Thoracoscopic procedures for intrathoracic diseases: The present status
Abstract
Thoracoscopic operations, alternatively termed as video-assisted thoracic surgery (VATS), are replacing the variety of surgical procedures which have been otherwise performed by open thoracotomy. The minimally invasive nature of the procedure, reduced postoperative pain, shortened hospital stay, and reduced cost, are the potential advantages of VATS. While these merits are being proven, the limits to this technique are also getting clearer. In fact, VATS has already become a standard treatment of choice in several diseases, such as bullectomy for spontaneous pneumothorax and biopsy for indeterminate nodule and diffuse interstitial lung disease, while others, such as major video-assisted lung resection for lung carcinoma and resection of metastatic lung tumour, await further evaluation of their roles in terms of oncological and technical aspects. Three issues that currently need to be addressed as the present role of thoracoscopy evolves are instrumentation, economics, indication, and end results in certain procedures.
INTRODUCTION
Thoracoscope is, by its original meaning, defined as an endoscope to observe intrathoracic space, like a bronchoscope for bronchial lumen and a gastrofibrescope for oesophageal, gastric, and duodenal lumina. But today this endoscope has also made it possible to perform various kinds of intrathoracic procedures which had been otherwise done with open thoracotomy.
The technique of thoracoscopy is attributed to Jacobaeus who described his experiences in 1910.1,2 However, before 1990 the role of thoracoscopy had been restricted to observation, biopsy, empyema irrigation, management of pneumothorax, sympathetic chain ablation, and removal of intrathoracic foreign bodies. Owing to the advent of video-image technology and instruments originally designed for laparoscopic use, thoracoscopic technique has been dramatically improved since its first introduction in 1990.3 At present, the indication has been extended to various kinds of procedures and their efficacy has been evaluated. However, the limitations to this procedure are also becoming clearer. In fact, surgeons do not seem to have reached a general consensus on indication and current role of this procedure; some indications remain controversial.4
Today the term, video-assisted thoracic surgery (VATS), is widely used to describe every procedure performed under thoracoscope in not only the thoracic space but also the chest wall and mediastinum. This review is intended to describe the basic principle, indications, contraindications, and practical technique of VATS in the present day so that not only thoracic surgeons but also anaesthesiologist and pulmonologist can provide enough information to patients who are possible candidates for VATS.
BASIC PRINCIPLES OF THE PROCEDURE: ELEMENTS OF VATS
The thoracic cavity is usually filled with expanded lung, and only very limited space is left when the lungs are aerated. To make thoracoscopic procedures possible, deflation (collapse) of the lung on the affected side is indispensable to provide the space for observation and working of surgical instruments. A collapsed lung and subsequent working space are the first elements of VATS. The second essential element is the scope and specially designed equipment. It is fortunate for thoracic surgeons that a humble gas insufflation is not necessary in VATS. One of the most characteristic differences between the thoracic and abdominal cavities, is the fact that the thoracic cavity is sustained by the bony frame of 12 pairs of ribs. Therefore, if one entire lobe is collapsed by one-lung ventilation, enough space is given for vigorous observation of the entire thoracic cavity and for manipulation of surgical devices without any insufflation of carbon dioxide, which is always necessary in laparoscopic surgery together with air-tight valved trocars.
The distinctive features of VATS compared with the open procedure should be well recognized. Thoracoscopic surgery greatly reduces the surgeon's ability to use tactile feedback. The second point is that the images are essentially lacking in three-dimensional information in spite of their high resolution quality. The size of operative view is also restricted. Therefore, surgeons are required to be very familiar with the three-dimensional sensation of the phantom images, and possess a detailed knowledge of the topographical anatomy in the thorax as well as the possibility of anatomical variations.
BASIC OPERATIVE SET-UP AND PROCEDURE
Set-up
A thoracoscopic approach requires an appropriate operating room environment that allows the thoracic surgeon the immediate potential to convert to an open thoracotomy if necessary. The basic operative set-up includes the following:
(1) General anaesthesia with double-lumen endotracheal tube (Bronchocath®, Malincroldt, Athrone, Ireland) or bronchial blocker tube (Univent tube®, Fuji systems Inc., Bunkyo, Japan);
(2) Equipment: thoracoscope (10 mm operative scope, straight or 30°); high-resolution video monitor; 2–4 intercostal access thoracoports; endoscopic stapling devices (Endopath Endocutter ETS,® ETS-FLEX,® Ethicon, Cincinnati, OH, USA; EndoGIA,® EndoGIA II,® US Surgical, Norwalk, CN, USA; see Fig. 1); endoscopic scissors; endoscopic forceps; endoscopic bags (EndoCatch,® US Surgical, Norwalk, CN, USA); and endoscopic dissector (Cotton Finger,® Kenz Medico, Kodama, Japan);

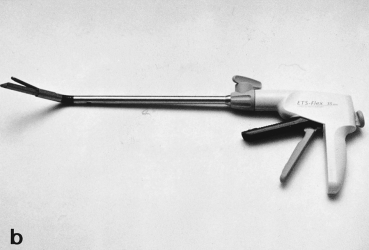
Endoscopic staplers used in video-assisted thoracic surgery. (a) EndoGIA II (US Surgical, Norwalk, CN) and (b) Endopath Endocutter ETS-Flex (Ethicon, Cincinnati, OH) (with permission of US Surgical and Ethicon).
(3) Open thoracotomy instrument tray, which must be opened and available on request.
Basic technique
Regardless of surgical procedures, there are several common principles in VATS. The patient is positioned in a full lateral decubitus position with the thorax surgically prepared in case conversion to an open thoracotomy is necessary during the course of the operation. A VATS procedure is started by choosing the best site for paramount observation of the thoracic cavity, usually it is in the 6–7 intercostal space on the midaxillary to posterior axillary line. The rest of the trocars are located according to the lesion of interest and ease of instrumental access. In most of the procedures, two to four trocar sites are necessary. However, the following rules should be applied whatever the procedure is:
(1) Trocars are placed far enough from the lesion to allow surgical manipulation and observation;
(2) Each trocar site is far enough away from each other to avoid the crossing of instruments;
(3) All trocar sites must be located within the same 180° arc to avoid the mirror imaging.
When considering these rules, the trocars are usually placed on three neighbouring corners of the lozenge with the target lesion on the remaining one (Fig. 2). For more complex procedures, such as lobectomy of the lung, access thoracotomy or utility thoracotomy is performed. I usually make a 5 cm long thoracotomy wound according to the lobe resected (Fig. 3).5 In manipulating the scope and instruments, it is important to move them serially, and not simultaneously or randomly.3
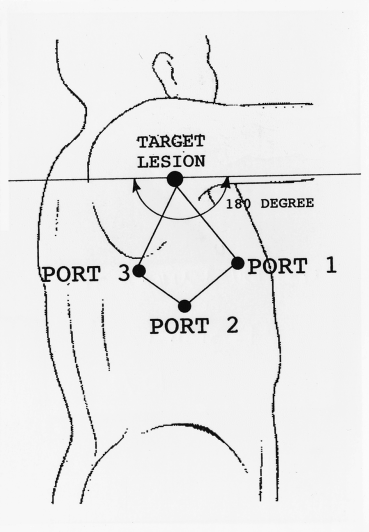
Location of three trocar incisions. Trocars are located on the three adjacent corners of lozenge with the lesion on the remaining one within the same 180° arc.
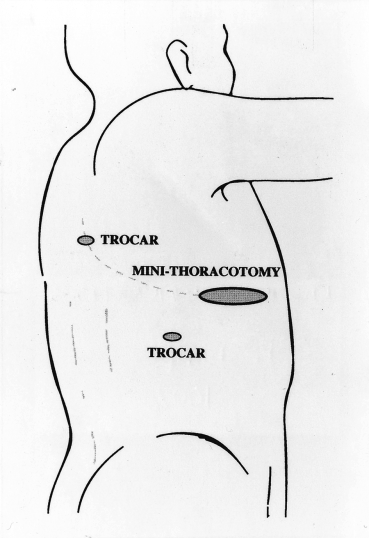
Minithoracotomy and two trocar sites for performing video-assisted upper lobectomy of the right lung.
CONTRAINDICATION FOR THORACOSCOPIC SURGERY
Video-assisted thoracic surgery procedures are not always possible; in several situations VATS is more likely to induce intra-operative and postoperative complications than open thoracotomy. Surgeons must know the technical and oncological limitations to VATS and provide detailed information prior to surgery.
Patients who cannot tolerate one lung ventilation well
Although patients at poor risk for surgery may best benefit from minimally invasive procedure, VATS cannot be safely done if patients cannot tolerate well one lung ventilation because of poor cardiopulmonary function. As the collapse of one whole lung is indispensable to ensure a good operative field and working space for the instrument, patients in whom there is significant decrease of arterial oxygen saturation after one lung ventilation are not good candidates for the VATS procedure.
Extensive adhesion in the thoracic cavity
If intrathoracic adhesion, mostly due to previous pleurisy, is extensive, it is impossible to have an enough working space. Lysis under thoracoscopy can be done, but it is usually time-consuming with significant amount of blood loss. There might be an even higher risk of damaging the visceral pleura and lung parenchyma, which may cause prolonged air leakage especially in the elderly with bullous lungs. Therefore, except for a localized one, the extensive symphysis of pleura should be respected as a contraindication and a case which should be converted to the open thoracotomy.
Complex anatomical variation
In the case of complex pulmonary resections, such as lobectomy, surgical orientation might be lost because of the anomalous branching of the pulmonary vessels and bronchus, which is usually noted during surgery. In such a situation, the procedure should be converted to open thoracotomy without hesitation.
Large lesions
Any large intrathoracic mass, wherever it is located, is difficult to retrieve from the thoracic cavity. Not only do they require enough extension of the trocar port or access thoracotomy, but they may be crushed during extraction, which may result in tumour dissemination.
Small thoracic cavity
Working space for instrumental manipulation and observation and intercostal access via the trocar port is limited in a small thoracic cavity.
Difficult location
For intrapulmonary and mediastinal lesions, there are some locations where movement of thoracoscopic instrumentation is quite difficult; in lesions located at the mediastinal aspect of the lung, and those close to hilum. Mediastinal masses adjacent to the vital structures such as great vessels are also not suitable for this technique because of danger of massive bleeding.
Minute nodules located deeply in the lung parenchyma
The exact location of tiny nodules which are deep under the overlying visceral pleura are difficult to define by thoracoscopic observation alone. When thorough palpation is necessary, open procedures are advised, although several alternative methods are available.6,7
BENEFITS OF THORACOSCOPIC SURGERY
The most obvious potential benefit of thoracoscopic surgery is the reduction of both acute and chronic postoperative pain. There has been an accumulation of data suggesting that the postoperative pain in VATS, especially in its acute period, is significantly reduced compared with that after open thoracotomy. For instance, Landreneau et al. demonstrated that less than one year after the procedure, the presence of pain, the perceived magnitude of the pain, and shoulder function were superior with VATS resection to a lateral thoracotomy.8 After one year there was no difference. Guidicelli et al. compared 44 VATS minithoracotomies with 23 open muscle-sparing thoracotomies and also found a statistically significant reduction in early (<1 week) postoperative pain in the minithoracotomy group.9
Other potential advantages of a VATS procedure are shorter operating time, decreased hospital stay, additional potential benefit gained by an earlier return to work, and reduced cost. Hazelrigg et al. made a cost analysis for thoracoscopic wedge resection of the lung in comparison with open wedge resection.10 They demonstrated that disposable instrument charges were elevated for the thoracoscopic procedures, but a shortened hospital stay and operative time for the VATS procedure resulted in the total charges being comparable. For spontaneous pneumothorax, Crisci and Coloni demonstrated the therapeutic efficacy of VATS and that in VATS the total economic cost is lower (22.7%) in comparison with traditional thoracotomy.11
The medical community is concerned to define what are the actual advantages of VATS procedures compared with the open procedures, and if there are advantages, how large these are. We need further clinical studies to define the advantages of VATS considering differences in medical environment such as admission policy and medical care insurance.
VATS-RELATED COMPLICATIONS AND ADVERSE EFFECTS
The incidence of complications in relation to VATS procedures is known to be acceptable and similar to that seen after open thoracotomy. The common forms of complication are similar to open procedures such as prolonged air leak, superficial wound infection, and bleeding requiring transfusion or rethoracotomy.12 Two issues are discussed.
Tumour implantation at the port site
Until now, local dissemination and implantation of most malignant tumours of the thorax to thoracotomy incisions have been infrequent. As the VATS procedures have been becoming more common for various kinds of diseases, reports describing the tumour implantation at the port site and pulmonary staple line is accumulating.13,14 Downey et al. reported 21 patients in whom tumour implants were thought to be directly related to VATS techniques.13 Their histological types were lung carcinoma in 10 cases, metastatic lung tumour in five, mesothelioma in five, and oesophageal carcinoma in one. They noted that considering the special nature of mesotheliomas, that they tend to grow into thoracotomy and chest tube incisions, the port site recurrence in VATS procedures might be highly expectable. These implants may be attributable to the technical features of VATS, as they are not seen so frequently in open procedures. The disruption of the tumour might occur at two occasions; at the time of pulling the mass through a relatively small incision; or removing the mass from the lung parenchyma. The former leads to deposit clumps of viable tumour cells in the chest wall and the latter to implantation either along the margin of resection or on the pleural surface. Some of these implants can be avoided when the specimen is extracted in special bags such as surgical gloves or EndoCatch® (US Surgical) and when the wound incisions are always protected by the metal or plastic ports. Clamping the surrounding tissue or tumour itself too closely or too tightly should be also avoided in manipulation.
This adverse phenomenon is likely to be greatly reduced in future if the surgeons involved in VATS realize the importance of preventing implants.
Prolonged air leakage
A disadvantage of parenchymal lung resection by VATS is the difficulty of performing a sealing test to detect a pleural tear causing an air leak by filling the thoracic cavity with water, as visual field is limited in VATS, particularly when the lung is fully inflated with positive pressure. Perhaps some of the points of air leak are overlooked for this reason. The use of special materials such as bovine pericardium (Peri-strip®, Bio-Vascular Inc, St Paul, MN, USA) may reduce the incidence of this complication.
VARIOUS PROCEDURES IN THORACOSCOPIC SURGERY
Various kinds of thoracic surgical procedures which have been otherwise performed by open thoracotomy are being replaced by VATS (Table 1). Some of them are already established as a standard treatment of choice, whereas others await further definition of their role. Procedures in which VATS techniques actually have a great technical impact are discussed in the following paragraphs.
Diagnosis of indeterminate pulmonary nodule and diffuse interstitial lung disease
Diagnostic approaches toward the indeterminate solitary pulmonary nodule have been bronchoscopy and percutaneous needle biopsy. Indeed, bronchoscopy is helpful for large central and intraluminal lesions but has a low diagnostic yield, approximately 10% for small, peripheral nodules.15 Transthoracic needle aspiration biopsy (TNAB) has reported diagnostic accuracy ranging from 43 to 97% for malignant lesions but is less effective for obtaining a definitive diagnosis in benign nodules.15 Thoracotomy is accurate for diagnosis but has significant morbidity and surgical burden. Based on the experiences of 242 patients with solitary pulmonary nodule who underwent thoracoscopic biopsy, Mack et al. reported virtually 100% sensitivity and 100% specificity of this procedure with no mortality and minimal morbidity such as atelectasis, pneumonia, and prolonged air leak.15 In fact, thoracoscopic biopsy by wedge resection is already playing a significant role for definite diagnosis with the least invasiveness to the patients, although nodules located deeply in the lung parenchyma necessitate a special technique for localization.6,7 The role of thoracoscopy as a diagnostic tool is established and included in the approaches available.
There are advantages in thoracoscopic parenchymal wedge biopsy for interstitial lung disease over conventional biopsy through a limited thoracotomy; the procedure is more simple, and samples can be taken from the most appropriate areas after full inspection of the entire lung rather than simply the area underlying the minithoracotomy site. Diagnostic accuracy is at least as good as for open biopsy.16 It is, however, still important to make a plan concerning the exact location for biopsy with a computed tomography (CT) scan prior to surgery.
Diagnosis and treatment of lung cancer
Diagnosis
Thoracoscopy is the only diagnostic modality to observe intrathoracic pathology. Some of the TNM factors indicate surgical inoperability; pleural dissemination (T4), malignant effusion (T4), intrapulmonary metastasis (T4 or M1), invasion to the vital structures (T4), and extensive mediastinal lymph node metastasis (N2 or N3). Pre-operative thoracoscopy might be helpful in detecting these findings without painful thoracotomy. We have performed exploratory thoracoscopy for 116 surgical candidates with lung carcinoma and found inoperable findings such as pleural dissemination, malignant effusion, and bulky lymphadenopathy in five patients (4.2%).17 Wain18 and Roviaro et al.19 showed a similar incidence of inoperable findings using exploratory thoracoscopy for lung carcinoma at 4.7 and 8.3%, respectively. Therefore, thoracoscopic observation of the affected side of the lung prior to thoracotomy is strongly advised, particularly if questionable findings such as pleural thickening are shown on CT. If case inoperability is established prior to thoracotomy, patients will benefit greatly as it obviates the need for painful exploratory thoracotomy.
Treatment
The role of thoracoscopy in the treatment of lung carcinoma has yet to be defined. It has been shown that lobectomy is a preferred mode of resection compared with limited resections such as segmentectomy and wedge resection, as patients are at good risk.20 Hilar and mediastinal lymph node dissection is also advised on an empirical basis because of the useful prognostic information that can be obtained and possible chance for cure.
As for an operative mode, wedge resections of peripheral tumours have been performed safely on high-risk patients with acceptable results.11 In fact, in these patients, a resection, although limited, is superior to radiotherapy and with the use of VATS the residual lung function is not impaired as much as after standard thoracotomy.21,22 Video-assisted lobectomy (VATS lobectomy) has been shown to be technically feasible using one minithoracotomy and a couple of port accesses.5,19 However, few reports have clearly shown that complete lymph node dissection can be done by VATS as it is with open thoracotomy. In fact, questionnaires to North American thoracic surgeons showed that VATS lobectomy was considered a ‘preferred’ or ‘acceptable’ therapy for lung carcinoma by only 19% of respondents, and rather ‘investigational’ or even ‘unacceptable’ by the rest.4 This indicates that not only the spread of cancer, but the ability to perform a ‘complete’ oncological operation without compromise (e.g., adequate regional lymph node dissection) was a significant concern among thoracic surgeons.
Recently, some reports have included peripheral T1N0M0 (Stage 1a) lung carcinoma as a candidate for VATS lobectomy with curative intent.19 We have investigated lymph node metastasis in 337 resected small (<3.0 cm in diameter), peripheral, non-small cell carcinomas, and found mediastinal lymph node metastasis in 16.3% of patients.23 Even for tumours less than 2 cm in diameter mediastinal metastasis was still seen in 11.5%. Therefore, because of the relatively high prevalence of lymph node involvement, complete hilar/mediastinal lymph node dissection should be routinely done regardless of tumour histology and size. At present in the National Cancer Center, Tokyo, the VATS lobectomy is indicated when mediastinal lymph node dissection is omitted from the scheduled surgery because of: (i) poor risk for surgery for patients due to conditions such as poor cardiopulmonary function, serious diabetes, and advanced age; and (ii) the nature of the tumour has a low possibility of spread to the mediastinum in histologic types such as in situ bronchioloalveolar carcinoma and squamous cell carcinoma of the peripheral origin without hilar lymph node involvement.
The indication of VATS for treatment of lung carcinoma is one of the greatest concerns among the thoracic surgical community. Further accumulation of data and discussion are necessary.
Resection of metastatic lung tumour
Pulmonary metastases, when found as the only site of metastatic disease in patients where the primary tumour has been controlled, are often best treated by metastasectomy. Standard surgical approaches for pulmonary metastasectomy have been thoracotomy or median sternotomy with parenchymal sparing resection such as wedge resection. Only metastatic lesions deeply located in the parenchyma or close to hilum require anatomic major pulmonary resection. Metastasectomy with VATS technique has the potential benefit of avoiding the morbidity associated with open approaches while allowing for resection equivalent to that performed with open techniques. However, two issues must be considered. First, VATS technique does not allow careful palpation of the lung parenchyma, which often detects small metastases not detected by CT scan. McCormack et al. retrospectively compared the radiological findings of CT scan with the surgical findings of 72 patients with primary colon cancer metastatic to the lung, and found the CT scan underestimated surgical findings in 42% of cases.24 Following this study, they did a prospective study to compare the value of CT scan, VATS exploration, and open thoracotomy in the management of pulmonary metastases.25 Patients presumed to have one or two ipsilateral pulmonary metastases on CT scan initially underwent VATS exploration and resection of all lesions. Subsequently, they underwent thoracotomy for complete palpation of whole lung, and any additional lesions found were removed. Eighteen patients of a planned 50 were treated before closure of the study, and additional benign lesions were found at thoracotomy in 22%, additional malignant lesions in 56%, and nothing in 22%. Because of a 56% failure rate of CT scan and thoracoscopy, the study was closed early. The results of this study send a warning not to overlook the minute metastatic lesions only discovered by palpation. Second, in performing multiple resections by stapling, the application of the stapler too close to the lesion may cause a problem. As palpation is limited in VATS, the application of the stapler at an adequate distance from the lesion is difficult, especially for those located deeply under the pleural surface. The role of VATS in therapeutic metastasectomy remains to be defined.
Spontaneous pneumothorax
Spontaneous pneumothorax is usually caused by the rupture of a subpleural bleb in the underlying lung and represents one of the most common elective applications of VATS. The goal of surgical intervention is to restore the full expansion of the lung and to prevent recurrence. Intercostal tube drainage of the pleural space is the accepted method of treatment in patients when the degree of lung collapse exceeds 25%. Failure of the lung to re-expand after tube drainage may be as high as 35%, and recurrent pneumothorax may occur in 40%. Currently the accepted indications for surgical intervention are persistent air leak, recurrent spontaneous pneumothorax, contralateral pneumothorax, and the first pneumothorax occurring in high risk occupations such as being a pilot. At present, the thoracoscopic approach toward spontaneous pneumothorax is the combination of bullectomy with partial parietal pleurectomy or pleural abrasion.26,27 Although parietal pleurectomy is currently not indicated in younger patients because of the future possibility of re-opening the pleural cavity for any reason, it may play an important role in preventing the relapse of the pneumothorax.
Thymectomy
Thymectomy is indicated for the relief of symptoms associated with myasthenia gravis (MG) and for the resection of thymus-related neoplasms such as thymoma, thymic carcinoma, and thymic carcinoid tumour. To date the best surgical approach to thymectomy remains undecided; the available surgical options include median sternotomy, median sternotomy combined with a transverse cervical extension (Jaretzki's maximal thymectomy28), partial sternotomy, transcervical approach, and more recently the video-assisted thoracoscopic approach. The selection of the appropriate approach depends upon the disease itself and performance of thymectomy. Apparently, thymomas invading the neighbouring structures such as lungs, great vessels, and pericardium and those of considerable size are best managed by resection with a transternal approach or at best with thoracotomy. Normal looking or slightly hyperplastic thymuses in MG and those with small encapsulated thymoma and benign conditions such as thymic cyst may be operable using other approaches for thymectomy. However, regardless of technique, it is generally agreed that thymectomy for MG should be complete as the exact relationship between the gland and the disease is unclear. Today, video-assisted thymectomy is being indicated mainly for thymectomy for MG. Thymomas of considerable size requiring thoracotomy of any size to be drawn from the thoracic cavity, or apparently invasive thymomas, are generally not considered candidates for this VATS technique from the technical and oncological viewpoint.
Video-assisted thymectomy can be done from either side of the thoracic cavity, but Yim et al. suggested that the right-sided approach is preferable because of the better recognition of subclavian and caval veins in relation to thymus.29 From three trocar ports, the entire thymic tissue including superior and inferior horns is dissected free from the pericardium and pleura of both sides with endoscopic instruments and removed in a plastic bag through one of the port incisions. The recently introduced Harmonic Scalpel® (Ethicon, Cincinnati, OH, USA) is preferably used for dissection (Fig. 4). In the case of thymoma, resection is approached from either side depending on the location of the mass, and again, larger and/or invasive thymomas should not be resected by VATS technique.

Harmonic scalpel® for dissection of the mediastinal fat tissue (Ethicon, Cincinnati, OH).
Mack et al. studied 33 patients with MG under-going VATS thymectomy and using meta-analysis, compared the efficacy with that of 9 previous reports in which other techniques were used. No difference was shown in terms of clinical improvement after thymectomy between the series and it was concluded that VATS thymectomy is as effective as the traditional open surgical approaches of thymectomy.30 Further investigation with long-term follow-up is needed to further clarify the role of video-assisted thymectomy for the management of MG and small-sized encapsulated thymoma.
Pericardial window for pericardial effusion and subsequent cardiac tamponade
Symptomatic pericardial effusions are common and may result from a variety of causes, of which malignancy is the single most common source constituting between 30–50% of causes. Because excessive accumulation of pericardial effusion causes cardiac tamponade and subsequent heart failure, medical and surgical intervention is necessary. For patients with short expected survival times, pericardiocentesis and the instillation of sclerosant agents may be indicated. However, they are accompanied by a number of complications.31 Surgical options include a subxiphoid resection, median sternotomy, anterolateral thoracotomy, or a thoracoscopic approach. Among these, the subxiphoid approach has been accepted widely because it is simple and can be performed with local anaesthesia in a debilitated patient.
The potential advantage of video-assisted pericardiectomy over the subxiphoid route is that it can allow for a more extensive pericardiectomy with full visualization of the pericardium and can be safely done even in a patient who has a past history of median sternotomy operation. However, the disadvantage of VATS pericardiectomy is the more complex anaesthetic management required, as very ill patients with far advanced malignancy cannot tolerate general anaesthesia.32
Video-assisted pericardiectomy is usually performed through the left thoracic cavity with three trocar ports. They are placed posterior to the posterior axillary line to enhance the distance from the pericardium for greater ease of instrumental manipulation as well as prevention of heart injury during trocar insertion. After the full retraction of the lung posteriorly, the pericardium anterior to the phrenic nerve is resected with endoscopic scissors to make a defect large enough. If there is posterior loculation of fluid, the posterior portion to the phrenic nerve can also be resected.
Video-assisted pericardial resection is safe and effective. It allows a wider pericardial resection than that usually permitted by the subxiphoid route. The simultaneous management of both pericardial and pleural abnormalities may be another advantage. However, as this procedure is often indicated for seriously ill patients with deteriorated cardiopulmonary function and the subxiphoid approach does not obviate general anaesthesia, the role of video-assisted pericardiectomy must be defined more clearly.
Acknowledgements
This work was supported by a Grant-in-Aid for Cancer Research (7–23) from the Ministry of Health and Welfare, Japan.