throughout the container and often spent some time with non-preferred leaf types; thus we are confident that the preferences shown are not due to any of the behavioural artefacts which can affect the interpretation of laboratory choice experiments ( Horton 1995).
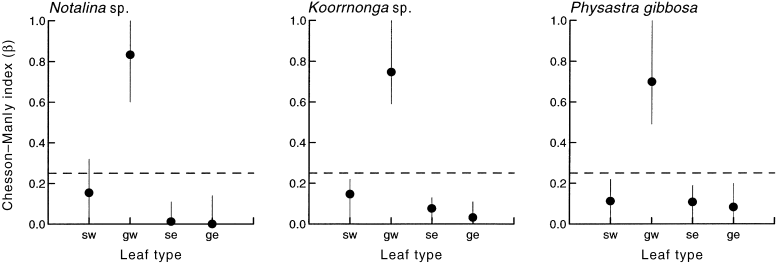
Feeding preferences of Koorrnonga sp., Notalina sp. and Physastra gibbosa indicated by the Chesson–Manly index, β (mean values ± 95% confidence intervals; n = 15) for the different leaf types. The 95% confidence intervals that include the value 0.25 indicate neither preference nor avoidance of that food type. sw, senescent willow; gw, green willow; se, senescent eucalypt; ge, green eucalypt.
Figure 2 shows the increase in shell height of P. gibbosa when fed different types of leaf litter and biofilm after 60 days. There was a significant difference in the growth rates of snails fed on different food types (F4,44 = 23.64, P < 0.001). Tukey’s test confirmed that snails fed on green willow showed significantly greater growth compared with those fed on the other food types (P < 0.05), but there was no significant difference in the growth rates of snails fed on senescent willow, senescent eucalypt, green eucalypt and the pebble with a biofilm (P > 0.05). Notalina sp. showed similar differences (F3,29 = 9.55, P < 0.001; Fig. 2). Tukey’s test showed significantly greater growth on green willow than on any other leaf type (P < 0.05).
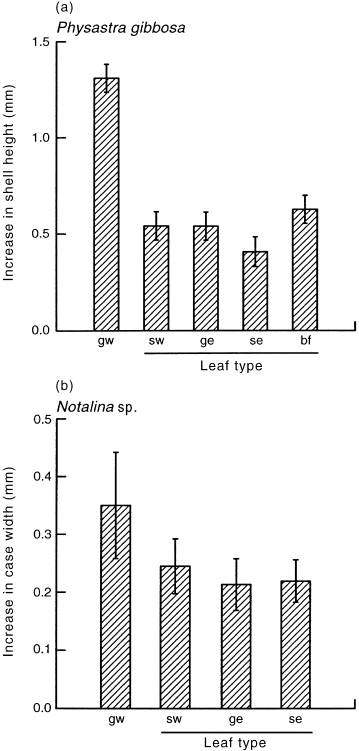
The mean (± SE) increase in shell height of (a) Physastra gibbosa and increase in case width of (b) Notalina sp. fed different food types for 60 days. The bold line shows those groups that are not significantly different at P = 0.05 using Tukey’s multiple comparison test. gw, green willow; sw, senescent willow; ge, green eucalypt; se, senescent eucalypt; bf, biofilm only.
DISCUSSION
All three species of invertebrate preferred green willow leaves, and two of the species, Notalina sp. and P. gibbosa, grew faster on green willow leaves than on any other food type. Whether Koorrnonga sp. would also grow fastest on its preferred food remains to be confirmed. Interestingly, growth rates did not differ among any of the other leaf types. This suggests that, while some of these leaf types were actively avoided, growth was still possible when they were the only food available. In the case of P. gibbosa, feeding on biofilm alone was also adequate to maintain growth at similar rates to those of the less preferred leaf types. The mechanisms for these preferences and differences in growth rates are unclear and two sets of hypotheses are commonly proposed: the physical structure of the different types of leaves and differences in their nutritional value, including the concentration of potentially deleterious secondary compounds.
Leaf structure may play an important role in determining preference and leaf toughness has been implicated as a deterrent to consumption of eucalypts by terrestrial herbivores (e.g. Ohmart et al. 1987 ). Hanlon (1981) noted that disruption of leaf surfaces by abrasion increased both the preference and the growth of the hydrobiid snail Potamopyrgus jenkinsi (= Potamopyrgus antipodarum, Ponder 1988) and noted that it preferred feeding on the abaxial surface of leaves where the cuticle and epidermis were thinner. Similarly we observed that P. gibbosa preferentially consumed material from the abaxial surface of willow, where it was often seen feeding underneath the cuticle. Koorrnonga sp. and Notalina sp. had less pronounced feeding orientations. Differences in leaf structure are unlikely to explain our results because we used leaf disks in our preference study which gave invertebrates easy access to the spongy and palisade mesophyll layers without having to break through the cuticle and epidermis. Even with this easy access, senescent willow and both green and senescent eucalypt were generally avoided.
The preference for green willow leaves is therefore more likely to be due to differences in nutritional conditions engendered by this leaf type. However, this preference is puzzling because, generally, we would expect green leaves to be avoided because they have higher levels of secondary compounds than senescent leaves. Green leaves of several species of willow have been shown to have high contents of phenolics ( Julkunen-Tiitto 1985) and the concentrations of substances such as condensed tannins have been shown to be negatively correlated with leaf consumption by detritivores ( Irons et al. 1988 ). Only a few studies have compared the consumption or processing of green and senescent leaves directly. Nolen & Pearson (1993), in tropical Queensland, found that Anisocentropus kirramus (Trichoptera: Calamoceratidae) actively selected between leaf types, consumed substantial quantities of green leaves of three of the five species examined but only processed green leaves for one species, Litsea leefeana (Lauraceae) more rapidly. Similarly, Otto (1974) noted that green leaves were generally fed on before senescent leaves in laboratory trials with a Swedish caddis shredder. Some field studies of litter breakdown also document faster processing of green leaves over abscissed material ( Stout et al. 1985 ), even though green leaves have higher initial concentrations of such substances as polyphenolics ( Campbell et al. 1992 ). Clearly, for some species, the nutritional benefits of consuming green leaves with their higher nutrient levels prior to abscission outweigh the costs of dealing with the higher concentrations of secondary compounds present in this material.
Most investigators have found that willow detritus becomes more attractive to invertebrates after it has been submerged for some time (review: Collier & Winterbourn 1986; Chergui & Pattee 1993; Lester et al. 1994b ). This may arise from increased microbial conditioning ( Collier & Winterbourn 1986) or from the removal of harmful secondary compounds that either affect invertebrate feeding directly ( Lester et al. 1994b ) or delay the microbial conditioning process ( Chergui & Pattee 1993). We did not quantify the microbial biofilm in our experiments but noticed that green willow material rapidly accumulated a much thicker slimy microbial biofilm than the other leaf types. However, we found that P. gibbosa fed exclusively on biofilm grew more slowly than when fed on green willow leaves. Either the leaf material supplies additional nutrition for this snail or the production of the biofilm may have been limited during the course of the growth experiment. Future research on the nutritional value of different forms of detritus should focus on the relative contributions of the leaf material itself and the biofilm that develops on it.
What are the implications of this research for the potential impacts of introduced willows on invertebrates in Australia? There is abundant evidence that, in the field, detritus from native and introduced willows can support high numbers of shredders and other invertebrates ( Pidgeon & Cairns 1981; Mutch & Davies 1984; Collier & Winterbourn 1986; Chauvet et al. 1993 ; Parkyn & Winterbourn 1997; Schulze & Walker 1997). So, although we found no deleterious effects of willow litter on invertebrate growth, we wish to amplify Parkyn & Winterbourn’s (1997) caution that these results do not justify the planting of willows to enhance the secondary productivity of Australian streams.
We only found increased growth rates on green willow leaves; abscissed willow leaves produced growth rates no better or worse than E. viminalis. Although there is a noticeable input of green leaves from S. fragilis in early spring, and unpredictably over summer after wind-storms in southeastern Tasmania (M.G. Read & L.A. Barmuta, personal communication, 1994), the majority of willow litter enters as abscissed material in autumn. Such material is processed more rapidly than E. viminalis ( Yeates 1994) and Pidgeon & Cairns (1981) found that S. babylonica leaves had virtually disappeared after 4 weeks and supported lower densities of invertebrates than native Eucalyptus blakelyi leaves at this time. These observations suggest that small streams that rely entirely on willows for their leaf inputs may run short of coarse particulate food matter during winter ( Pidgeon & Cairns 1981; Cummins et al. 1989 ).
Thus the net effect of this introduced species of tree on the structure and metabolism of Australian streams needs further, field-based investigation. Although Schulze & Walker (1997) found few differences between the invertebrate colonists of S. babylonica and E. camaldulensis detritus in sections of a large river in South Australia, Lester et al. (1994a) found that densities of invertebrates in small streams in southern New Zealand were depressed in stretches lined with S. fragilis which they speculated was due to the effects of willow root mats. The effects of riparian willows on benthos therefore ramify beyond the palatability of their leaves. Future descriptive and experimental research should therefore focus on the continuity of supply of detritus and the effects of habitat alterations wrought by the trees themselves. We suspect that these changes will be most marked in smaller streams where shading by the canopy and invasion of the stream bed by root mats is most marked.
Acknowledgements
This work was carried out under permit from the Inland Fisheries Commission of Tasmania and was supported by the School of Zoology, University of Tasmania, Honours Programme and resources made available by the Land and Water Resources Research and Development Corporation to L.A.B. and M.G. Read. We are grateful to Professor B. F. J. Manly (University of Otago) for making available drafts of his manuscript and software for computing the selectivity index and its confidence interval. Belinda Robson, Martin Read and Mark Wapstra (School of Zoology, University of Tasmania), assisted in experimental work and commented on drafts of this manuscript.




