Video-assisted thoracic surgery: A renaissance in surgical therapy
Abstract
Within a few years, video-assisted thoracic surgery (VATS) has become the accepted or preferred approach over a wide range of thoracic procedures. The authors review the development of this technique, the basic operative strategies and the current surgical indications. Technical pitfalls and future developments are also discussed.
HISTORICAL PERSPECTIVE
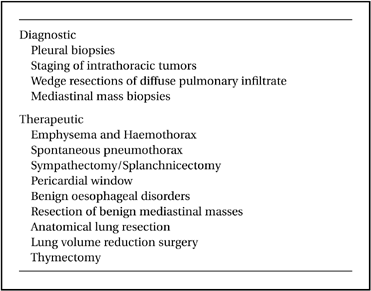
Thoracoscopy has been around for almost a century. Hans Christian Jacobaeus, a professor of internal medicine from Stockholm reported using a cystoscope to examine the thoracic cavity under local anaesthesia in 1910.1 He primarily used this technique to lyse adhesions in order to collapse the lungs in the treatment of tuberculosis (Fig. 1).2 The introduction of this approach was initially received with enthusiasm throughout Europe and America.3 However, in the 1940s, the advent of streptomycin led to a rapid decline in the use of thoracoscopy for tuberculosis. Thoracoscopy was used only sporadically as a diagnostic tool. In 1976, Lewis et al. reported direct diagnostic thoracoscopy on 40 patients using either a rigid bronchoscope or fibreoptic mediastinoscope under general anaesthesia with no mortality and minimal morbidity. The correct diagnosis was achieved in all patients.4 This stimulated a resurgence of interest in this neglected technique.
Showing the early use of thoracoscopy by HC Jacobaeus with the two cannula technique (Reproduction with kind permission of the Scoeity of Thoracic Surgeons).3
The improved Hopkins lens coupled with the development of solid state systems and microcameras in the 1980s led to the rapid development of video-endoscopic surgery. For the first time, the assistants were able to watch with the operator. The success of laparoscopic cholecystectomy gave impetus to the development of video-assisted thoracic surgery (VATS). Selective one-lung ventilation permits easy manoeuvrability of the telescope and instruments. The availability of specially designed endoscopic instruments, like the linear staple cutter, opened up new vistas for a spectrum of diagnostic and therapeutic thoracoscopic procedures.
BASIC PRINCIPLES AND OPERATIVE STRATEGIES
Conventional wisdom relates minimal access to limited exposure but with the advent of videoscopic surgery, this is no longer true. The thoracoscope attached to a videocamera unit provides a magnified view of the surgical field with a high resolution for details. The chest is the most suitable body cavity for the minimal access approach, not only because thoracotomy is a very painful incision, but also because once the lung is collapsed (with selective one lung ventilation), there is plenty of room for instrument manoeuvring.5 The use of carbon dioxide insufflation and hence valved ports, is unnecessary. Conventional thoracic instruments can be placed directly through small wounds into the chest. We prefer to use conventional instruments over the dedicated, disposable endoscopic ones whenever possible as they are familiar to the surgeons, easy to use and cost-effective.6 For some procedures, a utility thoracotomy is required for retrieval of the specimen. Therefore, VATS should be viewed as a spectrum with the purely endoscopic approach at one end and a video-assisted approach at the other end of a continuum.
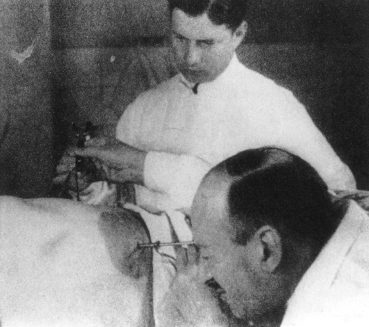
Although thoracoscopy could be performed under local anaesthesia, most VATS procedures are performed under general anaesthesia with selective one-lung ventilation. This is achieved using either a double lumen tube or a single lumen tube with a bronchial blocker. For most procedures, the patient is placed in the full lateral decubitus position with flexion of the operating table at the level of the nipples to open up the intercostal spaces on the operating side (Fig. 2).7 The patient is prepared and draped as for full thoracotomy.
Flexing the operating table opens up the intercostal spaces for instrumentation (Reproduction with kind permission of the Society of Endoscopic and Laparoscopic Surgeons of Asia).34
General exploratory thoracoscopy is usually performed by placing the telescope in the sixth intercostal space, mid-axillary line, unless otherwise directed by the location of the pathology. We recommend the ‘finger-clamp’ technique to enter the pleural cavity as in the insertion of a chest drain. The inferior placement of the telescope ensures a panoramic view of the chest. For simple procedures like pleural biopsy, an operating telescope (carrying an instrument channel) is recommended. For more complicated procedures, usually two additional instrument ports are required. Their positions would depend on the target pathology although some general principles should be followed: (i) instruments should be placed far apart from each other and from the telescope (triangulation strategy) to avoid ‘fencing’ during instrument manoeuvring; (ii) avoid paradoxical motion by positioning the telescope and instruments within the same 180° arc (i.e. approach the lesion from the same general direction).8 In situations where paradoxical motion is generated, it can be counteracted by rotating the camera 180°.
INDICATIONS FOR VIDEO-ASSISTED THORACIC SURGERY
A survey of North American thoracic surgeons showed VATS has become the preferred or accepted approach over a wide range of thoracic procedures.9 It is important to emphasize that VATS represents a new approach and not a new operation. Therefore, we adhere to the same basic surgical principles in VATS as we would for open thoracotomy.
Contraindications to VATS are relatively few. In addition to the general contraindications, like recent myocardial infarction and severe coagulopathy, specific contraindications include pleural symphysis and inability to tolerate selective one-lung ventilation. The former is relatively uncommon and moderate adhesions can usually be taken down using a combination of sharp and blunt dissection under videoscopic vision. Prior operation in the ipsilateral chest should not be regarded as a contraindication.10
Some of the common procedures performed by VATS are list in Table 1. As experience is gained, more and more operations are technically approachable by VATS. However, clinical judgment must be exercised so that we are not compromising the long-term benefit for the sake of the minimal access technique. It is important to point out that while there is now a wealth of literature on VATS, there are relatively few publications comparing a VATS procedure with its conventional (thoracotomy) counterpart in a randomized, prospective manner.11,12 Nonetheless, because of their low morbidity, good short- and long-term results, many VATS procedures are now well accepted as the approach of choice by the thoracic surgical community.9 Detailed discussion of each procedure is beyond the scope of this article and readers are referred to our specialized textbook on this subject.13 We present an overview of the subject in this review.
DIAGNOSTIC MODALITY
The use of VATS as a diagnostic modality is well established and this includes biopsy of the pleura, lung mass, diffuse lung infiltrate, mediastinal mass, pericardium and vertebral body.14 For simple procedures, the use of miniaturized instruments (‘needlescopic’ instruments of 2 mm or less in diameter) provides an attractive option which could further reduce postoperative discomfort.5 However, diminished illumination, reduced resolution and flexibility of the equipment render it difficult to control fine movements. The use of VATS in staging intrathoracic tumours requires some qualification. Video-assisted thoracic surgery is a useful adjunct to mediastinoscopy in the biopsy of several lymph node stations not accessible by the latter approach (like the aortopulmonary, para-oesophageal and inferior ligament nodes) for patients with primary lung cancer. However, mediastinoscopy should remain the principal diagnostic modality as VATS cannot pro-vide information on contralateral mediastinal node involvement (N3 disease or Stage IIIB). Nonetheless, VATS plays an important role in excluding patients with pleural metastasis from an unnecessary thoracotomy. It is now our routine to perform VATS exploration in all patients with intrathoracic malignancy, before a planned thoracotomy VATS exploration also provides information regarding chest wall invasion and leads to proper planning of the subsequent thoracotomy.15 Video-assisted thoracic surgery also plays a role in the secondary staging of lung cancer, for example in patients with Stage IIIA disease who have undergone a course of neoadjuvant chemotherapy and in whom repeat mediastinoscopy may be technically difficult and potentially hazardous.
THERAPEUTIC MODALITY
Pneumothorax
The tremendous success of VATS in the treatment of primary spontaneous pneumothorax has led to earlier referral by physicians and increased acceptance by patients for surgery.16 Stapled-resection of apical bullae followed by mechanical pleurodesis remains the most frequently used technique, although more cost-effective means of eliminating the bullae (like suturing or looping) have been developed.6 While primary spontaneous pneumothorax cases are easily approachable by VATS, treatment of secondary spontaneous pneumothorax (with established lung pathology like emphysema or pneumoconiosis) requires more clinical judgment.17 Patients with difficult adhesions to take down may be more suitable for thoracotomy, while those who are elderly with multiple co-morbidities may benefit more from a chemical pleurodesis (we prefer talc slurry) if the lung can be fully re-expanded.
Pleural effusions
For therapeutic procedures, the role of VATS in the drainage of loculated effusions (including empyema and haemothorax) and pleural debridement is well established.18 It is important to remember that if simple drainage is inadequate, VATS exploration should be recommended early before the empyema progresses from a fibrinopurulent phase to an organized fibrotic phase, resulting in restricted pulmonary function from encasement or fibrothorax. The use of VATS to guide the proper placement of a chest drain should not be under-rated.19
Sympathectomy and splanchnicectomy
Thoracodorsal sympathectomy is indicated for patients with palmar and axillary hyperhidrosis, and selected patients with reflex sympathetic dystrophy, and vasculopathies like Raynaud's or Buerger's disease.20 A segment of the sympathetic chain can be excised or ablated (for example with electrocautery) and the extent of intervention is dependent on the patient. For example, for palmar hyperhidrosis alone, only the T2 segment needs to be excised but if axillary hyperhidrosis is also a problem, the level of resection (or ablation) should be extended to include T4. Even with VATS, a variety of surgical techniques are available. We continue to favour full lateral decubitus positioning, general anaesthesia with selective one-lung ventilation using three ports. The first rib can be identified by noting the position of the subclavian artery (which crosses over the first rib to supply the upper extremities). The second intercostal vein usually crosses over the sympathetic chain just deep to the parietal pleura and this has to be cauterized or clipped. We prefer to divide the sympathetic trunk proximally just below the stellate ganglion. Some surgeons still prefer resecting part of the stellate ganglion (because in 10% of patients, sympathetic innervation to the upper extremity occur through this ganglion). However, because of the risk of Horner's syndrome, this has not been our practice.
The success rate of this procedure for hyperhidrosis is high and ranges 85–95% in large series from Taiwan.21,22 Compensatory truncal hyperhidrosis occurs in about 20% of our patients and is occasionally difficult to treat. This has to be fully explained to the patients before surgery.
Splanchnicectomy is indicated for patients with intractable visceral pain arising from the upper abdomen. The greater splanchnic nerve arises from T5 to T10 and lesser splanchnic nerve from T10 to T11 of the sympathetic chain. Similarly, the nerves can be excised or ablated. In over half the patients, a unilateral approach from the left is effective and should be tried first before resorting to a bilateral approach.23
Mediastinal cysts
Benign mediastinal cysts, like demoid cyst (Fig. 3), oesophageal duplication cyst, thymic cyst, and pericardial cyst (Fig. 4), are often surprisingly easy to resect using VATS. Decompression of the cyst with a needle may be necessary to facilitate mobilization. The phrenic nerve should be identified and carefully preserved. For oesophageal duplication cysts, a fibreoptic light source (from a bronchoscope) placed in the oesophagus can help identify this structure thoracoscopically during dissection. As a rule, for benign cysts, the tissue plane is fairly well preserved. In fact, any suspicion of tissue plane invasion by the tumour should call for conversion to a full thoracotomy for further dissection.24
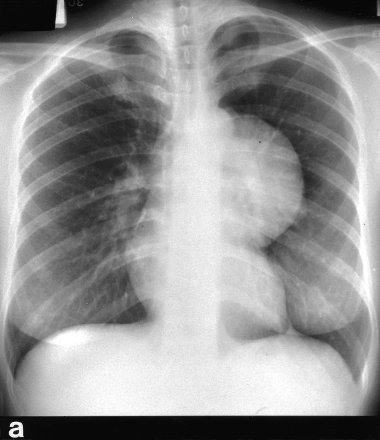
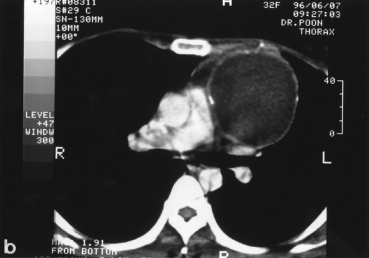
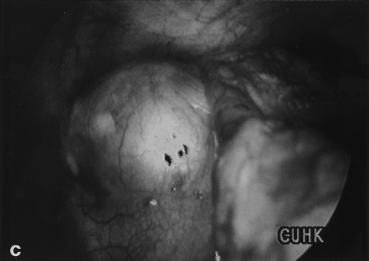

A young female presented with a large left sided mediastinal mass on CXR (a). CT scan shows calcified wall with cystic content (b). Thoracoscopic view of the cyst (c). Operative specimen of the large cyst wall (d).
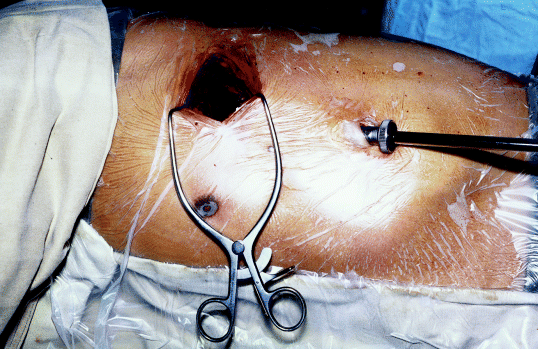
By opening up the intercostal spaces, a 4 cm ‘gap’ could be obtained between ribs. Only soft tissue retraction is usually necessary for the entire operation, including specimen retrieval (Reproduction with kind permission of the Society of Endoscopic and Laparoscopic Surgeons of Asia).34
Thymectomy
Thymectomy is an established therapy, in conjunction with medical treatment for generalized myasthenia gravis (MG). However, which technique to use continues to be a subject of controversy. Several surgical approaches are currently being used and these include transternal, transcervical, a combination of the two (for ‘maximal thymectomy’) and recently VATS.25 Our results, as well as the collective data of other centres have shown that there is little difference in symptomatic improvement to thymectomy regardless of which surgical approach was used.26 The complete remission rate following VATS seems to be slightly lower than the maximal thymectomy approach but we believe that was due to the relatively shorter term follow up of the VATS patients. The natural history of MG following thymectomy is that more patients will go into and stay in complete remission as time goes. Therefore, long-term follow up of these patients is important.26
Although the thymus could be approached from either side with VATS, we prefer the right-sided approach for several reasons: (i) The superior vena cava forms an easy landmark for dissection; (ii) the confluence of the two branchiocephalic veins which is a difficult area to dissect, can be easily approached from the right; and (iii) it is easier for a right-handed surgeon to dissect the thymus from below up from the right.27 Care has to be taken to completely dissect out the superior horns which can extend up into the neck. However, with firm, deliberate downward traction of the mobilized gland (after all the vascular branches and tributaries have been divided), the superior horns can be freed from its fascial attachment (a continuation of the pretracheal fascia). We have found a dental pledget mounted on a conventional curved clamp a very useful device for the blunt dissection of the thymus. Complete removal of the thymus, irrespective of the approach, is important for the success of this operation and therefore, we advocate that the VATS approach should only be performed by surgeons with considerable experience with this technique.
Video-assisted thymectomy definitely has several distinct advantages over the other approaches. It is much less painful than the transternal approach and recovery is much quicker. The thymus is essentially an anterior mediastinal structure and therefore, it is more direct to approach it from the chest than from the neck. The transcervical approach has the disadvantage of instrument crowding through a small, single access. Cosmesis, following VATS is excellent. Although this, on its own should only be regarded as a bonus rather than the main reason for choosing a particular surgical approach, the majority of MG patients are young females who care a lot about the surgical scars. As there is some evidence that the sooner the patient with generalized MG have surgery, the better the long term outcome, it follows that the VATS approach could encourage earlier acceptance of thymectomy by patients and earlier referral of these patients by their neurologists.27
Pericardial window
Video-assisted thoracic surgery provides a safe and effective approach to the drainage of pericardial effusion of both benign and malignant aetiology. Large pericardial windows can be created both anterior and posterior to the phrenic nerve for drainage.28 When pericardial effusion is associated with pleural effusion, the pericardium should be approached from the side with the pleural effusion (or with more effusion in bilateral disease), otherwise a right approach is usually preferred as the right haemithorax is larger than the left, giving a better perspective for VATS evaluation. Caution has to be exercised in the use of monopolar electrocautery to avoid touching the heart and precipitate dysrhythmia. Subxiphoid drainage remains a viable alternative approach which could be performed under local anaesthesia in patients who are very ill and debilitated and in those with a history of bilateral chest surgery or pneumonia where troublesome adhesions are anticipated. A video-assisted subxiphoid approach using a videomediastinoscope is a recent refinement of the old procedure.29
Anatomical lung resections
The application of VATS to anatomical lung resections continues to be a subject of considerable controversy. Even among surgeons practising VATS, only a few use this approach for lobectomy.30 Anatomical dissection performed through a minithoracotomy in an essentially closed chest raises questions about the safety of the technique; resection for intrathoracic malignancy casts doubt on adequate clearance; the long-term benefits of VATS over conventional thoracotomy approach are uncertain;11,31 and the high cost of the consumables and endoscopic equipment questions the cost effectiveness of this approach in the current era of cost containment.6
However, despite all the scepticism, intermediate term results from several centres performing VATS lobectomy have been very encouraging.30 The survival figures for lung carcinoma following VATS resection are at least as good, if not better than a similar group of patients following resection through a conventional thoracotomy. If these reports could be substantiated by a larger body of experience, it could have an important impact on the surgical management of thoracic malignancy. The reason for the improved survival remains unclear at present but there is circumstantial evidence that by minimizing chest wall trauma, the body inflammatory response is dampened and immune function better preserved.32
The actual technique of VATS lobectomy is by no means a unified approach. Dr Ralph Lewis in New Jersey, USA advocates the simultaneous stapling technique for the bronchus and pulmonary vasculature,33 while other surgeons, including ourselves, continue the individual ligation technique of the hilar structures. There is little consensus on the size of the minithoracotomy and more importantly, the use of a rib spreader. We recommend that the term VATS lobectomy should be reserved to the predominantly endoscopic technique with little or no rib spreading (Fig. 4),34 while the term minithoracotomy with video assistance (MVA) lobectomy should be used instead when rib spreading is routine and the surgeons operate by looking through the minithoracotomy. Mediastinal lymph node sampling or dissection by the VATS approach have been described.11,30
Regardless of the exact technique, it is generally agreed that careful patient selection is essential. Our own patient selection criteria for tumour resection include stage I non-small cell lung cancer (without evidence of endobronchial or chest wall involvement), tumour size less than 4 cm, and complete or near complete fissures (a thoracoscopic assessment).
We believe that VATS lobectomy is a feasible and safe procedure in experienced hands and may be of particular benefit to the elderly and patients with multiple co-morbidity who are otherwise poor-risk candidates for conventional thoracotomy. The exact role of this procedure, however, awaits its long-term results to be compared with thoracotomy.30
OTHER APPLICATIONS OF VIDEO-ASSISTED THORACIC SURGERY
Many thoracic procedures are being rediscovered through the thoracoscope, some of these also involve other surgical subspecialties. Thoracoscopic spinal surgery is receiving increasing attention by the orthopaedic community. Video-assisted surgery for spinal deformity is now feasible.35
Video-assisted thoracic surgery is also playing an important role in minimal access cardiac surgery. The left internal mammary artery (LIMA) can be harvested with thoracoscopic assistance through an anterior minithoracotomy.36 This incision is subsequently used for the anastomosis of LIMA to the left anterior descending (LAD) coronary artery in a procedure now referred to as minimally invasive direct coronary artery bypass grafting (MIDCABG). The thoracoscope has also found use in minimal access mitral valve surgery and a totally endoscopic approach to valve replacement and coronary revascularization (port access approach).37
FUTURE PROSPECTS
Cardiothoracic surgery is undergoing a rapid flux of evolution as the development of videothoracoscopy revolutionizes its practice. The question now is, does VATS, as we currently practise it, represent an end point that only requires minor refinements or is it an intermediate step to an even less invasive approach? We believe that both views may be correct as VATS represents a spectrum with a purely endoscopic approach at one end and a video-assisted approach (with a utility minithoracotomy) at the other. For the purely endoscopic procedures, there have been attempts to modify further the surgical access and mode of anaesthesia. The former resulted in the development of 2 mm ‘needlescopic’ instruments and the latter in therapeutic thoracoscopy under local anaesthesia. It is entirely possible that in the near future, simple thoracoscopic procedures could be performed as an outpatient procedure under local anaesthesia via an essentially percutaneous route with miniaturized instruments. However, it is important to remember that carefully conducted clinical trials should precede the general acceptance of a new technique or technology, no matter how attractive it may appear initially.5