Seed germination in Chrysophyllum sp. nov., a large-seeded rainforest species in north Queensland: effects of seed size, litter depth and seed position
Abstract
Leaf litter affects seed germination in many ways and past studies have shown greater impacts on relatively small seeds, both within and among species. In this shade-house experiment I examined the impact of forest litter on seed germination in Chrysophyllum sp. nov. (Sapotaceae), a large-seeded (2.4 g) rainforest tree from north Queensland. Seed mass varies more than 30-fold in this species, making it useful for studying the role of litter as a possible selective pressure in the evolution of seed size in large-seeded species. Seeds of varying size (small, medium, large) were sown in planting boxes containing one of three litter levels (low, medium, high) and placed either below or on top of the litter. Seed size and litter biomass had no significant impact on the number of germinating seeds or the time to germination but seeds placed below the litter germinated around twice as frequently, and 20% sooner, than seeds placed on top of the litter. There were no significant interactions between any of the three factors. This shade-house experiment suggests that leaf litter is not an important selective pressure in the evolution of seed size in this species. However, if litter disturbance under field conditions differentially affects the probability of seed germination in relation to seed size and/or litter biomass, then litter could still act as a selective pressure in the evolution of seed size in Chrysophyllum and other large-seeded species.
INTRODUCTION
Leaf litter affects seed germination in several ways. It creates a physical barrier through which shoots and roots must penetrate, alters the light, temperature and moisture regimes around the germinating seed, and may also be a source of allelopathic leachates ( Rice 1979; Sydes & Grime 1981a, 1981b; Vázquez-Yanes et al. 1990 ; Facelli & Pickett 1991). In addition, the cover provided by litter can protect seeds from discovery by vertebrate seed predators ( Myster & Pickett 1993; Cintra 1997).
Litter is well known to have widely varying impacts on the germination of different species within a community, and several studies have examined its role in determining contemporary patterns of relative species abundances (e.g. Beatty & Sholes 1988; Carson & Peterson 1990; Guzmán-Grajales & Walker 1991; Molofsky & Augspurger 1992). Some attention has been focussed on interspecific variation in seed size as a mechanism underlying these patterns (e.g. Keever 1973; Tao et al. 1987 ; Molofsky & Augspurger 1992; Myster 1994), but there are few studies examining the way in which litter affects germination across a range of seed sizes within a species ( Gross 1984; Winn 1985; Tripathi & Khan 1990). Seed mass often varies over an order of magnitude within a species ( Michaels et al. 1988 ), so that by differentially affecting germination success across a range of seed masses, litter may act as a selective pressure in the evolution of seed size within species (cf. Winn 1985).
In this greenhouse experiment I consider how the probability of seedling establishment in Chrysopyllum sp. nov., a large-seeded rainforest tree from north Queensland, varies with both seed size and litter quantity. The mass of individual seeds varies more than 30-fold in this species, making it ideal for studying the possible role of litter as a selective pressure in the evolution of seed size. I followed other workers (e.g. Molofsky & Augspurger 1992; Vázquez-Yanes & Orozco-Segovia 1992; Myster 1994) in placing seeds on the soil surface beneath varying quantities of natural litter, but in doing so only the relative ability of the shoot to penetrate upwards through the litter is considered. In reality, however, most newly dispersed seeds probably fall on top of the litter and are then gradually buried, so that the probability of seedling establishment is determined by the ability both of the shoot and of the root to penetrate the litter. Accordingly, I placed seeds both on top of and below the litter. Three specific hypotheses were tested: (1) that the probability and timing of germination varies with seed size; (2) that the probability and timing of germination varies with litter biomass; and (3) that the probability and timing of germination varies with seed position.
MATERIALS AND METHODS
Chrysophyllum sp. nov. (Sapotaceae, = RFK/3144 in Hyland & Whiffen 1993) is a canopy or subcanopy rainforest tree occurring in north Queensland from Cooktown to the Atherton Tableland, most frequently in upland forest on soils derived from granite. Trees reach reproductive maturity at 10 cm d.b.h. and the species is notable for its habit of mast-flowering and fruiting at intervals of 3–11 years ( Connell & Green 1999). In mast years masses of small cream-coloured flowers are born in the leaf axils and along the branches in January, and the large fleshy fruits (up to 6 cm diameter) ripen deep purple to black over the following 10 months and disperse in October. They contain between one and three seeds and the mean dry seed mass is 2.4 g (range 0.2–6.9 g; n = 275). Germination is hypogeal (sensuNg 1978). Seeds for this experiment were collected from a long-term study site at Davies Creek (17°05′S, 145°34′E; see Connell et al. 1984 ) in October 1994, during an episode of mast-fruiting.
The replicated unit in this experiment was a polystyrene box (46 × 35 × 18 cm) filled to a depth of 10 cm with a standard greenhouse potting mix (washed river sand, peat moss, vermiculite and nutrients). Each box had one litter level (low, medium or high), three seed size levels (small, medium and large) and one seed position level (sitting on top of the litter or on the soil below the litter), a design which allowed testing not only for the main effects but also for any interactions between them. Litter categories were determined from a field survey of standing litter biomass at the Davies Creek site in October 1994. A total of 200 samples were collected at 1-m intervals along four haphazardly placed 49-m-long transects. At each sampling station, a knife-edged sampling tube with an inner diameter of 38 mm was used to gather all the litter from within an 11-cm2 area, an appropriate scale for germinating Chrysophyllum seeds. The samples were air-dried and weighed and three experimental litter biomasses were chosen based on these data: low = 0.34 g/11 cm2 or 50 g/box of air-dry litter; medium = 0.85 g/11 cm2 or 125 g/box of air-dry litter; and high = 1.87 g/11 cm2 or 275 g/box of air-dry litter. Mixed forest litter was collected from Davies Creek several days before the start of the experiment and air-dried prior to its placement in the polystyrene boxes. Litter depths in these treatments were approximately 0.5 cm, 4.0 cm and 7.0 cm, respectively. Seed size categories were based on a survey of wet seed mass in 316 seeds; small seeds were 2.0–7.0 g (mean 5.3 g), medium seeds were 7.1–12.0 g (mean 9.2 g), and large seeds were 12.1–21.0 g (mean 13.8 g).
Twenty-one seeds were sown in three rows of seven in each box, each row containing either large, medium or small seeds. Each litter/seed position combination was replicated five times, for a total of 30 boxes (six treatment combinations × five replicates). The boxes were placed randomly within a 4 × 10 grid inside a shade-house transmitting 2% full sunlight (equivalent to the light environment found at ground level in rainforest where this species occurs) and watered for 15 min, twice daily. Seedling germination was monitored every 4 days from 9 November 1994 to 20 January 1995, with a final check on 7 May 1995 (by which time 73% of all seeds in the experiment had germinated). ‘Germination’ was defined as the first appearance of the shoot emerging from between the cotyledons or above the litter. At no time during the experiment did seeds initially placed on top of the litter become buried.
Two variables were considered for analysis: the percentage of seeds germinating over the 6 month period from November 1994 to May 1995, and the time to germination. The data were analysed as a three-way analysis of variance (litter quantity × seed position × seed size), and residuals were plotted against their corresponding estimates and inspected for homogeneity of variances. Percentage data were arcsin transformed prior to analysis. Only those seeds which germinated before 20 January 1995 (and therefore whose date of germination was accurately known) were included in the analysis of differences in time to germination (61% of all seeds in the experiment and 83% of all seeds germinating to May 1995), and the means of up to seven seeds per size category per box were used as replicates.
To assess the impact of leaf litter per se on germination in Chrysophyllum, a zero-litter treatment was added to the experiment as a control. Five replicate sets of 21 seeds were placed uncovered on the soil surface in polystyrene boxes, and monitored as for the other treatments.
RESULTS
Percentage germination
Seed size and litter quantity had no significant effect on the number of germinating seeds ( Fig. 1; Table 1; P > 0.05). Seed position was the only significant factor in the three-way ANOVA (P < 0.001; Table 1) and explained 42.2% of total variation in the analysis; more seeds germinated when placed below the litter (95.9 ± 1.7% (mean ± SE), pooled across litter quantity and seed size treatments) than when sitting on top of the litter (50.5 ± 5.5%; Fig. 1). There were no significant interactions between any of the three factors ( Table 1).
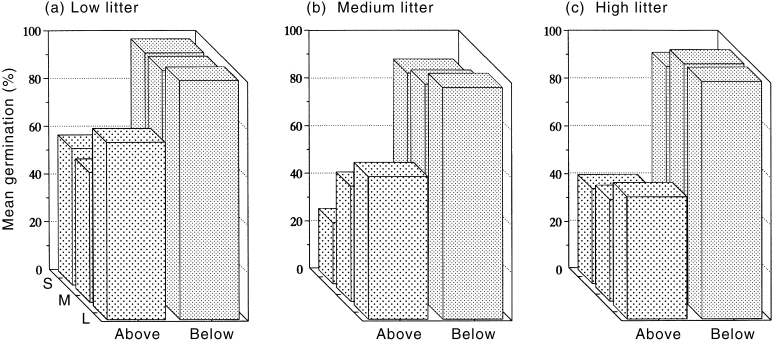
Mean per cent germination of small (S), medium (M) and large (L) Chrysophyllum sp. nov. seeds placed on top of and below (a) low litter, (b) medium litter and (c) high litter. Each bar is the mean of five replicates, each of seven seeds (see MATERIALS AND METHODS).
| Source of variation | d.f. | SS | MS | F | P |
|---|---|---|---|---|---|
| Seed size (S) | 2 | 0.60 | 0.30 | 1.70 | 0.190 |
| Litter quantity (L) | 2 | 0.52 | 0.26 | 1.47 | 0.236 |
| Seed position (P) | 1 | 10.58 | 10.58 | 61.73 | 0.000 |
| S × L | 4 | 0.11 | 0.03 | 0.16 | 0.957 |
| S × P | 2 | 0.10 | 0.05 | 0.30 | 0.743 |
| L × P | 2 | 0.32 | 0.16 | 0.89 | 0.413 |
| S × L × P | 4 | 0.17 | 0.04 | 0.24 | 0.917 |
| Error | 72 | 12.66 | 0.18 |
Time to germination
Seed size and litter quantity also had no significant effect on time to germination ( Fig. 2; Table 2; P > 0.05). Seed position was again the only significant factor in the three-way ANOVA (P < 0.001; Table 2) and explained 36.7% of total variation; seeds germinating below the litter did so sooner (39.8 ± 0.7 days, pooled across litter quantity and seed size treatments) than seeds germinating on top of the litter (50.8 ± 1.6 days; Fig. 2). There were no significant interactions between any of the three factors in the analysis ( Table 2).
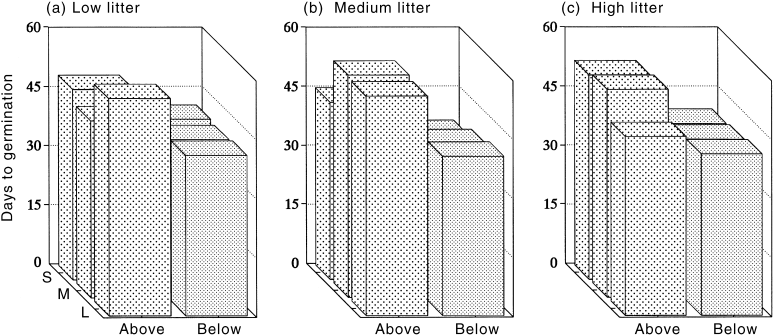
Mean time to germination of small (S), medium (M) and large (L) Chrysophyllum sp. nov. seeds placed on top of and below (a) low litter, (b) medium litter and (c) high litter. Each bar is the mean of five replicates, and each replicate is the mean of between one and seven seeds (see MATERIALS AND METHODS).
| Source of variation | d.f. | SS | MS | F | P |
|---|---|---|---|---|---|
| Seed Size (S) | 2 | 87.86 | 43.93 | 0.92 | 0.405 |
| Litter Quantity (L) | 2 | 4.55 | 2.28 | 0.05 | 0.954 |
| Seed Position (P) | 1 | 2090.67 | 2090.67 | 43.72 | 0.000 |
| S × L | 4 | 331.35 | 82.84 | 1.73 | 0.155 |
| S × P | 2 | 16.27 | 8.13 | 0.17 | 0.844 |
| L × P | 2 | 74.31 | 37.16 | 0.78 | 0.464 |
| S X L × P | 4 | 269.42 | 67.34 | 1.41 | 0.242 |
| Error | 59 | 2821.16 | 47.82 |
Litter per se had no effect on percentage germination; slightly more seeds germinated when placed below litter (95.9 ± 0.6%; n = 15 replicates polled across three litter biomass treatments) than when uncovered on bare soil (89.5 ± 1.7%; n = 5) but the difference was not significant (Mann–Whitney U = 27; P > 0.05). However, seeds germinated significantly faster when covered with litter (39.7 ± 1.0 days; n = 15) than on bare soil (44.2 ± 0.8 days; n = 5; Mann–Whitney U = 63; P < 0.05).
DISCUSSION
Three hypotheses were tested in this experiment: that the probability and timing of germination varies with seed size, litter biomass and seed position. The seed size hypothesis was rejected, which is at odds with many other intraspecific studies showing typically higher and/or more rapid germination in larger seeds (e.g. Griffin 1972; Ibikunle & Komolafe 1973; Ghosh et al. 1976 ; Howe & Richter 1982; Dunlop & Barnett 1983; Weller 1985; Tripathi & Khan 1990). The under- lying reason(s) for the lack of a seed size effect on the probability and speed of germination in Chrysophyllum is unknown but may be related to a lack of size- dependent variation in protein and carbohydrate concentrations, factors that have previously been linked to size-specific variation in germination ( Tripathi & Khan 1990).
The probability and timing of germination in Chrysophyllum was also independent of litter biomass. Other studies have demonstrated a lower probability of germination with increasing litter biomass but typically only for very small-seeded species (e.g. Hamrick & Lee 1987; Molofsky & Augspurger 1992; Peterson & Facelli 1992; Vázquez-Yanes & Orozco-Segovia 1992; Myster 1994). The probability of germination in species with very large seeds like Chrysophyllum may be independent of litter biomass simply because large seeds produce large and physically robust seedlings, capable of successfully germinating even in deep litter (e.g. Molofsky & Augspurger 1992; Myster 1994).
Seed position was the only factor which affected the probability and timing of seed germination – seeds under the litter germinated almost twice as frequently, and around 11 days sooner, than seeds placed on top of the litter. Leaf litter can physically impede seed germination in at least two ways: either by preventing the emergence of the shoot upwards through the litter or by preventing the downward penetration of the radicle into the soil. These results clearly demonstrate that only the latter is true for germinating Chrysophyllum seeds; more than 95% of seeds germinated with emergent shoots when placed below the litter but only half the seeds germinated when placed on top of the litter. Further, the lack of significant interactions between seed position and either litter biomass or seed size suggests that the probability of germination in Chrysophyllum is determined simply by the presence or absence of litter beneath the seed and is not a function of seed size or litter biomass. Two factors may have contributed to greater germination success of seeds placed below the litter. First, the shoots of Chrysophyllum seedlings may simply be stronger than their roots; second, leaf litter probably presents greatest impedance to structures trying to penetrate it in a downward (rather than upward) direction. At the end of the experiment I noted that the radicles of seeds placed below the litter were typically very straight, indicating they had penetrated the soil without obstruction. On the other hand, the radicles of seeds placed on top of the litter were typically looped and curved, indicating considerable obstruction before finally reaching the soil. Similar observations were made in the field. There seems to be a general consensus that very large-seeded species are little affected by leaf litter (e.g. Eriksson 1995), but the results from this experiment suggest this conclusion may be erroneous and be largely an artefact of experimental design. Past studies with large-seeded species have only sown seeds below the litter (e.g. Tripathi & Khan 1990; Molofsky & Augspurger 1992; Myster 1994), and the results of this experiment and observations like it (e.g. Koroleff 1954; Borchert et al. 1989 ) highlight the need for future studies to consider placing seeds both below and above the litter.
As in most species, the shoots of developing Chrysophyllum seedlings do not emerge until the radicle is already well formed and is anchoring the plant to its substrate. This probably explains some of the 11-day difference in mean germination time between seeds placed below and on top of the litter, in that seeds on top were slower to germinate simply because of the delay in the development of their radicles. Fowler (1986) also observed a delay in germination when Aristida grass seeds were sown on top of litter. However, in this case the delay was caused by the relatively slow movement of whole seeds downwards through the litter, not by the penetration of the radicle. It is also possible that seeds below the litter germinated sooner because the microclimate there fostered rapid development of the radicle and shoot. Data from the additional zero-litter treatment support this notion. Although litter did not increase the proportion of seeds germinating, the seeds did germinate around 5 days faster than seeds on bare soil. This result suggests that in some way leaf litter may promote germination in Chrysophyllum. This is at odds with many other studies, which have shown that leaf litter often inhibits germination through shifts in light wavelengths, allelopathic effects and the like (e.g. Vázquez-Yanes et al. 1990 ; review in Facelli & Pickett 1991).
These data suggest that the optimum litter microenvironment for germinating Chrysophyllum seeds is one where the seed is initially dispersed to bare soil and then quickly buried by litter, or where seed lands on top of the litter but is then rapidly brought into contact with the underlying soil through some localized disturbance. Both situations may frequently occur at the long-term study site at Davies Creek such that in mast years, a large proportion of Chrysophyllum seeds experience optimum conditions for germination. For example, bare ground accounted for 33% of the points sampled in the litter biomass survey. This survey was conducted within 2 weeks of the main period of fruit fall in 1994, suggesting that one-third of fruits fell into litter-free microsites. Further, Chowchillas (Orthonyx spaldingii, a ground-dwelling bird) are active local litter disturbers and elsewhere are estimated to turn over most of the litter in their home ranges at least once every month ( Jansen 1993). They often uncover bare soil (personal observation), and at Davies Creek, these localized disturbances (~15 cm diameter) are then quickly covered again through leaf fall and disturbance to neighbouring microsites ( T. C. Theimer & C. Gehring 1999). Even if local disturbances like this do not create completely bare patches, disturbance per se may still increase the probability of germination in Chrysophyllum seeds resting on top of litter under field conditions. In the complete absence of disturbance, litter in the shade-house experiment quickly became compacted and may have presented a greater degree of impedance to developing radicles than more disturbed (and therefore less compacted) litter might have presented under natural conditions. Furthermore, if litter disturbance differentially affects the probability of seed germination in relation to seed size and/or litter biomass, then the possibility exists that, despite the results of this experiment, litter could still act as a selective pressure in the evolution of seed size in Chrysophyllum and other large-seeded species. However, this idea needs to be tested experimentally under field conditions.
Acknowledgements
This work was funded through a National Science Foundation grant to Joseph H. Connell (DEB 92–20672).
I thank the Tropical Forest Research Centre, CSIRO, Atherton, for use of shade-house facilities, and Suzy Gerrard, Segun Osunkoya and Joe and Margaret Connell for assistance in monitoring the experiment. Joe Connell and Kitty Gehring read previous drafts of the manuscript.




